Abstract
In this study we aimed to evaluate the role of closed-suction drainage on the extent of epidural fibrosis (EF) after lumbar disc surgery and to define a new grading system of epidural fibrosis in these patients, based on magnetic resonance imaging. Seventy-nine patients (34 women, 45 men) with a unilateral, single-level lumbar disc herniation were included in this study. Forty-one patients in whom closed-suction drainage was implanted were compared with 38 patients in whom the drain was not implanted. We have used a new grading system for the extent of epidural fibrosis, on the basis of follow-up magnetic resonance imaging findings. Pain intensity was evaluated by visual analog scale (VAS), and the patients’ function and working ability were measured according to the Prolo functional-economic scale. We conclude that, in patients operated on for unilateral, single-level lumbar disc hernias, implantation of closed-suction drainage into the operation site results in less formation of EF radiologically and yields better clinical outcome.
Keywords: Epidural fibrosis, Closed-suction drainage, Lumbar disc surgery, Magnetic resonance imaging
Introduction
Failed back surgery syndrome (FBSS) is a clinical condition with which patients who undergo one or more surgical procedures for lumbosacral disease obtain unsatisfactory long-term relief of symptoms, with persistent or recurrent low back and/or leg pain. The primary factors leading to FBSS include iatrogenic instability, lateral and central spinal stenosis, arachnoiditis, recurrent disc herniation, and epidural fibrosis (EF) [10, 11, 18].
Approximately 10–24% of all cases of FBSS are the result of EF. Possible mechanisms of recurrent pain caused by EF include nerve root irritation, nerve root entrapment, direct dural compression, restriction of nerve root mobility, which increases susceptibility to small, recurrent disc herniations and osteophyte formation [10, 23]. Reoperation on scar often produces a poor surgical result and further scarring.
EF is part of the normal physiological tissue response to laminectomy and is an important cause of FBSS. This response may be extensive due to fibrous organization of hematomas, various other issues related to surgical trauma and technical failure [9].
Many materials have been used as a barrier for the invasion of fibrous tissue into the vertebral canal, but the ideal material hasn’t been found yet. The results achieved with these materials have been unsatisfactory, variable or preliminary. Closed-suction drains (CSD) (Wound drainage system, Medimark, Grenoble, France) may be used for the removal or emptying of postoperative collections at the operation site, and have an important role in surgical wound healing. For this reason most surgeons use them routinely.
In this prospective study we aimed to evaluate the postoperative EF, postoperative pain scales and scores, conducted by the same surgeon with the same technique in two groups of patients. In one group CSDs were used, and in the other group CSDs were not used. We have defined a new grading system for the extent of EF on the basis of follow-up magnetic resonance imaging (MRI) findings.
Patients and methods
Seventy-nine patients (34 women, 45 men) with a unilateral, single-level lumbar disc herniation, who were operated on by the same surgeon (0.S.) were included in this study. Forty-one patients in whom CSDs were implanted were compared with 38 patients in whom the drain was not implanted. Although we tried to have same number of patients in both groups, three patients in whom we did not implant CSDs were lost to follow-up and were excluded from the study.
Mean age was 46.44 years (range, 23–82 years; SD, 10.9). Surgical pathologies were divided into two groups according to the radiological and operative findings: (1) extruded disc and (2) protruded disc. Surgical approach was performed at the levels L2–3 (n=2), L3–4 (n=9), L4–5 (n=38) and L5–S1 (n=30). Past medical histories of all patients were unremarkable for hematologic or metabolic disorders. There was no other active disease. Full blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), activated thromboplastin time (APTT), fibrinogen, partial thromboplastin time (PTT), serum calcium, phosphate, and alkaline phosphatase levels were normal.
All operations were performed by the same neurosurgeon (O.S.). The operative reports described whether the nucleus pulposus had herniated through the annulus fibrosus or ruptured through a prominent bulge of the annulus. If the nucleus pulposus had extruded into the extradural canal, its anatomical relationship to the nerve root was recorded. Patients with spinal stenosis, infectious spondylitis, lateral recesses, neoplastic diseases and thickened ligamentum flavum were excluded. All patients were operated on in prone position under general anesthesia, and systemic prophylactic antibiotic therapy with cefazolin was administered intravenously in a dosage of 1 g at 2 h before surgery and 1 g every 6 h postoperatively, to a maximum of three doses.
After hemostasis, in all cases a 2 cm CSD with eight side-holes was placed at the operation site without negative-pressure generation, left only to gravity. The drains were maintained for 12 h postoperatively. After the drain was withdrawn, total drainage was noted, and the patient was mobilized. All of the patients were discharged from hospital 24–36 h post-surgery. The postoperative clinical follow-up period ranged 6–12 months (mean, 10.41 months; SD, 2.39 months), and the patients were invited to the hospital for reassessment. Pain intensity and disability were assessed both preoperatively and postoperatively. Pain intensity was evaluated by visual analog scale (VAS), the most commonly used measurement in many evaluation centers. Functional and working ability were measured for all of the patients according to the Prolo functional-economic scale, for which possible scores range from 10 (perfect) to 2 (incapacitated) and possible clinical outcomes are excellent (9–10), good (7–8), fair (5–6), and poor (2–4) [5, 6, 20, 21]. Both pain scales were conducted by a surgeon (M.V.A.) who was blinded to the surgery and present clinical status.
Magnetic resonance examinations were performed on the same MR machine (Siemens Magnetom Vision Plus, 1.5 T), using an 8-in. elliptical spine surface coil, with the patient supine and in head-first position, without knee support. Follow-up MRI was performed 6–12 months postoperatively (mean follow-up MRI time, 8.1 months; SD, 2.32 months). All examinations followed a standard protocol at each clinical site, as follows: (1) before contrast administration, two-dimensional axial T1-weighted spin-echo and T2-weighted turbo spin-echo sequences and sagittal T1-weighted spin-echo and T2-weighted turbo spin-echo sequences; (2) administration of 0.1 mmol/kg paramagnetic contrast material via slow intravenous push; and (3) axial and sagittal T1-weighted spin-echo sequences, completed within 15 min after administration of contrast material.
Both axial and sagittal images were obtained routinely; coronal images were obtained if necessary. Axial images were obtained in a gap-and-fill fashion, to allow complete coverage of the operative site and were contiguous. Axial images covered at least one level above the surgical site to one level below. Fat saturation was not used.
All MR images were interpreted independently by two neuroradiologists (O.K., O.Y.) blinded to the treatment arm and present clinical status. Presence of residual-recurrent disc herniation, EF, lumbar instability and infectious processes were evaluated carefully.
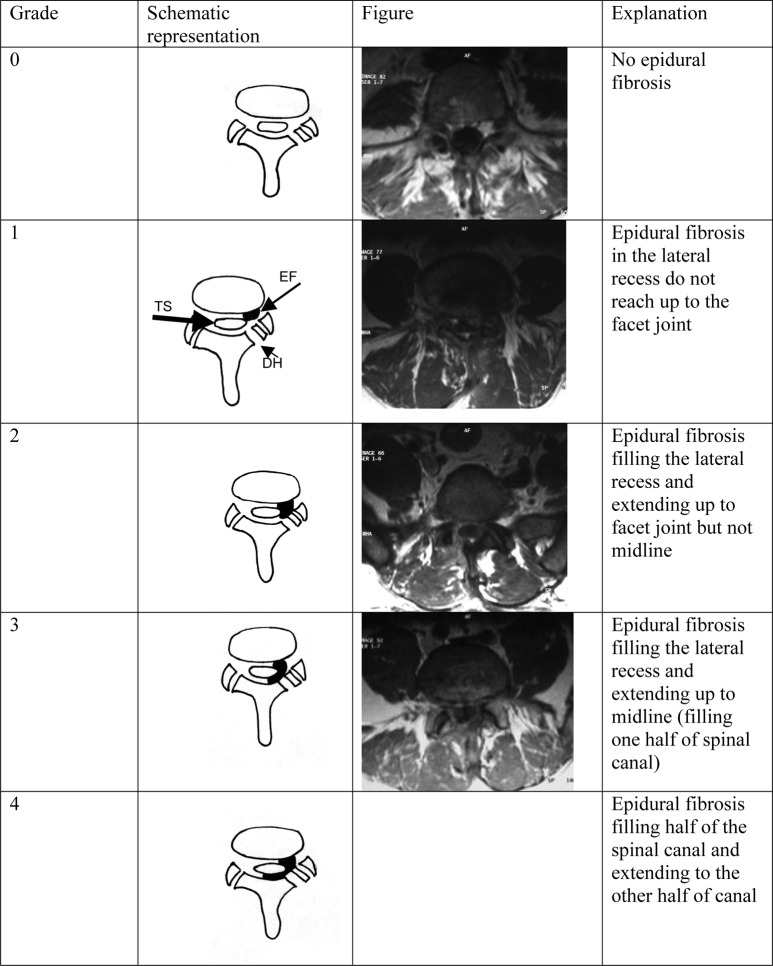
The criteria for identifying EF were as follows: EF is isointense to hypointense, relative to intervertebral discs on T1-weighted MRI. The MRI evaluation detailed the location and extension of EF on the basis of axial enhanced images. The presence or absence of mass effect is not a diagnostic criterion, since both EF and herniated discs may show mass effect. Retraction of the dural sac toward the side of the soft tissue is a criterion for EF. The extent of EF was graded on a scale of 0 to IV at each imaging section encompassing the surgical level. Since EF tends to form a curvilinear pattern surrounding the dural sac following the inside contour of lamina, we have used a new grading system that is a combination of systems defined by Annertz and Ross [1, 2, 3]. Our grading system for the extension of EF is explained and represented in Fig 1.
Fig. 1.
Left column shows grading system of epidural fibrosis and explanation; center column demonstrates schematic representation of our grading system; right column depicts the corresponding MR images (since we did not see any grade IV epidural fibrosis, there is no MR image for this category). (EF epidural fibrosis, TS thecal sac, DH defect of hemilaminectomy)
Statistical data
All continuous data are presented as mean ± SD and all categorical data as a percentage or number. Comparisons between groups were performed using the unpaired student t-test, x2 test, cross-tab analysis, descriptive analysis and independent samples test. Statistical significance was defined as p<0.05. All statistical analysis was done using SPSS computer software version 9.05.
Results
Periprocedural surgical complications, wound infection or discitis was not observed in our patient group. Mean length of hospital stay was 30 h. Mean amount of drainage was 25.85 cc ± 10.3 cc.
In the group in which CSDs were implanted, grade 0 EF was observed in 15 cases (36.6%), grade I in 20 cases (48.8%), grade II in six cases (14.6%) and grade III in none of the cases. In the group in which CSDs were not implanted, grade 0 EF was observed in none of the cases, grade I in seven cases (18.4%), grade II in 17 cases (44.7%) and grade III in 14 cases (36.8%). We did not observe grade IV EF in any of our cases. The mean EF grade was 0.78 (SD, 0.69) in the group in which CSDs were implanted, and 2.18 (SD 0.73) in the other group (p<0.0001). In the group in which CSDs were implanted, EF was less extensive than in the other group (Fig. 2).
Fig. 2.
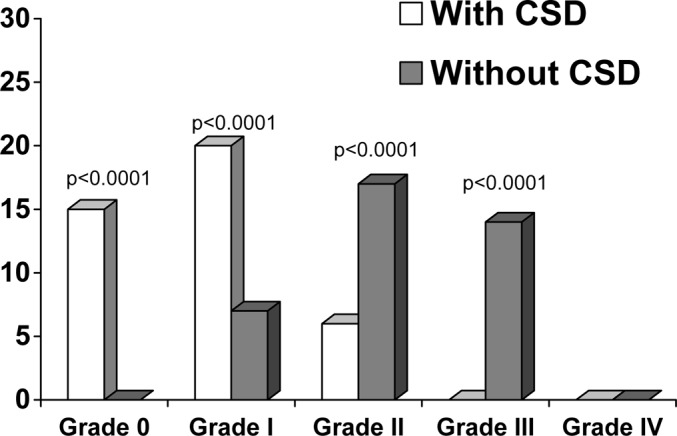
Graph showing the relation between epidural fibrosis and closed-suction-drain (CSD) implantation
Postoperative scores for VAS and the Prolo functional-economic scale are given in Table 1. Significant differences were determined (p<0.0001, t-test for paired samples) between preoperative VAS values (9.96±0.19) and postoperative VAS values (1.20±1.12) in the whole study group. The postoperative VAS score was 0.32 (SD, 0.52) for the group in which CSDs were implanted and 2.16 (SD 075) for the group in which CSDs were not implanted. Prolo-scale scores were 9.46 (SD, 0.78) for the CSD-implanted group and 7.13 (SD, 0.84) for the non-CSD group. Significant differences were determined between the CSD-implanted group and the non-CSD group (p<0.0001, t-tests for paired samples). Both scores of VAS and Prolo scale were better in the CSD-implanted group (Table 1).
Table 1.
Postoperative scores for visual analogue scale (VAS) and Prolo functional-economic scale, in combination with grades of epidural fibrosis, in patients with and without closed-suction drain (CSD)
| With CSD (n=41) | Without CSD (n=38) | |||
|---|---|---|---|---|
| Post-op VAS (Mean ± SD) |
Post-op Prolo (mean ± SD) |
Post-op VAS (mean ± SD) |
Post-op Prolo (mean ± SD) |
|
| Grade 0 | 0 | 10 | – | – |
| Grade I | 0.30 ± 0.47 | 9.5 ± 0.51 | 1.57 ± 0.53 | 7.86 ± 0.69 |
| Grade II | 1.17 ± 0.41 | 8 ± 0.63 | 1.94 ± 0.75 | 7.47 ± 0.62 |
| Grade III | – | – | 2.71 ± 0.47 | 6.36 ± 0.50 |
| Grade IV | – | – | – | – |
Residual-recurrent disc herniations were not observed, in postoperative follow-up MR images. For all MRI findings, the mean grade of EF was 1.46 (SD, 1.00) for neuroradiologist 1 and 1.38 (SD, 1.00) for neuroradiologist 2.
Discussion
EF is the cause of FBSS in 5–12% of the patients who undergo lumbar disc surgery. The EF rate in the Ebeling et al. series was 28% [8]. In various series the EF rate has been reported ranging from 10–75% [1, 2, 3, 14, 22, 23]. To date it has not been proven that EF formation causes radicular symptoms and low back pain postoperatively; however, anterior and posterior adhesions of the dura and nerve roots to EF tissues near spinal surgery sites are known to cause intractable pain [25, 26]. There is currently no effective medical or surgical therapy for EF.
LaRocca and Macnab termed this fibrosis “the laminectomy membrane” and found that fibroblasts originating from traumatized paraspinal muscles overlying the laminectomy site migrate to infiltrate and replace the epidural hematoma to form a dense scar [15].
Many methods have been studied to prevent EF. Several materials have been used in attempts to prevent the formation of EF. These materials include fat graft, absorbable gelatin sponge, Silastic sheeting, bone wax, steroids, polylactic acid, polymethylmethacrylate, carboxymethylcellulose, sodium hyaluronate, low-dose radiation, heparinized material, ADCON-L, urokinase, topical mitomycin c, tissue plasminogen activator (TPA), CO2 laser, among others [4, 7, 11, 12, 13, 17, 24]. Most heterologous materials are not effective, and have actually promoted a greater degree of epidural scarring compared with control specimens. Other materials deemed to be effective posed risks of serious complications, such as infection, mechanical compression of neural structures, and migration of the scar tissue around the margin of the material [15, 19, 24]. Neural compression by fat grafts has been reported [19]. ADCON gel is the most common procedure for preventing EF in current practice. But it is a foreign implant, expensive and requires additional time and surgical measures [7].
Meticulous techniques involving a small surgical wound, good hemostasis and small annular laminectomy incision are indisputable methods by which to decrease the incidence of EF [7, 27]. Fibroblast proliferation in the blood collection around the nerve root may provoke more-extensive fibrosis [2, 7, 8]. We implanted CSDs into the operation site in 41 of 79 cases after maximum hemostasis. We thought that prevention of hemorrhagic collection in the operation site might result in formation of less EF, as described by LaRocca and Macnab, and many others [2, 8, 15, 27]. It is theorized that rapid clearance of blood from the epidural area by CSD decreases the duration of contact of blood with the dura, and, consequently, the formation of EF may be reduced.
EF has been implicated as a factor contributing to continuing or recurrent radicular and/or low back pain. The association of EF and pain is controversial. Ross et al. suggested that there was a correlation between the extent of EF and the level of postoperative complaints. Jinkins et al. suggested that fibrosis in the epidural space may be less important in the pathogenesis of FBSS, and Coskun et al. did not find a relation between EF and pain scores or disability scores [2, 3, 5, 14, 16, 17, 23]. However, we have found a good correlation between scores for the VAS and Prolo functional-economic scale and the grade of EF by MRI. We suggest that fibrosis in the epidural space is important in the pathogenesis of FBSS. The good correlation between the radiological grade of EF and pain and disability scores suggests that the EF is not a radiological entity alone—it is also important for the patients’ complaints.
LIado et al. used expanded polytetrafluoroethylene (ePTFE) membrane in a clinical study to prevent formation of EF, and in 76% of their patients they did not see EF. Moreover, in patients in whom EF occurred, the EF was less extensive. They observed seromas in most patients in whom the ePTFE was implanted [17]. The formation of EF is the inevitable result of postoperative hemorrhages. We think that the CSD prevents postoperative hemorrhagic collection at the operation site and formation of seroma that gives rise to EF. In our study group, in 85.4% of the cases we did not see EF or the less-extensive form (grade I). However we did not see grade IV (most extensive type) EF in any of our cases.
For the most part, the rate of EF without its magnitude is given in the previous studies [1, 2, 3]. The grade of EF and occurrence rate was given first by Cervellini et al. [3]. In this study, we have evaluated the occurrence rate and EF grades in our patients. Cervellini et al. reported seeing mostly grade II EF. We observed grade 0 and I EF in CSD-implanted patients, and we observed grade II and III EF in non-CSD-implanted patients. Our results in patients without CSD are similar to those in Cervellini’s series. On the other hand, in patients with CSD, formation of EF was prevented or tended to be less extensive. This finding supports the idea that the cessation of contact between dura and blood prevents formation of epidural fibrosis.
The differentiation of EF and recurrent or residual disc herniation may be achieved with intravenous contrast-enhanced MRI. On un-enhanced T1-weighted MRI, EF and disc fragments have similar intensities. After intravenous administration of paramagnetic contrast material, scar tissue enhances prominently because of its blood supply, whereas disc enhances minimally because it has no blood supply. The formation of scar tissue takes place from 6 weeks to 6 months postoperatively, since it stabilizes by 6 months, with no further changes at 12 months [1–3, 14, 22, 23]. In this study the MRI evaluation follow-up time was 6–12 months (mean follow-up MRI time, 8.1 months, SD, 2.32), selected for the effectiveness of analysis.
Epidural fibrosis tends to form a curvilinear pattern surrounding the dural sac following the inside contour of lamina, but generally previous grading systems for the extension of EF were based on quadrants of spinal canal [1, 2, 3]. For this reason we have used a new grading system that is a combination of systems defined by Annertz and Ross [1].
Conclusion
In this study, the implantation of the CSD into the operation site in patients operated on for unilateral, single-level lumbar disc hernias, resulted in less formation of EF radiologically and yielded better clinical outcome. The closed-suction drain is a well-known, cheap material that is a way of preventing formation of seroma, without placing a foreign material into the body. To our knowledge, the use of CSDs for the prevention of EF has not been reported.
Grading of EF with our new MRI grading system showed a good clinical correlation between pain and disability scores.
References
- 1.Annertz Spine. 1995;20:449. doi: 10.1097/00007632-199502001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Aydin Surg Neurol. 2002;57:5. doi: 10.1016/S0090-3019(01)00677-2. [DOI] [PubMed] [Google Scholar]
- 3.Cervellini Neurosurgery. 1988;23:710. doi: 10.1227/00006123-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Colak Acta. 1996;Neurochir:162. doi: 10.1007/BF01411355. [DOI] [PubMed] [Google Scholar]
- 5.Coskun Eur Spine J. 2000;9:218. doi: 10.1007/s005860000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J Neurosurg. 1994;80:415. doi: 10.3171/jns.1994.80.3.0415. [DOI] [PubMed] [Google Scholar]
- 7.Dogulu F, Kurt G, Emmez H, Erdem O, Memis L, Baykaner K, Ceviker N (2003) Topical mitomycin C—induced inhibition of postlaminectomy peridural fibrosis in rabbits. J Neurosurg 99 [Suppl 1]:76–79 [DOI] [PubMed]
- 8.Ebeling J Neurosurg. 1989;70:397. doi: 10.3171/jns.1989.70.3.0397. [DOI] [PubMed] [Google Scholar]
- 9.Fritsch Spine. 1996;21:626. doi: 10.1097/00007632-199603010-00017. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel EM, Friedman AH (1996) The failed back surgery syndrome. In: Wilkins RH, Rengachary SS (eds) Neurosurgery. Mc Graw-Hill, New York, pp 3863–3870
- 11.Gerszten Neurosurgery. 1999;44:597. doi: 10.1097/00006123-199903000-00090. [DOI] [PubMed] [Google Scholar]
- 12.He Spine. 1995;20:557. doi: 10.1097/00007632-199503010-00010. [DOI] [PubMed] [Google Scholar]
- 13.Henderson Spine. 1993;18:1268. doi: 10.1097/00007632-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Jinkins AJNR Am J Neuroradiol. 1993;14:383. [PMC free article] [PubMed] [Google Scholar]
- 15.LaRocca J Bone Joint Surg Br. 1974;56:545. [PubMed] [Google Scholar]
- 16.Liu S, Boutrand JP, Bittoun J, Tadie M (2002) A collagen-based sealant to prevent in vivo reformation of epidural adhesions in an adult rat laminectomy model. J Neurosurg 97 [1 Suppl]: 69–74 [DOI] [PubMed]
- 17.Llado Eur Spine J. 1999;8:144. doi: 10.1007/s005860050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Neurosurg Clin N Am. 1991;2:899. [PubMed] [Google Scholar]
- 19.Nussbaum Neurosurgery. 1990;26:649. doi: 10.1097/00006123-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Pappas Neurosurgery. 1992;30:862. doi: 10.1227/00006123-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Price Pain. 1983;17:45. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 22.Ross Radiol Clin North Am. 1991;29:793. [PubMed] [Google Scholar]
- 23.Ross Neurosurgery. 1996;38:855. [PubMed] [Google Scholar]
- 24.Selcuklu Spine. 1993;18:165. doi: 10.1097/00007632-199301000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Songer Spine. 1990;15:550. doi: 10.1097/00007632-199006000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Songer Spine. 1995;20:571. doi: 10.1097/00007632-199503010-00012. [DOI] [PubMed] [Google Scholar]
- 27.WilliamsSpine 19783175663769 [Google Scholar]