Abstract
The aim of this study was to determine the influence of spinal fusion on ambulation and functional abilities in children with spina bifida for whom early mobilization was stimulated. Ten children (three males and seven females) with myelomeningocele were prospectively followed. Their mean age at operation was 9.3 years (standard deviation (SD): 2.4). Spinal curvature was measured according to Cobb. Pelvic obliquity and trunk decompensation were measured as well. The ambulation level was scored according to Hoffer, and functional abilities, as well as the amount of caregiver assistance, were documented using the Pediatric Evaluation of Disability Inventory. All patients were assessed before surgery and three times after surgery, with a total follow-up duration of 18 months after surgery. After spinal fusion, magnitude of primary curvature decreased significantly (p=0.002). Pelvic obliquity and trunk decompensation did not change. In spite of less immobilization as compared with other reported experiences, ambulation became difficult in three out of four patients who had been able to ambulate prior to surgery. Functional abilities and amount of caregiver assistance concerning self-care (especially regarding dressing upper and lower body, and self-catheterization) and mobility (especially regarding transfers) showed a nonsignificant trend to deterioration within the first 6 months after surgery, but recovered afterwards. From pre-surgery to 18 months after surgery, functional skills on self-care showed borderline improvement (p=0.07), whereas mobility did not (p=0.2). Mean scores on caregiver assistance improved significantly on self-care (p=0.03), and borderline on mobility (p=0.06), meaning that less caregiver assistance was needed compared with pre-surgery. The complication rate was high (80%). In conclusion, within the first 6 months after spinal fusion, more caregiver assistance is needed in self-care and mobility. It takes about 12 months to recover to pre-surgery level, while small improvement is seen afterwards. After spinal fusion, ambulation often becomes difficult, especially in exercise walkers. These findings are important for health-care professionals, in order to inform and prepare the patients and their parents properly for a planned spinal fusion.
Keywords: Myelomeningocele, Surgical treatment, Scoliosis, Spondylodesis, Ambulation level, Functional abilities
Introduction
Scoliosis is a serious and common problem in patients with myelomeningocele. The incidence varies from 50–80% in the literature [2, 7, 11]. Almost all children with thoracic or high lumbar lesions appeared to develop a scoliosis, with a decrease in incidence of 5% to 10% in those with sacral level lesion [7]. The most progression is often seen in early teenage years, but it can increase significantly in younger children as well, especially in curvatures of more than 40° [11]. Another serious spinal deformity that tends to show severe progression at an early age is lumbar kyphosis [2]. Progressive scoliosis often results in loss of truncal stability, especially in curvatures >40°, and when associated with pelvic obliquity ≥ 25°. This might endanger sitting balance, ambulation and activities of daily living (ADL) [1, 7]. Therefore, the main goal of surgical intervention is prevention of an unbalanced spine and to improve sitting balance. Additionally, but not of less importance, is the aim of maintaining or improving mobility, and ADL [8].
Few studies have been published on the functional consequences of spinal fusion [1, 7, 8, 10, 11] as compared with technical outcomes of surgery. Studies on functional consequences report a deterioration of ambulation level after surgery. However, no negative effects were found on ADL. After spinal fusion, patients are often immobilized in a plaster jacket or cast for quite a long time (20–24 weeks), and they are not allowed to ambulate until the cast has been removed [8]. This must have consequences for functional abilities. Mazur et al. [8] reported weakening of upper extremity muscle strength during the immobilization period, and attempts at using crutches became more difficult for their patients. They suggested that early intense physical therapy to prevent muscle atrophy and to promote ambulation and transfers might prevent patients from deterioration after spinal fusion. However, this requires strong internal fixation material and less reliance on external support. The literature on the course of functional abilities after spinal fusion is scarce [1, 8].
The aim of this prospective study was to evaluate the course of ambulation, functional skills and the amount of caregiver assistance needed regarding self-care and mobility, from pre-surgery to 18 months post-surgery, in children with myelomeningocele for whom early mobilization (12 weeks after surgery) was stimulated.
Patients and methods
From January 1998 to December 1999, ten consecutive patients with myelomeningocele, younger than 18 years of age, underwent surgical treatment for spinal deformities at the Wilhelmina Children’s Hospital, University Medical Center Utrecht, The Netherlands. All these patients have been followed-up in the outpatient spina bifida clinic of the hospital, and they were treated in the department of orthopedics because of development of progressive kyphosis, scoliosis, or both. All patients (three males and seven females) were willing to participate in the present prospective study approved by the medical ethics committee of our hospital. Informed consent was obtained from all the parents. One patient had kyphosis in the lumbar area, eight children had scoliosis (one thoracic, six thoracolumbar, and one lumbar scoliosis), and one boy was referred for treatment of recurrent deformation. He developed a pseudarthrosis at the thoracolumbar junction after a prior posterior fusion.
The clinical assessments were performed 1 day before surgery, and 6 months, 12 months and 18 months after surgery, by the same physical therapist (M.S.). Radiographs of the spine were performed at all four measurements.
Measurements
Spinal curvatures, pelvic obliquity, and trunk decompensation were measured using roentgenograms. The scoliosis or kyphosis angle was measured according to Cobb with the spinal rotation meter [13]. The degrees of pelvic obliquity were measured as the angle between the iliac crest and the horizontal line from radiographs taken with the patient in sitting position [14]. Trunk decompensation was measured as centimeters of deviation from the vertical line drawn from the seventh cervical vertebra to horizontal crests from radiographs. The same orthopedic surgeon (J.E.H.P.) performed all the radiographic measurements.
Gender, presence of shunted hydrocephalus, neurosegmental motor level, intelligence quotient (IQ), and age at operation, were documented. Sitting height was measured. Ambulation level was scored according to the criteria of Hoffer et al. [6], as shown in Table 1.
Table 1.
Scoring of ambulation based on Hoffer criteria
| Score | Community walker | Ambulating outdoors with or without braces but using wheelchair for longer distances |
| 1 | ||
| 2 | Household walker | Using braces or crutches for indoors and using wheelchair outdoors |
| 3 | Exercise walker | Walking only in therapeutic situations |
| 4 | Non-walker | Wheelchair-dependent |
Functional abilities and the amount of caregiver assistance concerning self-care and mobility were scored using the Dutch version of the Pediatric Evaluation of Disability Inventory (PEDI) [3, 5]. The PEDI is a validated and reliable parental questionnaire that measures functional skills (FS) and caregiver assistance (CA) in three domains: self-care, mobility and social function. For the purposes of this study, we used the scores on the self-care (FS and CA) and mobility (FS and CA) domains, as outcome measures. Results were calculated as a scaled score ranging from 0 to 100. In healthy children (≥7.5 years of age) all functional skills should be mastered leading to a score of 100. Regarding caregiver assistance, a higher score indicates a higher level of independence.
In seven patients, surgical release of tethered cord was carried out by a pediatric neurosurgeon within 3 months prior to spinal fusion. Surgical techniques used for correction of kyphosis, scoliosis, or both, are listed in Table 2. Three patients had undergone a two-stage anterior-posterior procedure. The same pediatric orthopedic surgeon (J.E.H.P.) performed all the operations.
Table 2.
Patient characteristics (M male, F female, R right, L left, Ant anterior, Pos posterior)
| Patient No. | Gender | Age | Motor level | Ambulation score | Deformity | Convexity and localization of primary curvature | Cobb angle (degrees) | Pelvic obliquity (degrees) | Type, and localization, of spinal fusion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- surg. | Post-surg. 18 months | Pre-surg. | Post-surg. 18 months | Pre-surg. | Post-surg. 18 months | |||||||
| 1 | M | 9.3 | T12 | 4 | 4 | Scoliosis | R: T4–L4 | 39 | 35 | 0 | 0 | Ant: T12–L5 + Pos: T3–S1 |
| 2 | F | 9.1 | L3–L4 | 4 | 4 | Scoliosis | L: T12–L5 | 50 | 28 | 8 | 11 | Ant: T9–L4 |
| 3 | F | 12.0 | L2 | 4 | 4 | Scoliosis | R: T4–T12 | 60 | 32 | 0 | 5 | Pos: T3–S1 |
| 4 | F | 8.4 | ↑ T11 | 4 | 4 | Kyphosis | T12–S1 | 90 | 109 | 0 | 6 | Ant: T12–L5 + strut-graft |
| 5 | F | 11.1 | L3 | 3 | 4 | Scoliosis | R: T7–L2 | 87 | 32 | 0 | 5 | Ant: T11–L5 + Pos: T3–S1 |
| 6 | M | 9.3 | T12 | 3 | 4 | Scoliosis | L: T11–L4 | 47 | 20 | 15 | 0 | Ant: T12–L4 + Pos: T3–S1 |
| 7 | F | 10.6 | S1 | 2 | 4 | Scoliosis | R: T12–L3 | 40 | 27 | 0 | 7 | Ant: T12–S1 |
| 8 | F | 3.4 | L5 | 3 | 3 | Scoliosis | L: T11–L5 | 43 | 27 | 15 | 10 | Ant: T12–L5 |
| 9 | F | 10.0 | ↑ T11 | 4 | 4 | Scoliosis | R: T6–L4 | 75 | 40 | 36 | 0 | Pos: T11–S1 |
| 10 | M | 10.3 | ↑ T11 | 4 | 4 | Pseudo-arthrosis | R: T12–L1 | 58 | 40 | 41 | 25 | Ant: T12–L1 + Strut-graft |
2=household walker
3=exercise walker
4=non-walker
All the children were cared for in a regular bed for an average of 7 days after the final operation, and than immobilized in a bi-valved total-contact orthosis (hip spica with both legs) for a period of 12 weeks. This orthosis allowed upright posture in a wheelchair and regular inspection for pressure sores. Afterwards, patients received a customized body-jacket, for an additional 12 weeks. In this period, 12 weeks post-surgery, early mobilization was started. During physical-therapy sessions, transfer training was started and patients were encouraged to ambulate as well as they could tolerate within their body jacket. Six months after surgery, mobilization was completely free.
Statistical analysis
Due to small sample sizes and data distribution, non-parametric Friedman tests were used for analyzing the four repeated measurements on all variables, with the exception of ambulation level (Cobb angle of primary curvature, pelvic obliquity, trunk decompensation, sitting height, and PEDI scores). When differences between the measurements appeared to be significant (p<0.05), the Wilcoxon signed rank test was used to detect which specific follow-up measurements significantly differed from each other. Data are presented as mean and standard deviation, or median and interquartile range (25th–75th percentile) (IQR), when appropriate. As the number of walking patients prior to surgery was too small (n=4) for statistical analyses, these data are presented in a descriptive way. Descriptive analysis was performed with the Statistical Package of the Social Sciences (SPSS 9.0).
Results
General characteristics are presented in Table 2. The mean age at operation was 9.3 years (SD 3.4). All patients had shunted hydrocephalus and Chiari II malformation. Their mean IQ was 72.7 (range: 60–95). One patient had assisted mechanical ventilation (tracheostomy), because of brainstem dysfunction (case 9). Two girls had lumbosacral lesions (cases 7 and 8). Both had restricted walking abilities prior to surgery due to hypertonia in the lower extremities.
Median values of Cobb angle, pelvic obliquity, trunk decompensation, and sitting height, before and after surgery, are shown in Table 3. Magnitude of primary curvature (Cobb angle) decreased significantly after operation. Significant changes were seen between measurements 1 and 2 (p=0.03), 1 and 3 (p=0.02), and 1 and 4 (p=0.02), respectively. Within 18 months after surgery, some loss of correction occurred. A preoperative scoliosis angle of 54° (interquartile range, 25th–75th percentile, of 42.3–78.0°) was primarily reduced to 26° (IQR, 22.5–38.0°). At final follow-up, correction deteriorated to 32.0° (IQR, 27.0–40.0°). The mean percentage of correction from pre-surgery to 6 months post-surgery was 38.9% (SD 25.9%), and decreased to 31% (SD 15.7%) 18 months after surgery. Sitting height improved after surgery. Significant changes were seen between measurements 1 and 2 (p=0.04), 1 and 3 (p=0.04), and between month 1 and month 4 (p=0.04) and between month 2 and month 4 (p=0.03).
Table 3.
Results before and after surgery with regard to Cobb angle of primary curvature, amount of correction, pelvic tilt, trunk decompensation, and sitting height. Values are presented in median and IQR, as they were not normally distributed. (IQR interquartile range)
| Measurement | Before surgery | 6 months after surgery | 12 months after surgery | 18 months after surgery | p value |
| 1 | 2 | 3 | 4 | ||
| Cobb angle (degrees) | |||||
| Median (P50) | 54.0 | 26.0 | 32.0 | 32.0 | 0.002* |
| IQR (P25–P75) | 42.3–78.0 | 22.5–38.0 | 26.8–39.8 | 27.0–40.0 | - |
| Pelvic obliquity (degrees) | |||||
| Median (P50) | 4.0 | 3.5 | 6.0 | 5.5 | 0.82 |
| IQR (P25–P75) | 0.0–20.3 | 0.0 15.0 | 0.0–20.0 | 0.0–10.3 | - |
| Trunk decompensation (cm) | |||||
| Median (P50) | 0.8 | 0.0 | 0.8 | 1.0 | 0.58 |
| IQR (P25–P75) | 0.0–2.0 | 0.0–1.3 | 0.0–1.1 | 0.0–2.0 | - |
| Sitting height (cm) | |||||
| Median (P50) | 63.0 | 66.6 | 69.5 | 70.6 | 0.003* |
| IQR (P25–P75) | 55.0–67.8 | 58.8–70.3 | 58.2–73.9 | 65.9–74.4 | - |
*Statistically significant values
In three out of four patients who were able to walk prior to surgery, ambulation level deteriorated after surgery. Three were exercise walkers prior to surgery (score 3). Two of them walked with the aid of an advanced reciprocating gait orthosis (ARGO) and a posterior walker (cases 6, 8), and the other patient used ankle foot orthosis and crutches (case 5). Two out of three exercise walkers (score 3) lost their walking ability permanently (cases 5, 6) and became non-walkers (score 4). One girl (case 8) was operated on at a young age (3.4 years) because of a rapid development of a severe scoliosis (43°) with pelvic obliquity (15°), resulting in an unbalanced sitting posture. After surgery, she lost the ability to walk in therapeutic situations (score 3) temporarily, but regained it within 1 year after surgery. Another girl (case 7), a household walker (score 2) prior to surgery, became a non-walker (score 4). She developed a (right) hip luxation within 6 months after spinal surgery due to severe hypertonia in her leg, despite tethered-cord release prior to spinal fusion.
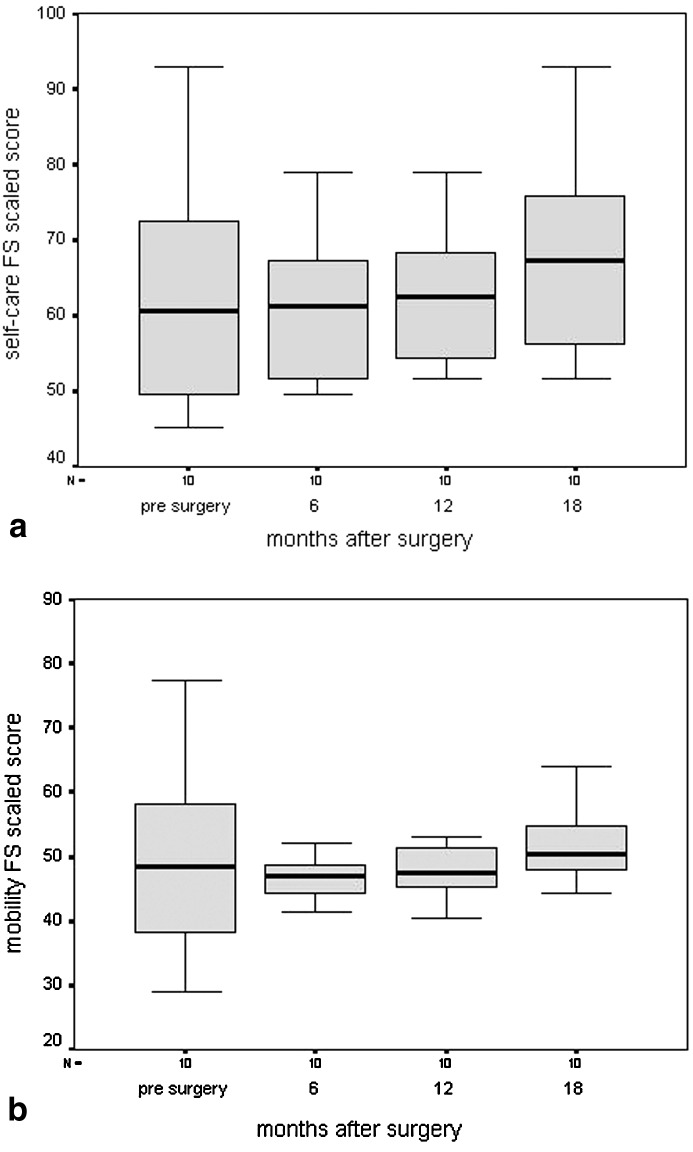
The mean PEDI-scaled scores (SD) on functional skills self-care showed a nonsignificant tendency to deteriorate from pre-surgery (measurement 1) to 6 months after surgery (measurement 2). Most problems were seen in items concerning dressing upper and lower body, and bowel and bladder management, such as inability to perform self-catheterization. After 6 months, scores showed a significant improvement (p<0.01: measurements 2 and 4, and 3 and 4), and restored to pre-surgery level at 12 months. The changes are shown in Fig. 1a. Eighteen months after surgery, functional skills on self-care showed borderline improvement (p=0.07) compared with pre-surgery level. The same trends were seen for functional skills on mobility (Fig. 1b). Deterioration 6 months after surgery mainly concerned items regarding transfers. From first measurement to 18 months after surgery, functional skills on mobility did not improve significantly (p=0.2).
Fig. 1.
Box plots of functional-skills scaled scores with regard to a self-care and b mobility during the four assessments. The whiskers represent the highest and lowest values within 1.5 times the interquartile range (box length, IQR). The lower horizontal line of the box indicates the 25th percentile. The upper horizontal line indicates the 75th percentile. The mid horizontal bar in the box indicates the median. (FS functional skills)
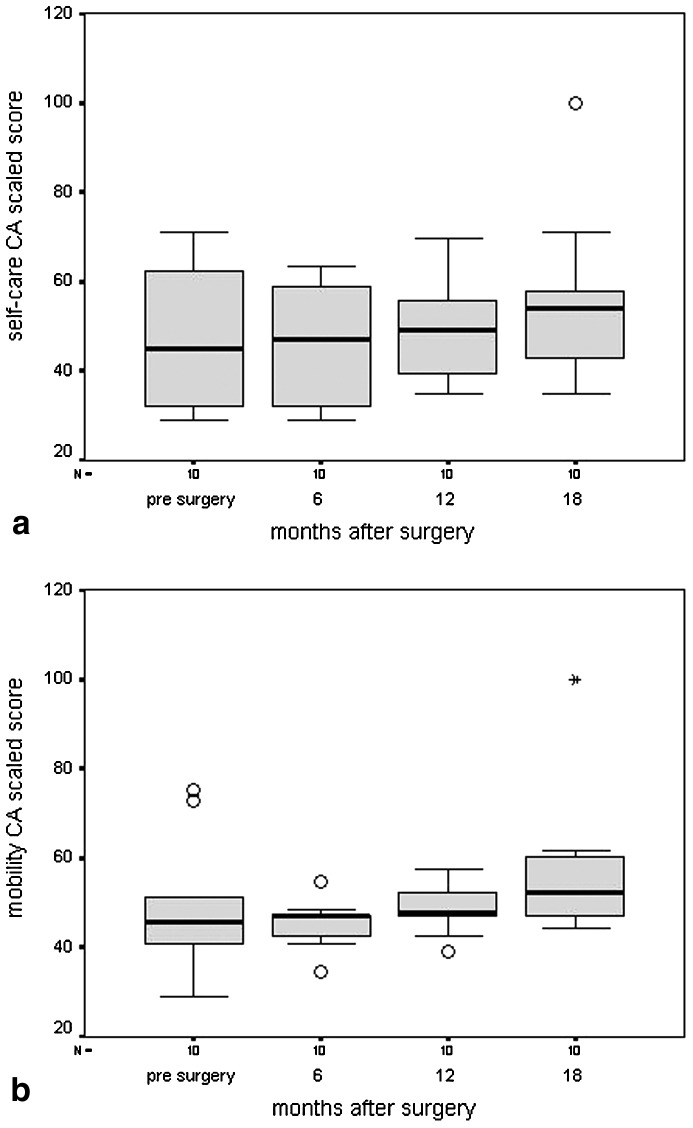
Changes for caregiver assistance regarding self-care and mobility are shown in Fig. 2a and Fig. 2b, respectively. The higher the score on caregiver assistance, the higher the level of independence, meaning that less caregiver assistance was needed 18 months after surgery compared with pre-surgery. Mean scores on caregiver assistance improved significantly on self-care (p=0.03), and they were borderline on mobility (p=0.06). PEDI scores improved from baseline to 18 months follow-up, in all but one patient. In the girl that developed a hip luxation (case 7), scores on all PEDI domains deteriorated.
Fig. 2.
Box plots of caregiver-assistance scaled scores with regard to a self-care and b mobility during the four assessments. The O symbol indicates an outlier between 1.5 and 3 times the interquartile range from the box and the asterisk * indicates an extreme lying more than 3 times the interquartile range from the box. (FS functional skills; for explanation of box plot see legend to Fig. 1)
Complications were seen in eight out of ten patients. In one girl, case 4 with severe lumbar kyphosis, no correction was obtained. Due to resorption of the strut-graft, her kyphosis showed progression from 90° before, to 109° after surgery. In three other patients (cases 2, 5 and 6) the instrumentation came loose, leading to some loss of correction in one patient (case 2). One of the patients (case 10) had osteomyelitis. His instrumentation had to be removed after 6 months, but he did not lose correction. Three out of ten patients (cases 1, 7, and 8) increased their curvature above or below the fusion mass. In two patients (cases 3 and 9), no complications were seen.
Discussion
This study describes the convalescence of children with myelomeningocele after spinal fusion, with special emphasis on ambulation, functional abilities and amount of caregiver assistance needed. Our study suggests that functional abilities on self-care and mobility showed a tendency to decrease within the first 6 months after surgery, but gradually improved to pre-surgery level within 12 months, and improved further at 18 months after surgery. The same trend was seen in the amount of caregiver assistance. Eighteen months after surgery less caregiver assistance was needed on self-care compared with the pre-surgery level. Caregiver assistance on mobility did not change significantly. Ambulation became difficult in three out of four patients who were able to ambulate prior to surgery.
Our main interest was not to evaluate the efficacy of different surgical procedures on scoliosis, but to follow-up the course of functional abilities after spinal fusion followed by immobilization. We evaluated the group as a whole and did not distinguish for different types of fusion, as all our patients were immobilized in a standardized way, for the same period in all patients.
As in other series, we found a significant correction of primary curvature after surgery, in all but one patient. In our study, the median curvature at operation was 54° and was corrected to 38.9%. The severity of the curvature at final follow-up (median: 32°) is comparable with that shown in current literature [1, 2, 9, 12, 14]. As in these series, some loss of correction is seen at final follow-up (median, 6°), mostly due to hardware problems. As expected, we found a significant increase in sitting height compared to pre-surgery, as this is caused by correction of the scoliosis. Moreover, we also found an increase in sitting height within the follow-up period (measurements 2–4). This means that spinal growth is still possible after fusion, probably secondary to the ongoing growth outside fused levels.
Although spinal alignment improves significantly after surgery, morbidity is high in most series. In our study, problems in terms of loss of correction, progression above or below fusion mass, or osteomyelitis occurred in eight out of ten patients.
Mazur et al [8] studied functional status and ambulation after spinal fusion in 49 children with spina bifida. They found deterioration in ambulation in 67% of patients who had undergone combined anterior and posterior fusion, in 57% following anterior fusion, and in 27% following posterior fusion alone. Muller et al [10] reported that 57% (8/14) of their patients, lost some of their ambulation capacity after spinal fusion. In spite of early transfer and ambulation training as suggested by Mazur et al [8] (12 weeks versus 20–24 weeks), we also found deterioration of ambulatory skills in patients who were able to walk prior to surgery. The possibility that these findings were biased by factors other than spinal fusion cannot be ruled out. Permanent deterioration of ambulation was seen in three out of four patients who were able to walk prior to surgery. One girl with sacral-level paralysis (case 7) lost ambulation due to progressive hypertonia, and two patients (cases 5 and 6) had thoracic and a high lumbar lesion level. In these patients the spine was fused down to the pelvis. High-level lesion patients often need a mobile lumbosacral junction to thrust their limbs forward. Therefore, this might be one of the reasons that they lost their ambulation skills. On the other hand, it remains unclear whether or not walking abilities would have been lost anyway in these patients. It is known that most children with high-level myelomeningocele loose the ability to walk during their teenage years, due to high energy consumption when walking with aids, and as a result of growth or obesity [4].
Few studies report on the influence of spinal fusion on functional status. These series report that spinal fusion does not adversely affect motor skills or activities of daily living (ADL) [1,8, 10]. Our data are partially in agreement with these findings, as we also found no adverse effects on ADL in the long-term. However, a temporarily decrease in functional abilities within the first 6 months after surgery was found. After surgery, all patients were wearing a hip spica for a period of 3 months, and a body jacket for an additional 3 months. In the first 3 months, patients were completely dependent with regard to self-care and mobility. Although transfer training started as soon as the hip spica was removed (12 weeks after surgery), mobility items were still limited due to the body jacket. This also restricted them in abilities such as dressing upper and lower body, as well as in performing their self-catheterization. Twelve months after surgery, all PEDI scores gradually improved to pre-surgery level. Moreover, 18 months after surgery, we found a significant improvement in scores in self-care, as well as a decrease in the amount of caregiver assistance needed, compared with the pre surgery level. Regarding overall mobility, nonsignificant changes were found. Askin et al. [1] also studied the influence of spinal fusion on functional status in 20 patients with four different neurological disorders. They found a tendency for overall abilities in all patient groups to deteriorate for the first 6 months. They returned to their pre-surgery level at 12 months. Their series included five children with myelomeningocele. For these children, 6-month assessments were not available as these patients were still in postoperative braces. At 12 months after surgery all patients had returned to their pre-surgery levels in ADL. However, walking abilities deteriorated in all of them. Our data are in agreement with these findings.
When interpreting our findings, it should be kept in mind that the sample size of our study is small. Therefore, long-term prospective follow-up studies have to be continued with larger numbers. On the other hand, our results confirm other small series and the scarce literature on this subject. Hence, as advances are made in technology to improve spinal fusion in children with myelomeningocele, future studies should not only focus on the effectiveness of surgery in terms of spinal curvature, pelvic obliquity, and complications. It is indispensable to evaluate the effect of such major surgery on children with spina bifida and their families.
This addresses the issue that the functional consequences of spinal fusion are measured in different ways, making comparison of results difficult. Moreover, outcome measures used in these series [8, 10] are often clinician oriented and might not reflect the abilities that are important to patients themselves. To overcome this problem, we used the Pediatric Evaluation of Disability Inventory to evaluate functional abilities and the amount of caregiver assistance needed pre-surgery and post-surgery. The PEDI is a very long, time-consuming questionnaire based on a structured interview with caregivers. This makes it less suitable for routine follow-up. Recently, Wai et al. [15] developed the Spina Bifida Spine Questionnaire (SBSQ), an apparently valid and reliable self-administered questionnaire to assess the effectiveness of surgical and other therapeutic interventions for children with spina bifida and scoliosis. In retrospect, this instrument could have been more appropriate for our study. However, it was not available when we started our study in 1998. The SBSQ will be a useful instrument for future follow-up studies on the effectiveness of spinal surgery in patients with spina bifida.
Conclusions
Within the first 6 months after spinal fusion, more caregiver assistance is needed compared with pre-surgery, especially with regard to dressing the upper and lower body, as well as bowel and bladder management and transfers. It will take about 18 months before patients and their parents experience the beneficial effects that spinal fusion can have on functional abilities. After surgery, walking abilities often become difficult especially for exercise walkers, although other factors such as ongoing neuraxial disease or natural history cannot be ruled out. These findings are important for health-care professionals, in order to inform and prepare the patients and their parents properly for a planned spinal fusion.
Acknowledgements
We would like to thank the families and children who participated in this study. We also acknowledge Tim Takken for his support in the data-analysis and advising in the preparation of this manuscript
References
- 1.Askin Spine. 1997;22:44. doi: 10.1097/00007632-199701010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Banta Spine. 1990;15:943. doi: 10.1097/00007632-199009000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Custers JW, Wassenberg-Severijnen JE, van der Net J, Vermeer A, Hart HT, Helders PJ (2002) Dutch adaptation and content validity of the “Pediatric Evaluation of Disability Inventory.” Disabil Rehabil 20:250–258 [DOI] [PubMed]
- 4.Findley Arch Phys Med Rehabil. 1987;68:518. [PubMed] [Google Scholar]
- 5.Hayley SM, Coster WJ, Ludlow LH, Haltiwanger JT, Andrellos PJ (1992) “Pediatric Evaluation of Disability Inventory” (PEDI). Development, standardization and administration manual. New England Medical Center, Boston
- 6.Hoffer J Bone Joint Surg Br. 1973;55:137. [PubMed] [Google Scholar]
- 7.Kahanovitz Spine. 1981;6:494. doi: 10.1097/00007632-198109000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Mazur J Pediatr Orthop. 1986;6:568. doi: 10.1097/01241398-198609000-00008. [DOI] [PubMed] [Google Scholar]
- 9.McMaster J Bone Joint Surg Br. 1987;69:20. doi: 10.1302/0301-620X.69B1.3818727. [DOI] [PubMed] [Google Scholar]
- 10.M Acta Paediatr. 1992;81:173. doi: 10.1111/j.1651-2227.1992.tb12197.x. [DOI] [PubMed] [Google Scholar]
- 11.MSpine 1994191478153821 [Google Scholar]
- 12.Parsch J Pediatr Orthop. 2001;10:10. [Google Scholar]
- 13.Pruijs Acta Orthop Belg. 1995;61:107. [PubMed] [Google Scholar]
- 14.Sponseller Clin Orthop. 1999;364:117. doi: 10.1097/00003086-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Wai J Pediatr Orthop. 2000;20:765. doi: 10.1097/00004694-200011000-00013. [DOI] [PubMed] [Google Scholar]