Abstract
Low back pain (LBP) in children was considered for many years to be a rare condition revealing a serious disease, but in the last two decades, epidemiological studies have shown that the prevalence of nonspecific LBP in children is high. This study was aimed at analyzing the prevalence, severity, consequences and associated factors of LBP in children. A cross-sectional study was undertaken in two preparatory schools in the city of Monastir, Tunisia, in April 2002. This study included a total of 622 children and adolescents—326 females and 296 males—with a mean age of 14 years (range: 11–19 years). They completed the questionnaire in the presence of the physician. For the first 201 questionnaires collected, the corresponding children and adolescents underwent a spine medical examination, with evaluation of pain by visual analog scale if LBP was present. A stepwise logistic regression analysis was carried out to determine the risk factors associated with LBP and chronic LBP. The cumulative lifetime prevalence of LBP was 28.4%. Eight percent of the subjects suffered from chronic LBP. LBP was responsible for 23% of school absenteeism and 29% for sports absenteeism. Medical care requirement was observed in 32.2% and psychological symptoms in 75%. Stepwise logistic regression analysis showed that three factors were associated with LBP: school failure (held back 1 year), odds ratio (OR) =2.6 (95% confidence interval [CI], 1.96–3.44), family history of LBP (parental or sibling LBP), OR=3.80 (95% CI, 2.94–5.92), dissatisfaction with school chair (in height and comfort), OR=3.40 (95% CI, 2.24–5.29). Two factors were associated with chronic LBP: dissatisfaction with school chair, OR=1.62 (95% CI, 1.46–3.32) and football playing, OR=3.07 (95% CI, 2.15–5.10). The prevalence of LBP among Tunisian schoolchildren and adolescents is high. This requires preventive measures and longitudinal studies, which are very important from the standpoint of public health.
Keywords: Low back pain, Children, Prevalence, Risk factors
Introduction
Low back pain (LBP) in children and adolescents was considered for many years to be rare and an indication of serious disease [1]. In the last two decades, epidemiological studies have shown that the prevalence of nonspecific LBP in children is high, reaching that of adults by the end of the growth period. LBP in childhood is also associated with some risk factors [9, 10, 17, 26, 28, 32]. We present the first Tunisian study of LBP in children and adolescents, with the aim of determining its prevalence, severity, medical and functional consequences and the associated risk factors. We also examined whether indicators from a medical examination correspond to self-reported LBP.
Material and methods
This cross-sectional study was based on an investigation carried out in April 2002 on 640 schoolchildren and adolescents recruited from two preparatory schools, randomly selected from the five schools of Monastir, Tunisia. The children represent all the students of these two schools, but the participation rate was 98% (2% of the children were absent the days of the inquiry). We did not observe any objection to participation, and the students were very motivated, because it was the first time this type of inquiry had been done. The two schools were representative of the preparatory schools of Monastir, and the social class of the children is the same (middle class). The number of subjects needed for our study was calculated after an inquiry among 116 schoolchildren. This inquiry gave an LBP prevalence rate of 28%.
The number of subjects needed (n) was calculated according to the formula: n=(ε/e) 2 p (1−p), where ε is the reduced gap (=1.96≅2); e is the precision (=5%); and p is the prevalence (=28%). The alpha level was fixed at 5%. Thus, the number of subjects needed was 323 children. The schoolchildren and adolescents filled in a questionnaire inspired by and derived from the Salminen [20] and Troussier [28] LBP questionnaires. A physician presented the questionnaire and explained the items, using a drawing to precisely indicate the location of the low back. He also helped the children to fill in the questionnaire, if necessary. The questionnaire was composed of 20 items and self-administered with an easy yes/no response format. These items evaluated the perceived characteristics of back problems, functional limitations, children’s activities and psychological parameters. This questionnaire, validated elsewhere among 72 pupils suffering from LBP [4], gave a good reliability (kappa coefficients between 0.70 and 1.00). For the first 201 questionnaires collected, the corresponding children had a specific spine medical examination. We searched for morphological abnormalities of the lumbar spine (kyphosis, hyperlordosis), contracture on palpation of the paravertebral muscles and pain on palpation of the spine. We examined the mobility of the lumbar spine. We measured the Schober index and the finger-foot index. If LBP was present, an evaluation of pain by a visual analog scale was performed. Only 622 questionnaires were analyzed (18 were not completed). Statistical analysis was performed using the chi-square test, Student’s t-test, the Fisher exact test and the analysis of variance (ANOVA) at the significance level p<0.05. A stepwise logistic regression analysis was carried out to determine the risk factors associated with LBP and chronic LBP. LBP severity was divided into three categories [28]: occasional LBP, frequent LBP, which lasts more than 3 months but occurs less than once a week, and chronic LBP, which lasts more than 3 months and occurs more than once a week.
Results
The mean age of our population was14.1 years±1.3 years [S.D.] (range, 11–19 years).There were 326 females and 296 males.
LBP prevalence
The cumulative lifetime prevalence of LBP was 28.4% (n=177, 95% confidence interval (CI): 25–32%). The point prevalence of LBP (LBP occurring in the course of the week preceding the inquiry) was 13%. The LBP prevalence rates were 47%, 24% and 29% (n=51) for occasional, frequent and chronic categories, respectively. The cumulative prevalence rate of the chronic LBP among the whole population was 8% (95% CI: 6–10%).
LBP consequences
Medical care requirements
Medical care requirements (medical consultation and physiotherapy) were observed in 32.2% of the LBP patients. The cumulative prevalence of the medical consequences in the entire cohort was 9.2%. The medical consequences of chronic, frequent and occasional LBP were 58.5%, 23.2% and 20.5%, respectively.
Functional limitations
The functional consequences of LBP were evaluated by school and sports absenteeism. Among LBP sufferers 23% of the subjects indicated that LBP was severe enough to force them to miss school, and 29% were prevented from playing sports. Among chronic LBP sufferers 41% missed school and 45% were prevented from playing sports.
Daily life consequences
The consequences on daily life were assessed according to the posture resulting in LBP (experienced pain in a particular posture), which occurred among LBP sufferers in 67% in the sitting position and in 40% in the standing position.
Psychological consequences
Anxiety, tiredness, sleeplessness and depression—which have a quite different meaning in spoken Arabic—were revealed in the item pertaining to psychological troubles. These symptoms were observed in 49%, 75% and 80% of healthy children, LBP patients and chronic LBP children, respectively.
LBP-associated factors
The LBP- and chronic LBP-associated factors that were studied included age, gender, height, weight, body mass index (BMI), school failure (held back for a year), school chair, the home-to-school journey, the satchel (carriage by hand or on the shoulders, relative weight of the satchel by the weight of the child), family history of LBP (parental or sibling LBP), TV watching, whether the child is right-handed or left-handed, smoking, history of injury and exercise.
Univariate analysis
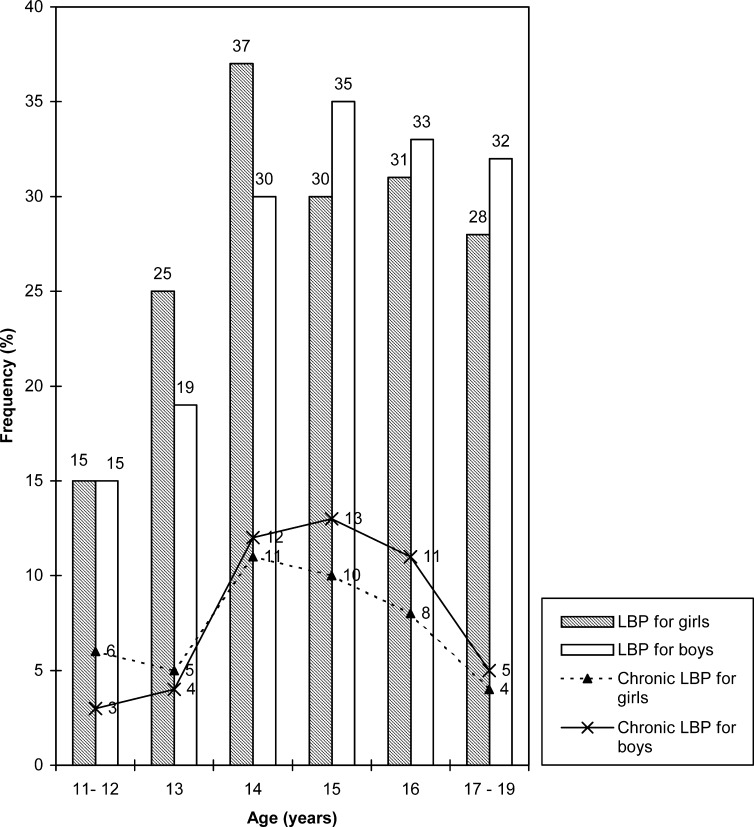
The mean age of the LBP and chronic LBP subjects was 14.07 years±1.42 years SD (range: 11–19 years) and 14.41 years±1.08 years (range: 11–19 years), respectively. There was no significant difference in mean age between the whole population, the LBP sufferers and the chronic LBP patients. The prevalence rates of LBP and chronic LBP increase with age and were at the maximum at age 15 years (37%) and age 14 years (12%), respectively. The maximum prevalence rates of both LBP and chronic LBP are also at age 14 years (30.7%) and age 15 years (26%) for girls and boys, respectively (Fig. 1). Chronic LBP prevalence rates for girls and boys were 9.5% and 6.8%, respectively (p=0.26). Of the subjects with LBP, 38% were dissatisfied with the school chairs, compared with 17.5% of those without LBP. This difference was significant (p=10-5). Dissatisfaction with the school chair was also associated with chronic LBP. Of the subjects with LBP, 11.2% were dissatisfied with the school chairs, compared with 4.8% of those without LBP. The average home-to-school journey in all the cohort lasted 20 min (range: 2–90 min), with 82% walking to school. Actually measuring the satchel’s weight, we found the average weight to be 6.8%± 2.5% (range: 3–20%) of the LBP child’s weight. Satchel weight as well as the way it was carried, by hand or on the shoulders, was not associated with LBP or chronic LBP. Of the LBP subjects, 45.7% had a family history of LBP, and 22.3% of the children without LBP had a family history of LBP. This difference was significant (p=10-6). Family history of LBP was also associated with chronic LBP (p=0.019). All the associated factors with LBP or chronic LBP tested are shown in (Table 1 and Table 2).
Fig. 1.
Distribution of low back pain (LBP) and chronic LBP according to age and gender
Table 1.
Low back pain (LBP) and chronic low back pain (CLBP), associated factors in univariate analysis
| LBP (p) | CLBP (p) | |
|---|---|---|
| Age Gender |
0.018 0.23 |
0.08 0.26 |
| Height Weight Body mass index |
0.0032 0.10 0.68 |
0.32 0.78 0.58 |
| School chair* School failure** |
10−5 0.016 |
0.05 0.07 |
| Home–school journey Satchel weight |
0.51 0.38 |
0.32 0.42 |
| LBP family history*** | 10−6 | 0.01 |
| TV watching Right- or left-handed in writing Smoking Spine injuries Sports injuries |
0.218 0.58 0.82 0.34 0.40 |
0.138 0.25 0.46 0.54 0.55 |
*Dissatisfaction with school chair
**Held back 1 year
***Parental or sibling LBP
Table 2.
Low back pain (LBP) and chronic low back pain (CLBP), associated sports activities in univariate analysis
| LBP (p) | CLBP (p) | |
|---|---|---|
| Football Basketball Handball Swimming |
0.8 0.0076 0.17 0.0006 |
0.03 0.3 0.1 0.07 |
| Volleyball Bowling |
0.1 0.027 |
0.44 0.01 |
| Gymnastics | 0.45 | 0.57 |
Multivariate analysis
Twelve variables were introduced in the model for a stepwise logistic regression analysis for LBP and 13 were introduced in the model for chronic LBP. These analyses showed that three factors were associated with LBP: school failure, family history of LBP and dissatisfaction with the school chair. Two factors were associated with chronic LBP: dissatisfaction with the school chair, and football playing (Table 3). These factors explain 23% and 21% of the variance of LBP and chronic LBP, respectively. The only risk factor associated with both LBP and chronic LBP was dissatisfaction with the school chair.
Table 3.
Low back pain (LBP) and chronic low back pain (CLBP), associated factors in multivariate analysis (OR odds ratio, NS non-significant)
| LBP | CLBP | |
|---|---|---|
| School failure* |
p<0.05 OR=2.60 (1.96–3.80) |
NS |
| LBP family history** |
p<0.01 OR=3.80 (2.94–5.92) |
NS |
| Football | NS |
p<0.01 OR=3.07 (2.15–5.10) |
| Dissatisfaction with school chair |
p<0.01 OR=3.40 (2.24–5.29) |
p<0.05 OR=1.62 (1.46–3.32) |
*Held back 1 year
**Parental or sibling LBP
Objective findings
The study of the objective findings with the aim of determining whether indicators from a medical examination corresponded to self-reported LBP was performed with 201 children. This subgroup was representative of the whole population. The mean age was 14.15 years±1.31 years S.D. (range: 11–19 years). Children were considered as having objective findings when the physician triggered pain or discomfort in palpation or mobilization of the spine, or when he found spine-curvature abnormalities. The analysis of the objective findings showed that 63 out of the examined 201 (31%) children and adolescents suffered from LBP.
Discussion
In our study, the LBP cumulative lifetime prevalence rate was 28.4%. The physician was not blinded with respect to the study group. Therefore, correspondence might be overestimated. The LBP cumulative lifetime prevalence rate in the literature varied from 21% to 74% [3] (Table 4). The LBP lifetime prevalence in the study of Kovacs [12] was 50.9% for boys and 69.3% for girls, with a point prevalence of 17.1% for boys and 33% for girls. This is higher than our point prevalence. Of our cases, 53% had severe, frequent or chronic LBP, and 8% of the total population suffered from chronic LBP. This result was in accordance with that related to the Salminen [20] and Duggleby [8] studies, which found 7.8% and 8.1%, respectively. Approximately one-third of the patients (8% of the cohort) had limited daily activities. Another third (9% of the cohort) had sought medical help.
Table 4.
Low back pain prevalence in the literature
| Author | Year | Age | N | Cumulative prevalence (%) |
|
Salminen |
1992 |
14 |
1,377 |
30.3 |
| Balagué | 1993 | 10–16 | 117 | 32.5 |
| Troussier | 1994 | 6–20 | 1,178 | 41 |
| Burton | 1996 | 15 | 216 | 51.4 |
| Harreby | 1999 | 13–16 | 1,389 | 58.9 |
| Our study | 2003 | 11–19 | 622 | 28.4 |
Of the cases with LBP, the sitting position was a factor in 67%. Inadequate school furniture and the high number of hours spent in sitting position are the possible precipitating factors of LBP among schoolchildren, as observed by other authors [15, 18, 21, 22]. As for the psychological symptoms, they can be an etiological factor or the consequence of LBP, mainly when it takes a chronic evolution. The psychological factors play a role in the experience of LBP. In fact poor self-perception of health (health belief) could be a factor behind reporting LBP [25]. Furthermore, pain perception and psychological factors were associated with LBP [24]. Adverse psychosocial factors and the presence of other preexisting somatic pain symptoms (abdominal pain, headaches, and sore throats) were also predictive of future LBP for Jones [11]. Poor well-being, in particular poor self-perceived fitness, was associated with LBP among adolescents in the study of Sjolie [23]. The psychological factors were significantly associated with reported nonspecific LBP and its consequences in the study of Balagué [2]. LBP prevalence was not gender-dependent in our study and occurred mostly during the growth period: age 14 years for girls and age 15 years for boys. Like Salminen [19] and Troussier [28], we found that school failure was associated with LBP. School failure can also result from LBP. The satchel’s relative weight among LBP patients was 6.8%. In only one-fourth of the patients did this percentage exceed 10%, which is considered a high-risk factor for LBP [2, 20, 31]. However, Jones [11] found that there was little evidence for an increase in short-term risk associated with mechanical load across the range of weights commonly carried by children to school. Balagué [2] demonstrated that sibling history of LBP was significantly associated with reported nonspecific LBP and its consequences. Salminen [21], Brattberg [5] and other cross-sectional studies [5, 7–9, 19] have likewise demonstrated the influence of genetic factors in the occurrence of LBP in children and adolescents.
Regarding sports activities, basketball, swimming and bowling were found to be associated with LBP, but football and bowling were associated with chronic LBP. Exercise has been considered a risk factor for LBP and chronic LBP, especially if intense and competitive [13, 14, 16, 27]. As for the analysis of the LBP-associated risk factors and after a stepwise logistic regression analysis, only dissatisfaction with the school chair was associated with both LBP and chronic LBP. Dissatisfaction with the school chair concerned height and comfort, as evaluated by the children. The school chairs were, in fact, very uncomfortable—old-fashioned with a standard form and height. Thus, they were not adapted for children of various ages (11–19 years) and wide-ranging heights (115–192 cm). Coleman et al. [6] studied the preferred settings for lumbar support height and depth of 123 subjects. The mean preferred height setting was 190 mm above the compressed seat surface. The mean depth setting (horizontal distance from front of seat to lumbar support point) was 387 mm. Van Dieen [30] found that dynamic office chairs offered a potential advantage over fixed chairs, but the effects of the task were more obvious than the effects of the chair. Van Deursen [29] also demonstrated that rotary dynamic stimuli on low back pain during prolonged sitting, especially low-frequency ones, reduced pain during prolonged sitting. Concerning the objective findings in our cases, they were retained by the physician after his impression of a simple clinical spine examination. There are, in fact, very few clinical signs that can help to single out schoolchildren with LBP. Indeed, in a total of 392 children aged 9, Gunzburg [9] found that of the 19 clinical parameters taken down during the medical examination, only one was significantly more prevalent in the group of children reporting LBP: pain on palpation at the insertion site on the iliac crest of the iliolumbar ligament. In short, there are many factors associated with nonspecific LBP in children, and they need to be studied from a multidisciplinary standpoint that includes biopsychosocial factors, developmental, educational, and cultural background [3].
Conclusion
LBP cumulative lifetime prevalence among Tunisian schoolchildren and adolescents was 28.4%, and chronic LBP prevalence was 8%. The only factor associated with both LBP and chronic LBP was the dissatisfaction with the school chair, its comfort and height. This requires preventive measures and longitudinal studies, which are very important from the standpoint of public health.
Acknowledgements
Professor Adel Rdissi’s help with the editing is gratefully acknowledged
References
- 1.AfshaniRadiographics 1991112691827529 [Google Scholar]
- 2.Balagu Spine. 1995;20:1265. doi: 10.1097/00007632-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Balagu Lancet. 2003;361:1403. doi: 10.1016/S0140-6736(03)13148-0. [DOI] [PubMed] [Google Scholar]
- 4.Bejia Rheumatology. 2003;24:119. [Google Scholar]
- 5.Brattberg G (1994) The incidence of back pain and headache among Swedish school children. Qual Life Res 3 [Suppl 1]: S27–S31 [DOI] [PubMed]
- 6.Coleman Ergonomics. 1998;41:401. doi: 10.1080/001401398186900. [DOI] [PubMed] [Google Scholar]
- 7.Coste Rev Rhum Mal Osteoartic. 1989;56:861. [PubMed] [Google Scholar]
- 8.Duggleby Disabil Rehabil. 1997;19:505. doi: 10.3109/09638289709166043. [DOI] [PubMed] [Google Scholar]
- 9.Gunzburg Eur Spine J. 1999;8:439. doi: 10.1007/s005860050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harreby Eur Spine J. 1999;8:444. doi: 10.1007/s005860050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones Pediatrics. 2003;111:822. doi: 10.1542/peds.111.4.822. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs Pain. 2003;103:259. doi: 10.1016/S0304-3959(02)00454-2. [DOI] [PubMed] [Google Scholar]
- 13.Kujala Med Sci Sports Exerc. 1996;28:165. doi: 10.1097/00005768-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kujala Am J Sports Med. 1997;25:363. doi: 10.1177/036354659702500316. [DOI] [PubMed] [Google Scholar]
- 15.Leboeuf-Yde Spine. 1998;23:2207. doi: 10.1097/00007632-199810150-00012. [DOI] [PubMed] [Google Scholar]
- 16.Legolf Rev Rhum Engl Ed. 1998;65:43. [Google Scholar]
- 17.Olsen Am J Public Health. 1992;82:606. doi: 10.2105/ajph.82.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piko Orv Hetil. 1999;140:1297. [PubMed] [Google Scholar]
- 19.Salminen Acta Paediatr Scand Suppl. 1984;315:1. [PubMed] [Google Scholar]
- 20.Salminen Acta Paediatr. 1992;81:1035. doi: 10.1111/j.1651-2227.1992.tb12170.x. [DOI] [PubMed] [Google Scholar]
- 21.Salminen Spine. 1995;20:2101. doi: 10.1097/00007632-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sheldon J Orthop Sports Phys Ther. 1994;9:105. doi: 10.2519/jospt.1994.19.2.105. [DOI] [PubMed] [Google Scholar]
- 23.Sjolie Eur Spine J. 2002;11:582. doi: 10.1007/s00586-002-0412-z. [DOI] [PubMed] [Google Scholar]
- 24.Staes Acta Paediatr. 2003;92:444. doi: 10.1111/j.1651-2227.2003.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 25.Szpalski Eur Spine J. 2002;11:459. doi: 10.1007/s00586-002-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taimela S, Kujala UM, Salminen JJ, Viljanen T (1997) The prevalence of low back pain among children and adolescents. A nationwide, cohort-based questionnaire survey in Finland. Spine15;22(10):1132–1136 [DOI] [PubMed]
- 27.Troussier B, Balagué F, Phelip X (1998) Risk factors of non specific low back pain in children and adolescents. Rev Rhum Engl Ed 65[Suppl 3]: 49–57
- 28.Troussier B, Marchou-Lopez S, Pironneau S, Alais E, Grison J, Prel G et al (1999) Back pain and spinal alignment abnormalities in schoolchildren. Rev Rhum Engl Ed 66(7–9):370–380 [PubMed]
- 29.van Eur Spine J. 1999;8:187. doi: 10.1007/s005860050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Ergonomics. 2001;44:739. doi: 10.1080/00140130110045794. [DOI] [PubMed] [Google Scholar]
- 31.Verni J Sports Med Phys Fitness. 1999;39:61. [PubMed] [Google Scholar]
- 32.Viry P, Creveuil C, Marcelli C (1999) Nonspecific back pain in children. A search for associated factors in 14-year-old schoolchildren. Rev Rhum Engl Ed 66(7–9):381–388 [PubMed]