Abstract
Cervical corpectomy is a frequently used technique for a wide variety of spinal disorders. The most commonly used approach is anterior, either with or without plating. The results for single-level corpectomy are better than in multilevel procedures. Nevertheless, hardware- or graft-related complications are observed. In the past, constrained implant systems were developed and showed encouraging stability, especially for posterior screw and rod systems in the lumbar spine. In the cervical spine, few reports about the primary stability of constrained systems exist. Therefore, in the present study we evaluated the primary stability of posterior screw and rod systems, constrained and non-constrained, in comparison with anterior plating and circumferential instrumentations in a non-destructive set-up, by loading six human cadaver cervical spines with pure moments in a spine tester. Range of motion and neutral zone were measured for lateral bending, flexion/extension and axial rotation. The testing sequence consisted of: (1) stable testing; (2) testing after destabilization and cage insertion; (3a) additional non-constrained screw and rod system with lateral mass screws, (3b) with pedicle screws instead of lateral mass screws; (4a) constrained screw and rod system with lateral mass screws, (4b) with pedicle screws instead of lateral mass screws; (5) 360°set-up; (6) anterior plate. The stability of the anterior plate was comparable to that of the non-constrained system, except for lateral bending. The primary stability of the non-constrained system could be enhanced by the use of pedicle screws, in contrast to the constrained system, for which a higher primary stability was still found in axial rotation and flexion/extension. For the constrained system, the achievable higher stability could obviate the need to use pedicle screws in low instabilities. Another benefit could be fewer hardware-related complications, higher fusion rate, larger range of instabilities to be treated by one implant system, less restrictive postoperative treatment and possibly better clinical outcome. From a biomechanical standpoint, in regard to primary stability the constrained systems, therefore, seem to be beneficial. Whether this leads to differences in clinical outcome has to be evaluated in clinical trials.
Keywords: Cervical spine, Constrained implants, Corpectomy, Primary stability
Introduction
Posterior screw and rod systems for spinal fusion procedures are increasingly used. In the cervical spine they showed superior biomechanical stability in comparison with posterior plating [19], whereas the posterior plates had stability benefits compared with anterior plating [11, 42]. The effect of constrained (rigid) systems in the lumbar spine has already been evaluated [7, 34, 50], whereas studies for the cervical spine are rare. The higher stability of pedicle screws in comparison with lateral mass screws [26, 27] led to a frequent use in the lumbar spine [1, 4, 5, 6, 23, 32, 35] for various spinal disorders, including spondylolisthesis, scoliosis or degenerative conditions, and to a lesser degree in the cervical spine [2, 3]. Acceptable complication rates were observed [18, 57]. The goal of this study was to evaluate the primary stability potential of posterior constrained and non-constrained screw and rod systems with lateral mass and pedicle screws in comparison with anterior plating and circumferential instrumentations in a cervical spine single-level corpectomy model.
Material and methods
In this study six human cadaveric specimens (two males, four females, mean age 80 years, range 66–92 years), consisting of C2 to at least T1 were used. After examination of the specimen, plain radiographs were taken, to exclude structural damage. When no abnormality was detected, specimens were then stored frozen at −20°C in triple-sealed plastic bags until preparation. The muscle tissue was removed, while all ligaments and bony structures were preserved. During preparation and testing, the specimens were kept moist with saline solution to prevent dehydration. As studies showed [37, 55], handling specimens as described does not affect their biomechanical properties.
After preparation of the specimen, bone mineral density was measured in the vertebral bodies of C4 and C6 by quantitative computed tomography (XCT 960, Stratec Medizintechnik, Pforzheim, Germany). Short screws were then partially driven into the cranial and caudal vertebrae to obtain a better anchorage of the vertebrae in the polymethylmethacrylate. The ending vertebrae were then embedded in polymethylmethacrylate (Technovit 3040, Heraeus Kulzer, Wehrheim, Germany). The motion-analysis system was fixed to the C4 and C6, by laterally screws inserted into the vertebral bodies.
The corpectomy C5 was created with ordinary clinical instruments (rongeurs, high-speed air drill) with a width of at least 16 mm, while the posterior longitudinal ligament was preserved. For accommodation of the cage with force sensor, a small hole was drilled in the adjacent endplates. The miniature load cell (type 8413, Burster Präzisionsmeßtechnik, Gernsbach, Germany) was placed in a purpose-built cage based on a routinely used cage (ADD, Ulrich Medizintechnik, Ulm, Germany), with spikes at the superior and inferior end, which prevented the cage from slipping. Adjustment of the cage to the desired graft height was provided by either a screw thread at the lower end of the cage or by use of two stainless steel tubes of different lengths.
The testing sequence consisted of:
Stable testing of the intact specimen (intact)
Testing after destabilization and cage insertion (cage)
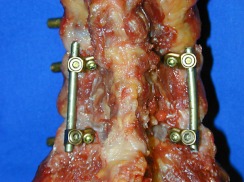
Additional Cervifix [22] (Synthes, Umkirch, Germany) instrumentation with 3.5 mm lateral mass screws (NC-LM) (Fig. 1)
Cervifix with 3.5 mm pedicle screws, instead of lateral mass screws (NC-P)
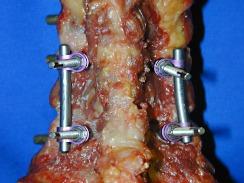
Neon occipito-cervical system [43, 44] (Ulrich Medizintechnik, Ulm, Germany) with 4.0 mm cannulated lateral mass screws (C-LM) (Fig. 2)
Neon with 4.0 mm pedicle screws, instead of lateral mass screws (C-P)
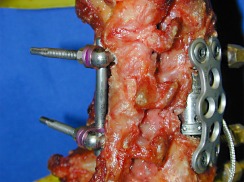
360° set-up (Fig. 3): a combination of the anterior plate and the constrained screw and rod system with pedicle screws (C-P)
Anterior plate (OAP) (Osmium, Ulrich Medizintechnik, Ulm, Germany)
Fig. 1.
Non-constrained screw and rod system with lateral mass screws (NC-LM)
Fig. 2.
Constrained screw and rod system with lateral mass screws (C-LM)
Fig. 3.
Circumferential instrumentation (360°)
After destabilization, the specimens were mounted in the spine tester with free movement in all directions. The force sensor in the cage was then adjusted to 40 N, thereby providing equal preload with respect to the individual neutral position of every cervical spine.
The spine tester used was described in detail previously [54]. A high-resolution, non-contacting ultrasound motion-analysis system (Zebris, Isny, Germany, resolution 0.06°) was used for measuring segmental motion. Loads were applied as pure moments in alternating sequences for right/left lateral bending (±Mx), flexion/extension (±My) and right/left axial rotation (±Mz) with ±2.5 Nm for all directions. Every instrumentation was tested with three cycles. The first two cycles were needed to precondition the specimens and to minimize viscoelastic effects, whereas the third was recorded, and range of motion (ROM) and neural zone (NZ) were evaluated [56]. The test set-up was described and used in a previous study [46]. The testing procedure was conducted according to the recommendations for the standardization of in vitro stability testing of spinal implants [56].
For statistical analysis, a nonparametric test (Wilcoxon signed rank test) was chosen due to missing normal distribution. Calculations were performed by commercial statistics software (StatView 4.0, Abacus Concepts, USA). Adjustment for multiple testing was not performed, as this would have resulted in a great loss of information. Consequently, a p<0.05 was not rated as significant, but rather as a tendency towards a relevant difference of the results compared.
Results
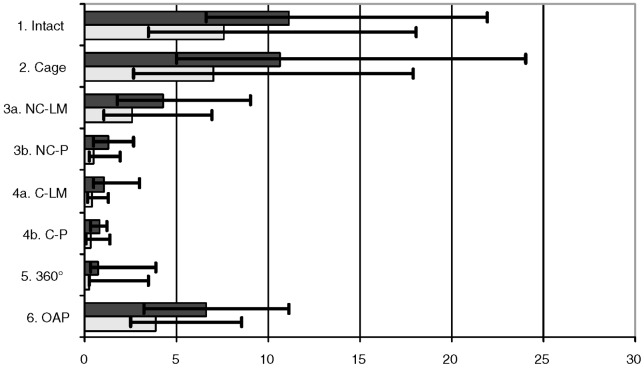
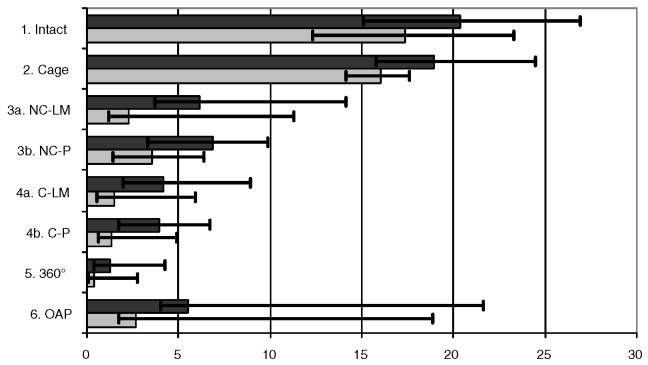
For lateral bending the highest median ROM was found in the intact state, closely followed by the cage (Fig. 4). Consequently, except for the cage, all the instrumentations showed a p<0.05 compared with the intact state and the cage (Table 1). This was the same for the OAP, which had the third-highest ROM, when compared with the other instrumentations. The non-constrained system with pedicle screws (NC-P) and the other posterior instrumentations showed no differences among each other, but had a benefit against the non-constrained system with lateral mass screws (NC-LM) (Table 1). In contrast, for the constrained system no distinct difference was observed between pedicle and lateral mass screws.
Fig. 4.
Median ROM (dark plot) and NZ (bright plot), minimal and maximal values for lateral bending in degrees. 1.) stable testing, 2.) testing after destabilization and cage insertion, 3a) additional non-constrained screw and rod system with lateral mass screws 3b) with pedicle screws instead of lateral mass screws; 4a) constrained screw and rod system with lateral mass screws 4b) with pedicle screws instead of lateral mass screws, 5.) 360°, 6.) anterior plate
Table 1.
Median range of motion (ROM) values for lateral bending in degrees (first line) and p values
| 1. Intact | 2. Cage | 3a. NC-LM | 4a. C-LM | 3b. NC-P | 4b. C-P | 5. 360° | 6. OAP | |
|---|---|---|---|---|---|---|---|---|
| ROM | 11.2 | 10.7 | 4.3 | 1.1 | 1.3 | 0.8 | 0.8 | 6.7 |
| 2. Cage | 0.4631 | – | – | – | – | – | – | – |
| 3a. NC-LM | 0.0277 | 0.0277 | – | – | – | – | – | – |
| 4a.C-LM | 0.0277 | 0.0277 | 0.0277 | – | – | – | – | – |
| 3b. NC-P | 0.0277 | 0.0277 | 0.0277 | 0.6858 | – | – | – | – |
| 4b. C-P | 0.0277 | 0.0277 | 0.0277 | 0.1380 | 0.0679 | – | – | – |
| 5. 360° | 0.0277 | 0.0277 | 0.0277 | 0.4631 | 0.3454 | 0.3454 | – | – |
| 6. OAP | 0.0277 | 0.0277 | 0.0464 | 0.0277 | 0.0277 | 0.0277 | 0.0277 | – |
1. Stable testing
2. Testing after destabilization and cage insertion
3a. Additional non-constrained screw and rod system with lateral mass screws
3b. Non-constrained system with pedicle screws instead of lateral mass screws
4a. Constrained screw and rod system with lateral mass screws
4b. Constrained system with pedicle screws instead of lateral mass screws
5. 360° set-up
6. Anterior plate
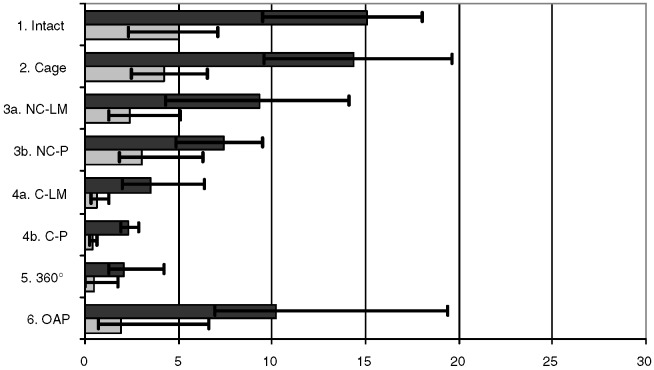
The intact state and the cage showed in flexion/extension, again, close median ROM values and indistinct p values, as in lateral bending (Fig. 5, Table 2). In contrast to lateral bending, in the non-constrained system no difference for the various screws was observed (NC-P vs NC-LM p>0.05), although a slightly higher median ROM occurred for the pedicle screws (Table 2). This led to a p>0.05 for the C-LM vs NC-LM comparison, but p<0.05 for C-LM vs NC-P. The screw type again had no effect in the constrained group (p>0.05). The stability of the anterior plating and the non-constrained screw and rod system showed no difference, whereas the 360° set-up had a p<0.05 compared with all other instrumentations.
Fig. 5.
Median ROM (dark plot) and NZ (bright plot), minimal and maximal values for flexion/extension in degrees. 1.) stable testing, 2.) testing after destabilization and cage insertion, 3a) additional non-constrained screw and rod system with lateral mass screws 3b) with pedicle screws instead of lateral mass screws; 4a) constrained screw and rod system with lateral mass screws 4b) with pedicle screws instead of lateral mass screws, 5.) 360°, 6.) anterior plate
Table 2.
Median range of motion (ROM) values for flexion/extension in degrees (first line) and p values
| 1. Intact | 2. Cage | 3a. NC-LM | 4a. C-LM | 3b. NC-P | 4b. C-P | 5. 360° | 6. OAP | |
|---|---|---|---|---|---|---|---|---|
| ROM | 20.4 | 19.0 | 6.2 | 4.2 | 6.9 | 4.0 | 1.3 | 5.5 |
| 2. Cage | 0.7532 | – | – | – | – | – | – | – |
| 3a. NC-LM | 0.0277 | 0.0277 | – | – | – | – | – | – |
| 4a.C-LM | 0.0277 | 0.0277 | 0.1730 | – | – | – | – | – |
| 3b. NC-P | 0.0277 | 0.0277 | 0.7532 | 0.0277 | – | – | – | – |
| 4b. C-P | 0.0277 | 0.0277 | 0.0277 | 0.6002 | 0.0277 | – | – | – |
| 5. 360° | 0.0277 | 0.0277 | 0.0277 | 0.0277 | 0.0277 | 0.0464 | – | – |
| 6. OAP | 0.0464 | 0.0464 | 0.4631 | 0.0464 | 0.9165 | 0.0277 | 0.0277 | – |
1. Stable testing
2. Testing after destabilization and cage insertion
3a. Additional non-constrained screw and rod system with lateral mass screws
3b. Non-constrained system with pedicle screws instead of lateral mass screws
4a. Constrained screw and rod system with lateral mass screws
4b. Constrained system with pedicle screws instead of lateral mass screws
5. 360° set-up
6. Anterior plate
The anterior plate did not have, in axial rotation, more stability than the intact state or the cage. At the same time, both screw-and-rod systems had p<0.05 compared with intact state and cage (Table 3), but only the constrained system and the 360° set-up had a benefit compared with the anterior plate. There was a difference for the pedicle screws in the non-constrained group (NC-P vs NC-LM p<0.05), in contrast to the constrained system (NC-LM vs NC-P p>0.05). The constrained system, independent of screw type, and the circumferential set-up had a lower median ROM (p<0.05) than the non-constrained system (Fig. 6).
Table 3.
Median range of motion (ROM) values for axial rotation in degrees (first line) and p values
| 1. Intact | 2. Cage | 3a. NC-LM | 4a. C-LM | 3b. NC-P | 4b. C-P | 5. 360° | 6. OAP | |
|---|---|---|---|---|---|---|---|---|
| ROM | 15.1 | 14.4 | 9.4 | 3.5 | 7.4 | 2.3 | 2.1 | 10.3 |
| 2. Cage | 0.6750 | – | – | – | – | – | – | – |
| 3a. NC-LM | 0.0464 | 0.0277 | – | – | – | – | – | – |
| 4a.C-LM | 0.0277 | 0.0277 | 0.0277 | – | – | – | – | – |
| 3b. NC-P | 0.0277 | 0.0277 | 0.0464 | 0.0277 | – | – | – | – |
| 4b. C-P | 0.0277 | 0.0277 | 0.0277 | 0.1056 | 0.0277 | – | – | – |
| 5. 360° | 0.0277 | 0.0277 | 0.0277 | 0.0277 | 0.0277 | 0.3454 | – | – |
| 6. OAP | 0.2489 | 0.1159 | 0.2489 | 0.0277 | 0.0747 | 0.0277 | 0.0277 | – |
1. Stable testing
2. Testing after destabilization and cage insertion
3a. Additional non-constrained screw and rod system with lateral mass screws
3b. Non-constrained system with pedicle screws instead of lateral mass screws
4a. Constrained screw and rod system with lateral mass screws
4b. Constrained system with pedicle screws instead of lateral mass screws
5. 360° set-up
6. Anterior plate
Fig. 6.
Median ROM (dark plot) and NZ (bright plot), minimal and maximal values for axial rotation in degrees. 1.) stable testing, 2.) testing after destabilization and cage insertion, 3a) additional non-constrained screw and rod system with lateral mass screws 3b) with pedicle screws instead of lateral mass screws; 4a) constrained screw and rod system with lateral mass screws 4b) with pedicle screws instead of lateral mass screws, 5.) 360°, 6.) anterior plate
The median BMD was 179.9 mg/cm3 in the vertebral bodies of C4, with a range of 99.3–303.6 mg/cm3 and 194.4 mg/cm3 (range 159.4–274.1 mg/cm3) for C6.
Discussion
The anterior plate showed a comparable stability to the non-constrained screw and rod system for flexion/extension and axial rotation. A difference was observed only for lateral bending (Figs. 4, 5, 6). This is in contrast to the results from a multilevel study [46], where the results for the anterior plating were worse. It seems that the anterior plate reaches its limits when a higher instability is to be treated. Paramore et al. [38] found that anterior plate length is predictive of plate failure. Sasso et al. [45] found 94% successful fusions for a two-level corpectomy, whereas they had construct failures in five of seven patients (71%) with a three-level corpectomy. ElSaghir et al. [13] found more hardware-related problems for anterior plating in double-level than in single-level corpectomy. Whether the limiting number of corpectomy levels for anterior plating is 2 or 3 has not been definitively established.
The tested anterior plate was unlocked, which can affect the primary stability. Spivak et al. [49] showed a higher primary stability and after-cyclic loading for locked (constrained) anterior plates. Lowery and McDonough found significantly fewer failures for constrained anterior plates than non-constrained [31]. Conversely, Epstein et al. [15] had the highest failure rate for single-level anterior cervical corpectomy and plating (ACF) when using a constrained system, in comparison with semi-rigid and dynamic plating. This could be due to graft settling and “stress shielding” of the constrained plate, in which the plate acts as a barrier. This prevents changes of the length of the anterior column, possibly leading to pseudarthrosis in the case of graft settling. Dynamic, or “translational,” systems that are supposed to reduce these effects still have a plate- or graft-related complication rate of 9.5% [17]. The benefit of dynamic plates seems to be the incorporation of the graft into the system, by keeping it loaded. Brodke et al. [9] found an axial load transfer of more than 60% to the graft for locked and dynamic plates with a full-length graft. When graft subsidence occurred, the transmitted load to the graft was significantly lower, which led to significant stiffness differences in flexion/extension, but not for lateral bending where the locked plates were more stable. In axial rotation the graft loading had no effect on stiffness, as the graft does not provide additional resistance in axial torsion. Therefore, the stabilizing potential of dynamic anterior plating for higher instabilities seems questionable. The combination of a posterior implant and a dynamic anterior plate in difficult cases (e.g., morbidly obese patients) could lessen or avoid plate-related problems [16]. However, then the question arises as to whether a combined procedure is required. Do Koh et al. [11] found no significant improvement of stability in combined anterior-posterior fixation in comparison with anterior graft and posterior plating. This is in line with our results, in which the circumferential instrumentation had no distinct benefit for stability in lateral bending and axial rotation, when comparing with the constrained system with pedicle screws (Figs. 4, 6). The difference in flexion/extension (Fig. 5) is due to the anterior plate, which had its greatest stabilizing effect in flexion/extension. As the clinical results and complication rates differ widely between single and multilevel corpectomy, there seems to be no need for such a complex procedure in single-level instability when using anterior graft and posterior instrumentation. This is reflected when comparing the results of this study and the multilevel [46] one.
The question as to whether “stress shielding” for posterior implants occurs is not answered. Some expect a favorable environment for fusion by dynamic devices [47]. Others [53, 58] prefer constrained systems in the lumbar spine. Stress shielding for posterior implants is probably prevented by the fact that the so-called constrained or rigid implants are not what they are supposed to be in vivo. Although, within the system, a rigid interface connection may be achieved up to the mechanical limiting force, which overcomes the screw/nut stability, the biological bone–metal (screw–bone) interface is already under physiological loads, semi-rigid, especially after cyclic loading. This, theoretically, allows the graft to settle. Thereby, in contrast to anterior plates, the most rigid implant could be used without unloading the graft, keeping it under axial load and still giving enough stability to keep the alignment and simplify postoperative treatment.
The performance of anterior plating in this study corresponds to the clinical results in single-level corpectomy with fusion rates ranging 86–100%. Nevertheless, complication rates of 0–57% are reported [10, 12, 21, 33, 48, 51, 52], although a definite separation between single-level and multilevel results is not always feasible in these studies. Up to 20% hardware-related problems for anterior single-level procedures occur, such as hardware failure, graft extrusion and pseudarthrosis [13, 15, 17]. A re-operation is not always mandatory, but the outcome for patients can be affected. This raises the controversial question as to whether a more rigid fixation can enhance clinical outcome and reduce complication rates, or if constrained implants prevent fusion by stress shielding. Johnston et al. suggested a benefit to higher segmental stiffness, due to stiffer constructs of larger rod diameters, in a goat thoracic model for short [25] and long [24] segment fusions. Similar results were also found by other authors [20, 34]. Besides improved fusion rates, stiffer constructs could also lead to a lower rate of early failures, which was found for plate and screw failure in anterior plating [31]. The influence of spinal fusions on accelerated adjacent-segment degeneration—a subject of controversy [29, 30, 39, 41]—is probably not mainly dependent on implant rigidity but, rather, on motion concentration by the bone graft [28]. Nevertheless, spinal fusions could increase degeneration and should, therefore, be limited to as few levels as possible. This is better facilitated by a higher primary stability due to rigid implants.
The non-constrained system with pedicle screws had advantages over the lateral mass screws in lateral bending and axial rotation (Table 1, 3), in contrast to the constrained system, in which no difference between the screw types was obvious, independent of motion direction (Figs. 4, 5, 6). This was in contrast to results of a multilevel study [46] that found no benefit for the non-constrained system with pedicle screws in flexion/extension and axial rotation, but did find one for the constrained system. The question is why the stability enhancement by pedicle screws is not found for both systems in both models?
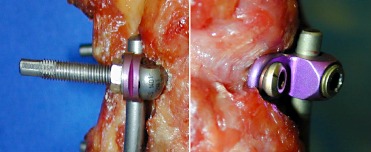
The posterior screw and rod system can be divided into three parts that determine stability. First, the screw–bone interface includes the tightening of the screw, which depends on the bone stock; screw length and screw trajectory. This point seems, in this setting, secondary, because the bone stock was constant, as the same specimens were used and the screw trajectories were also identical. The second determining point is the material properties, including diameter, of the rods or screws. Deformation of the rods or screws was not seen under the induced moments. Still, this could have affected primary stability. If this had been the major reason for the various stabilities achieved, the question would be why the stabilizing effect of the pedicle screws was not found in the single-level and multilevel model for both systems. Therefore, the third point, the stability of the screw–rod interface, seems to be the key one. In the constrained system, the connection of screw and rod is made by a nut compressing the connector to the screw head. It is tightened over a thread fastened to the screw head (Fig. 7). This is highly reproducible, independent of the tightening of the screw in the bone stock. This rigid fixation does not allow any motion at the screw–rod interface, as long as the acting forces do not exceed the torsional force of the screw–nut connection. For the non-constrained system the screw–rod connection is achieved by tightening the connector to the bone by the screw (Fig. 7). The connector acts similarly to a shim. Its stability is highly dependent on the tightening between screw and bone. In osteoporotic bone, for example, the lower degree of tightening of the screw into the vertebra affects not only the screw–bone interface but consequently also the screw–rod interface. The connector can toggle between bone and screw-head in a certain range of motion, until the end of the toggle range, where the connector can regain stability by tilting. The total stability of the system is a consequence of the three parts mentioned above, as long as the chain is not disconnected, e.g., by screw or rod failure. The material, screw and rod diameters are inherent to the system; therefore, only different screw trajectories and lengths can change primary stability.
Fig. 7.
Screw–rod connection: constrained system left and non-constrained system right
Since, in vivo, the forces acting on the implant are not known exactly, it seems, from a biomechanical point of view, safer to choose a more rigid implant to prevent failure [20, 24, 25, 34]. If similar stability can be achieved by different systems or screws, the easiest technique with the lowest risk should be used. Nevertheless, clinical results and outcomes do not depend only on primary biomechanical stability. Restrictive postoperative treatment can enhance clinical outcome. Bernhardt et al. [8] found similar results in lumbar spine fusions for instrumentation with and without pedicle screws; however, the postoperative regime was different—6 weeks treatment with a thoracolumbosacral half-hip spica cast for those without instrumentation, and no orthosis for those with pedicle screw instrumentation. A higher primary stability can affect postoperative treatment by restricting dangerous deflections of the spine before bony union occurs. In our opinion, this is a point that has to be considered when choosing a therapeutic strategy, especially as the trend goes to faster rehabilitation. Implant failures can still lead, in the end, to solid bone fusion or asymptomatic pseudarthrosis, although this is frequently associated with a poor clinical outcome [14, 36, 40] and should, therefore, be reduced as well as possible.
Besides the general limitations of biomechanical in vitro studies, we have to mention that no randomization of the testing order was done in this study. This was due to technical reasons. We wanted to evaluate instrumentations in the same specimen to minimize inter-individual differences, and, additionally, we wanted to use the same screw trajectories. As the screw diameters of the two posterior systems were different (3.5 mm and 4 mm), it was predetermined that the non-constrained system had to be tested first. Nevertheless, the constrained system tested after the non-constrained system showed higher stability and the anterior plate in flexion/extension had similar indistinct values compared with the non-constrained system, thereby limiting the possibility of an order effect. Nevertheless, such an effect cannot be totally excluded. Additionally, the bigger screw diameter of the constrained system could also have an influence on the higher stability achieved by this system compared with the non-constrained system.
Conclusions
This biomechanical study shows that the major limiting factor—besides different screw sizes—of primary stability between constrained and non-constrained screw and rod systems is the screw–rod interface, which leads to a generally lower total system stability for the non-constrained system. Although enhancement for the NCS can be achieved by using pedicle screws in the single-level model, the effect is overcome in the multilevel model. This makes the use of pedicle screws and their risk of complications questionable, when the same primary stability is achievable by a rigid system with lateral mass screws. No definitive answer has been found as to whether higher primary stability leads to higher fusion rates or lower complications, especially when comparing different anterior plating systems with posterior screw and rod systems. Moreover, the impact of stress shielding for posterior implants, the contribution to adjacent segment degeneration and the effect of anterior dynamic systems are also not known. Therefore, the ideal implant for cervical single-level and multilevel corpectomies has yet to be found. More basic and clinical studies are needed before these questions can finally be answered by evidence, not by surgeons’ preference.
Acknowledgment
Support was provided by Ulrich Medizintechnik, Ulm, Germany
References
- 1.Abdu Spine. 1994;19:710. doi: 10.1097/00007632-199403001-00011. [DOI] [PubMed] [Google Scholar]
- 2.Abumi Spine. 1997;22:1853. doi: 10.1097/00007632-199708150-00010. [DOI] [PubMed] [Google Scholar]
- 3.Abumi J Spinal Disord. 1994;7:19. doi: 10.1097/00002517-199407010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Aebi M (1988) Correction of degenerative scoliosis of the lumbar spine. A preliminary report. Clin Orthop 80–86 [PubMed]
- 5.AebiSpine 1987125443660081 [Google Scholar]
- 6.Ani Spine. 1991;16:S302. doi: 10.1097/00007632-199106001-00025. [DOI] [PubMed] [Google Scholar]
- 7.Ashman Spine. 1989;14:1398. doi: 10.1097/00007632-198912000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Bernhardt M, Swartz DE, Clothiaux PL, Crowell RR, White AA 3rd (1992) Posterolateral lumbar and lumbosacral fusion with and without pedicle screw internal fixation. Clin Orthop 109–115 [PubMed]
- 9.Brodke Spine. 2001;26:1324. doi: 10.1097/00007632-200106150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Brown Spine. 1988;13:236. doi: 10.1097/00007632-198803000-00003. [DOI] [PubMed] [Google Scholar]
- 11.DoSpine 2001261511148640 [Google Scholar]
- 12.ElerakyJ Neurosurg 1999903510413123 [Google Scholar]
- 13.ElSaghir Arch Orthop Trauma Surg. 2000;120:549. doi: 10.1007/s004020000153. [DOI] [PubMed] [Google Scholar]
- 14.Emery Spine. 1997;22:2622. doi: 10.1097/00007632-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Epstein Surg Neurol. 2001;56:73. doi: 10.1016/S0090-3019(01)00523-7. [DOI] [PubMed] [Google Scholar]
- 16.Epstein J Spinal Disord Tech. 2002;15:221. doi: 10.1097/00024720-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Epstein Spinal Cord. 2003;41:153. doi: 10.1038/sj.sc.3101418. [DOI] [PubMed] [Google Scholar]
- 18.Gaines J Bone Joint Surg Am. 2000;82:1458. doi: 10.2106/00004623-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Grubb Spine. 1997;22:1948. doi: 10.1097/00007632-199709010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Gurr J Orthop Res. 1989;7:838. doi: 10.1002/jor.1100070610. [DOI] [PubMed] [Google Scholar]
- 21.Herman J Neurosurg. 1994;80:963. doi: 10.3171/jns.1994.80.6.0963. [DOI] [PubMed] [Google Scholar]
- 22.Horgan Neurosurgery. 1999;44:1267. [PubMed] [Google Scholar]
- 23.Horowitch Spine. 1989;14:461. doi: 10.1097/00007632-198904000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Johnston Spine. 1990;15:908. doi: 10.1097/00007632-199009000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Johnston Spine. 1995;20:2400. doi: 10.1097/00007632-199511001-00006. [DOI] [PubMed] [Google Scholar]
- 26.Jones Spine. 1997;22:977. doi: 10.1097/00007632-199705010-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kotani Spine. 1994;19:2529. doi: 10.1097/00007632-199411001-00007. [DOI] [PubMed] [Google Scholar]
- 28.Krag Spine. 1991;16:S84. doi: 10.1097/00007632-199103001-00014. [DOI] [PubMed] [Google Scholar]
- 29.Lee Spine. 1988;13:375. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Leong Spine. 1983;8:793. doi: 10.1097/00007632-198310000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Lowery Spine. 1998;23:181. doi: 10.1097/00007632-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 32.Marchesi Spine. 1992;17:S304. doi: 10.1097/00007632-199208001-00016. [DOI] [PubMed] [Google Scholar]
- 33.MayrJ Neurosurg 2002961011795694 [Google Scholar]
- 34.McAfee Spine. 1989;14:919. doi: 10.1097/00007632-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 35.McAfee Spine. 1991;16:S422. [PubMed] [Google Scholar]
- 36.Newman Spine. 1993;18:2380. doi: 10.1097/00007632-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Panjabi J Orthop Res. 1985;3:292. doi: 10.1002/jor.1100030305. [DOI] [PubMed] [Google Scholar]
- 38.Paramore J Neurosurg. 1996;84:957. doi: 10.3171/jns.1996.84.6.0957. [DOI] [PubMed] [Google Scholar]
- 39.Penta Spine. 1995;20:743. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 40.Phillips Spine. 1997;22:1585. doi: 10.1097/00007632-199707150-00012. [DOI] [PubMed] [Google Scholar]
- 41.Rahm J Spinal Disord. 1996;9:392. [PubMed] [Google Scholar]
- 42.Richman Spine. 1995;20:2192. doi: 10.1097/00007632-199510001-00003. [DOI] [PubMed] [Google Scholar]
- 43.Richter Eur Spine J. 2000;9:417. doi: 10.1007/s005860000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richter Spine. 2002;27:1724. doi: 10.1097/00007632-200208150-00008. [DOI] [PubMed] [Google Scholar]
- 45.Sasso Spine. 2003;28:140. doi: 10.1097/00007632-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt Spine. 2003;28:1821. doi: 10.1097/01.BRS.0000083287.23521.48. [DOI] [PubMed] [Google Scholar]
- 47.Seifert Spine. 1999;24:2206. doi: 10.1097/00007632-199911010-00006. [DOI] [PubMed] [Google Scholar]
- 48.Seifert Neurosurgery. 1991;29:498. [PubMed] [Google Scholar]
- 49.Spivak Spine. 1999;24:334. doi: 10.1097/00007632-199902150-00005. [DOI] [PubMed] [Google Scholar]
- 50.Steffee Spine. 1993;18:1160. doi: 10.1097/00007632-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Tippets Neurosurgery. 1988;22:1008. doi: 10.1227/00006123-198806010-00006. [DOI] [PubMed] [Google Scholar]
- 52.Tominaga Surg Neurol. 1994;42:408. doi: 10.1016/0090-3019(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 53.Wetzel Spine. 1999;24:1138. doi: 10.1097/00007632-199906010-00014. [DOI] [PubMed] [Google Scholar]
- 54.WilkeEur Spine J 19943917874556 [Google Scholar]
- 55.Wilke Anat Rec. 1998;251:15. doi: 10.1002/(SICI)1097-0185(199805)251:1<15::AID-AR4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 56.Wilke Eur Spine J. 1998;7:148. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yahiro Spine. 1994;19:2274S. doi: 10.1097/00007632-199410151-00004. [DOI] [PubMed] [Google Scholar]
- 58.Zdeblick Spine. 1993;18:983. doi: 10.1097/00007632-199306150-00006. [DOI] [PubMed] [Google Scholar]