Abstract
The ability of bone substitutes to promote bone fusion is contigent upon the presence of osteoinductive factors in the bone environment at the fusion site. Osteoblast progenitor cells are among these environmental osteoinductive factors, and one of the most abundant and available sources of osteoblastic cells is the bone marrow. As far as biological conditions are concerned, the vertebral interbody space appears as a favorable site for fusion, as it is surrounded by spongy bone, theoretically rich in osteogenic cells. This site may, however, not be as rich in osteogenic precursor cells especially at the time of grafting, because decortication of the vertebral end plates during the grafting process may modifiy cell content of the surrounding spongy bone. We tested this hypothesis by comparing the abundance of human osteogenic precursor cells in bone marrow derived from the iliac crest, the vertebral body, and the decorticated intervertebral body space. The number of potential osteoblast progenitors in each site was estimated by counting the alkaline phosphatase–expressing colony-forming units (CFU-AP). The results, however, demonstrate that the vertebral interbody space is actually poorer in osteoprogenitor cells than the iliac crest (P<0.001) and vertebral body (P<0.01), especially at the time of graft implantation. In light of our results, we advocate addition of iliac crest bone marrow aspirate to increase the success rate of vertebral interbody fusion.
Keywords: Bone substitutes, Calcium phosphate ceramics, Osteogenic precursor cells, Spinal fusion
Introduction
Posterolateral and anterior spinal fusions are valuable surgical approaches for the management of spinal disorders such as spondylolisthesis, stenosis, and degenerative disc disease. Autologous bone graft is the preferred approach to obtain a solid spinal fusion. Iliac crest harvesting is however often associated with complications such as increased operative time, blood loss, and pain at the donor site [6]. Furthermore, spinal fusion with autologous bone graft may fail. Novel bone substitutes are therefore increasingly sought after.
Most bone substitutes are osteoconductors that lack osteoinductive properties. Therefore, these substitutes can only promote bone fusion when osteoinductive factors are provided by the bone environment at the implantation site. These osteoinductive factors include the osteoblast progenitor cells. The latter can be found, for instance, in the periosteum, the peritrabecular connective tissue, and the bone marrow. The most abundant and readily available source is the bone marrow [1, 7, 8].
Combination of bone substitutes, such as ceramics, with bone marrow has shown significant osteogenic potential in vitro and in vivo [4, 10 ]. In a rabbit model of posterolateral fusion, our group [3] found a significant increase in bone formation when bone marrow aspirate was added to ceramics. Moreover, Curylo et al. [2] observed a 30% greater increase in bone volume when bone marrow aspirate was added to autogenous iliac crest graft in a rabbit model of posterolateral fusion.
Altogether these results strongly suggest that addition of bone marrow to graft material can greatly facilitate bone formation at the fusion site. As bone growth may be enhanced to a greater extent if the implantation site is rich in osteogenic precursor cells, the local bone environment has to be taken into account when characterizing the implantation site, i.e., in determining the necessity of adding bone marrow.
The various possible sites for spinal fusion can be divided mainly into two categories with respect to the biomechanical context: the posterolateral and anterior sites. This paper concerns only anterior sites. They are theoretically rich in osteogenic precursor cells as they are mainly surrounded by spongy bone, which is considered to be rich in osteogenic precursor cells. The presumed abundance of osteogenic cells at the interbody site may lead one to believe that addition of bone marrow aspirate from the iliac crest to graft substitutes is not required for a successful bone fusion. This site may, however, not be as rich in osteogenic precursors as expected, especially at the time of grafting, because decortication and bleeding in the disc space during the grafting process may modify the cell content of the surrounding spongy bone.
We tested this hypothesis by comparing the abundance of human osteogenic precursor cells in bone marrow derived from the iliac crest, the vertebral body, and the decorticated intervertebral body space. This enabled us to estimate whether or not addition of bone marrow aspirate to graft material at the vertebral interbody fusion site was of interest.
Materials and methods
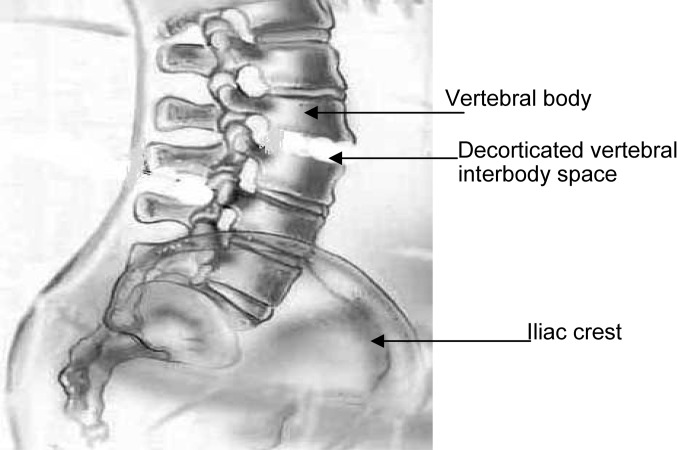
Bone marrow from the iliac crest, the vertebral body, and the decorticated intervertebral body space was collected by aspiration in 12 patients (7 women and 5 men) with scoliosis during anterior spinal surgery for fusion (Fig. 1). Mean patient age was 31 years.
Fig. 1.
Sites of aspiration. Two milliliters of bone marrow was aspirated from three different sites, i.e., the iliac crest, the decorticated vetebral interbody space, and the vertebral body. The richness in osteoprogenitor cells of all three sites was compared.
Aspiration technique
Bone marrow aspiration was performed under anesthesia before the surgery. Harvesting from the posterior iliac crest required that a 2-mm stab incision be made through the skin. The aspiration needle was advanced into the intramedullary cavity. The obturator was removed, and a 10-ml syringe containing 1 ml of heparinized normal saline was attached to the needle. Drawing back the plunger created negative pressure which caused marrow to flow into the syringe. Two milliliters was thus collected.
For the vertebral body samples, bone marrow aspiration was performed using a thoracoscopic approach and required a long aspiration needle. The needle was advanced into the vertebral body and 2 ml of bone marrow was aspirated.
At the interbody fusion site, 2 ml of bone marrow was aspirated from the disc space after decortication and preparation of the site for the grafting process.
Alkaline phosphatase–expressing colony-forming units assay
Alkaline phosphatase is a marker of the early stages of osteoblastic cell differentiation. The number of potential osteoblast progenitors can therefore be estimated by counting alkaline phosphatase–expressing colony-forming units (CFU-AP) [5].
All bone marrow samples were suspended in 5 ml of alpha minimum essential medium (αMEM) (Gibco, Eragny, France). To isolate mononucleated cells, each bone marrow suspension was subjected to two centrifugation steps.
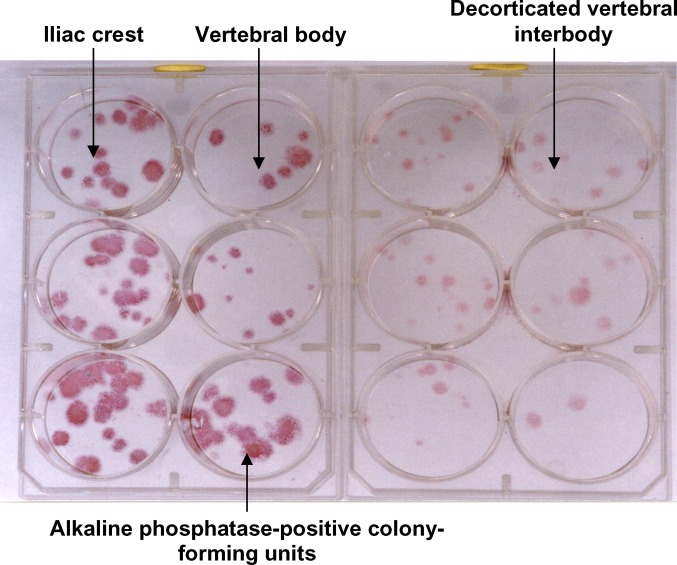
Briefly, samples were first subjected to a density gradient centrifugation. They were layered onto Ficoll-Hypaque solution (D=1.077 g/ml; Seromed, France) and centrifuged at 700 g for 10 min. The buffy coat thus obtained, containing the mononuclear osteoprogenitor cells, was resuspended in 5 ml αMEM and subjected to a second centrifugation step at 160 g for 6 min. Each sample was plated onto a six-well plate at 500×103 cells per well and cultured with αMEM supplemented with 10% fetal calf serum (Dutscher, Brumath, France). Plates were incubated at 37°C in 5% CO2. Medium was changed twice a week. After two weeks, in situ staining was performed to determine alkaline phosphatase activity (Fig. 2). CFU-AP were manually counted in each well, and the mean count for each site of aspiration was determined.
Fig. 2.
Bone marrow samples from the iliac crest, the vertebral body, and the decorticated vertebral interbody (disc space), plated onto six-well dishes at 500×103 mononucleated cells per well and maintained in culture for 2 weeks. The numbers of alkaline phosphatase–expressing colony-forming units in samples from the three different sites were compared.
Results
The number of mononucleated cells and prevalence of CFU-AP in 2-ml aspirates varied significantly from one harvest site to another in each patient (Fig.3). The mean number of mononucleated cells in 2-ml aspirates from the iliac crest ranged from 7 million to 9 million; in the vertebral body, it ranged from 5 million to 7 million. In contrast, the mean number of mononucleated cells in 2-ml aspirates from the decorticated vertebral interbody was around 1 million.
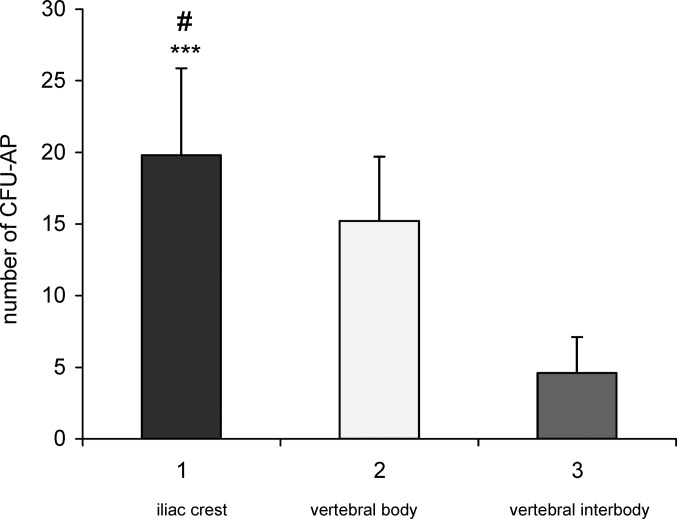
Fig. 3.
Prevalence of alkaline phosphatase–expressing colony-forming units (CFU-AP) as a function of the site of aspiration. Prevalence of CFU-AP in aspirates from the iliac crest, the vertebral body, and the vertebral interbody was compared in 12 patients. The number of osteoprogenitor cells is significantly greater in the iliac crest than in the vertebral body (***P<0.001) and decorticated vertebral interbody (#P<0.002).
The prevalence of CFU-AP was defined as the number of CFU-AP per 500×103 plated mononucleated cells. Only alkaline phosphataseexpressing cell clusters that were 3 mm in diameter or larger were counted. The mean number of CFU-AP per 500×103 mononucleated cells was 19.8 (SD±6.08) in aspirates from iliac crest, 15.4 (SD±4.50) in aspirates from the vertebral body, and 4.2 (SD±2.50) in aspirates from the decorticated vertebral interbody.
Statistically, the osteoprogenitor cells were significantly more abundant in the iliac crest than the vertebral body (P<0.01) and decorticated vertebral interbody (P<0.001).
Discussion
With respect to the biological context, the vertebral interbody appears as a favorable site for arthrodesis, as it is surrounded by spongy bone, which is theoretically rich in osteogenic cells.
We investigated whether addition of bone marrow aspirate to graft materials in vertebral interbody fusion may be of interest to increase the success rate of interbody fusion. We compared abundance of osteoprogenitor bone marrow cells in the iliac crest (used as a reference), the vertebral body, and the decorticated vertebral interbody. We chose the iliac crest as a reference as it is the most commonly used source of bone marrow aspirate. The vertebral body was chosen for comparison as it is easily accessible during surgery and near to the grafting site, i.e., the vertebral interbody.
Aspiration volume was considered a determining parameter in this study. Indeed, Muschler et al. [7] observed that aspiration volume affects the number and concentration of osteoblast progenitor cells in marrow aspirates. Thus, we used the same aspiration volume for all samples. The chosen aspiration volume of 2 ml is clinically relevant as it allows the highest concentration of osteoblast progenitor cells to be collected.
The current study provides an accurate criterion of the richness in osteoblast progenitor cells from three different aspiration sites. In the literature, the richness of the aspirate is most commonly determined by the number of mononucleated cells and prevalence of CFU-AP among mononucleated cells. Because not all mononucleated cells have the capability of differentiating into osteoblasts, we only considered the prevalence of CFU-AP after 2 weeks of culture to estimate the number of osteoblast progenitor cells.
A number of probable reasons may explain why the vertebral interbody site was poorer in osteoprogenitor cells than the iliac crest. First, peripheral blood may have washed osteoprogenitor cells away from the decorticated vertebral interbody site. Moreover, dilution by peripheral blood during the decortication process may have modified cell content of the interbody grafting site.
The richness in osteoprogenitor cells in the iliac crest aspirates was also significantly higher than that of the vertebral body aspirates.
The number of osteoprogenitor cells may increase if local bone marrow is highly cellular and loosely connected, allowing bone marrow cells in the marrow space to flow into the aspiration needle. Moreover, it has been reported [7] that the concentration of bone marrow–derived cells obtained by aspiration depends on the rate of dilution with local blood. Dilution rate may be affected by the cancellous bone density in blood vessels and sinusoids.
Consequently, a high cellularity or a lower number or lower size of blood vessels and sinusoids could account for the higher concentrations of osteoprogenitors observed in iliac crest aspirates compared to vertebral body aspirates.
Conclusion
Osteoprogenitor cells are more abundant in the iliac crest than the vertebral interbody space and vertebral body. The vertebral interbody space, which is theoretically rich in osteoprogenitors, becomes poorer at the time of grafting due to local bleeding. From a clinical point of view, it would therefore seem advisable to perform an iliac crest bone marrow puncture to enhance vertebral interbody fusion.
References
- 1.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development: bone repair and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curylo LJ, Johnstone B, Petersilag CA, Janicki JA, Yoo JU. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit: posterolateral spine fusion model. Spine. 1999;24:434–439. doi: 10.1097/00007632-199903010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Delécrin J, Deschamps C, Romih M, Heymann D, Passuti N. Influence of bone environment on ceramic osteointegration in spinal fusion: comparison of bone-poor sites and bone-rich sites. Eur Spine J. 2001;10:S110–S113. doi: 10.1007/s005860100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einhorn TA. Current concepts review: enhancement of fracture-healing. J Bone Joint Surg. 1995;77A:940–956. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 5.George FM, Hironoti N, Cynthia AB, Kirk AE. Age and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 6.Lukasz JC, Brian J, Cheryl AP, Joseph AJ, Jung UY. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit posterolateral spine fusion model. Spine. 1999;24:434–439. doi: 10.1097/00007632-199903010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Ohgushi H, Goldberg VM, Caplan AI. Hetertopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7:568–578. doi: 10.1002/jor.1100070415. [DOI] [PubMed] [Google Scholar]
- 9.Rickard DJ, Kassem M, Hefferan TE, Sakar G, SelsbergTC, Riggs BL. Isolation and characterisation of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 10.Toquet L, Rohanizadeh R, Guicheux J, Couillaud S, Passuti N, Heymann D. Osteogenic potential in vitro of human bone marrow cells cultured on macroporous biphsic calcium phosphate ceramic. J Biomed Mater Res. 1999;44:98–108. doi: 10.1002/(sici)1097-4636(199901)44:1<98::aid-jbm11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]