Abstract
Low back pain persisting or appearing after a technically successful lumbar fusion challenges clinicians. In this context, the sacroiliac joint could be a possible source of pain, but the frequency of its responsibility is not really known. We used sacroiliac anesthetic blocks, the gold standard for diagnosis, to determine this frequency. Our second goal was to search predictive factors for a positive block. Our prospective series consisted of 40 patients with persistent low back pain after a technically successful fusion who received a sacroiliac anesthetic block under fluoroscopic control. The diagnostic criterion was a relief of more than 75% of the pain on a visual analog scale. We found a 35% rate of positive blocks. The only criterion that characterized these patients was a postoperative pain different from the preoperative pain in its distribution ( p =0.017). A free interval of more than 3 months between surgery and appearance of the pain had an indicative value ( p =0.17). An increased uptake in the sacroiliac on bone scintigraphy or a past history of posterior iliac bone-graft harvesting had no significant value ( p =0.74 and p =1.0, respectively). The sacroiliac joint is a possible source of pain after lumbar fusion. The anesthetic block under fluoroscopic control remains the gold standard.
Keywords: Fusion, Sacroiliac joint, Block, Bone scintigraphy, Donor site pain
Introduction
Lumbar surgical fusion is restricted to patients with severe chronic low back pain. A recent multicentric study showed that this operation brought more relief than a classic conservative treatment [6]. However, despite a careful selection of patients, the failure rate ranges from 10–30% according to the various studies.
Among all the different sources of persistent pain, the responsibility of the sacroiliac joint was evoked by several authors. The first reported cases were of pelvic instability linked to damage to the posterosuperior sacroiliac ligaments, resulting from removal of iliac bone grafts from the posterior iliac crest [3–11]. This complication seems to be very rare [10]. Frymoyer et al. have evoked the possibility of an accelerated degeneration of the sacroiliac joint due to the lumbar fusion. This hypothesis was not confirmed when they found that the incidence of the degenerative change in the sacroiliac joint and sacroiliac flexion-extension range of motion were similar in fused and unfused patients, and were unrelated to the complaint of pain. Most recently, attention was focused on the frequency of the donor site pain, concerning up to 30% of patients for Heary et al. [9]. Not only could this syndrome be misdiagnosed as a sacroiliac syndrome because of the pain distribution, but, according to a recent study, the pain could arise from the sacroiliac joint itself [4]. Actually, the iliac-crest graft harvesting could potentially initiate a violation to the synovial part of the sacroiliac joint [19]. The prevalence of such an inner table disruption would be high in patients with sacroiliac pain after posterior iliac bone-graft harvesting [4]. It has been evoked that this disruption could lead to painful, severe degenerative changes in the joint. However, none of these studies used sacroiliac anesthetic blocks under fluoroscopy.
Blocks are considered the gold standard for the diagnosis of sacroiliac syndrome. Simple blocks [16] or double blocks [13] can be used. Only blocks allow a correct appreciation of the frequency of sacroiliac syndrome in a population of patients suffering from persistent low back pain after lumbar fusion. Simple sacroiliac blocks were already used in a small series of ten cases of persistent pain after lumbar fusion [12]. Four blocks were positive, confirming that the sacroiliac joint could be the source of residual pain. But the real frequency of this syndrome is not known yet. The primary goal of our study was to evaluate the frequency of sacroiliac syndrome in a large series of fusion patients with persistent postsurgical low back pain, with the use of anesthetic blocks under fluoroscopy. The second goal was to search for predictive factors for a positive block. The studied factors were:
Results of sacroiliac bone scintigraphy
Presence or absence of iliac crest bone-graft harvesting
Presence or absence of an interval free of pain after fusion
The character of post-fusion pain, identical or not to the preoperative pain in terms of distribution
Material and methods
Patients
This prospective study started in 1996. Sixty-one patients treated by lumbar fusion came for consulting in our medical center between 1996 and 2002, for persistent back pain after surgery; 45 had pain meeting the following criteria: unilateral (or with unilateral prevalence) persistent pain for more than 6 months; compatible distribution with a sacroiliac origin [5]; not radiating below the knee; tenderness of the sacroiliac sulcus at palpation; and no evidence of lumbar cause (in particular, no degeneration of the adjacent disc on MRI, and no pseudarthrosis). Sciatic pain radiating below the knee, a work-related injury, litigation or a psychiatric disease were considered exclusion criteria. In patients who have had a lumbar fusion, increased uptake in the sacroiliac has already been described as related to altered spinal biomechanics [15] or to sacroiliitis [8]. The pain persistence of more than 6 months in our patients made sure that the hyperfixation of the sacroiliac area was not due to a normal process of bone reconstruction after bone graft harvesting.
Various parameters were recorded: side pain; fused segments including L5–S1; presence of a free interval (period of significant pain relief after surgery of at least 3 months, followed by a pain recurrence); character of post-fusion pain, which could be similar to or different from the preoperative pain in terms of distribution; past-history of iliac crest bone-graft harvesting and its side (the postoperative pain could be either on the same side as the bone graft harvesting or on the opposite side).
Investigations
A lumbar and sacroiliac bone scintigraphy with sacroiliac quantification was first performed. The result of sacroiliac uptake was reported according to a side-by-side percentage ratio. The positive criterion was an increased uptake on the painful sacroiliac area, more than 7% compared with the other side [14]. From 1999, bone scintigraphy was replaced by SPECT (single-photon-emission computerized tomography). Shortly afterwards, an anesthetic sacroiliac block was conducted under fluoroscopic control. The block itself was performed with the patient prone on the fluoroscopy table. The skin overlying the presumed symptomatic sacroiliac joint was anesthetized with lidocaine 1%, using a short needle. After carefully avoiding anesthetizing the periarticular ligaments, the joint was entered with a 20-gauge, 50-mm needle at its lower part. One ml of nonionic contrast material was injected to confirm intra-articular placement of the needle, and the joint was then anesthetized with 2 ml of 2% lidocaine (Fig. 1). The pain was measured before the block according to a visual analog scale of 10 cm (“What is your average pain this morning?”), then 15 min following the block, after the patient had taken a 5 min walk in the street, up steps, and then sat down. The block was considered positive when the contrast was injected strictly into the joint and when the pain relief was up to 75%. A positive block confirmed the diagnosis of sacroiliac syndrome.
Fig. 1.
Sacroiliac arthrography performed before the injection of anesthetic
Statistical methods
Contingency tables were analyzed using the Fisher exact tests. A p value<0.05 was considered as significant.
Results
There were five unsuccessful blocks due to the impossibility of a correct contrast injection right into the sacroiliac joint, in spite of what seemed a correct needle position. This left 40 patients included in the study. They were 14 males and 26 females with a mean age of 48±11 years. Their preoperative diagnoses were: low back pain with or without referred pain (27 cases); disc herniation (four cases); L5–S1 spondylolisthesis (three cases); lumbar stenosis (three cases); lumbar scoliosis (two cases); and fracture (one case). The arthrodesis had been performed 3.8±3.7 years before enrollment in the study.
The fusion levels are shown in Fig. 2. There were 36 posterolateral fusions (two with no instrumentation) and four anterolateral fusions.
Fig. 2.
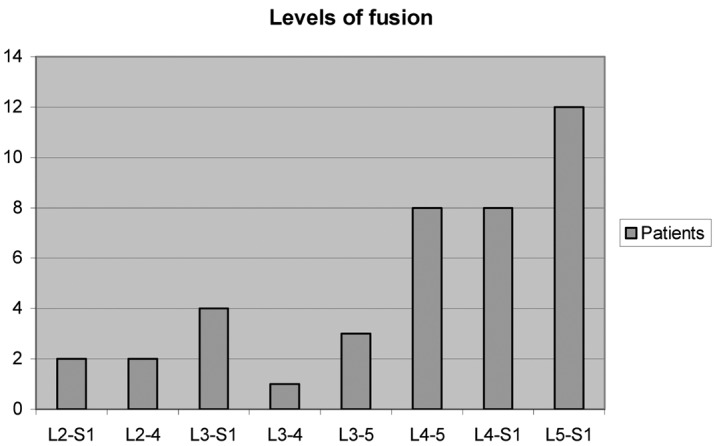
Levels of fusion
Fourteen blocks (35% of the patients, 95% confidence interval (CI): 20.2–49.8%) were positive. Trying to characterize the patients with a positive block, we found that the only significant element was the presence of postoperative low back pain in the sacroiliac area that was different from the preoperative pain (Table 1). An interval free of pain of more than 3 months had only an indicative value (Table 2) and all the other elements were not significant (Tables 3, 4, 5). Among 20 patients who had had a posterior, iliac crest bone-graft harvesting, there were 15 with ipsilateral post-fusion pain and five with contralateral pain. The 15 patients might be labeled as having “donor site pain syndrome.” The five others were considered as having had no iliac crest bone-graft harvesting on the painful side. They were counted with the 17 other patients having had bone graft from other origin (lamina and spinous processes or allografts). No information about bone graft harvesting was available for three other patients. Because only five of these patients had a positive block, such a history does not increase the possibility of a sacroiliac syndrome (Table 1).
Table 1.
Results of the anesthetic block according to the type of post-fusion pain, similar to or different from the preoperative pain
| N =40 | Positive blocks | Negative blocks |
|---|---|---|
| Different pain (15) | 9 (60%) | 6 (40%) |
| Similar pain (25) | 5 (20%) | 20 (80%) |
Fischer’s test: 0.017
Table 2.
Results of the anesthetic block according to the length of the pain-free interval
| N =40 | Positive blocks | Negative blocks |
| Free interval >3 months (16) | 8 (50%) | 8 (50%) |
| Free interval <3 months (24) | 6 (25%) | 18 (75%) |
Fischer’s test: 0.176
Table 3.
Results of the anesthetic block according to the level of fusion
| N =40 | Positive blocks | Negative blocks |
|---|---|---|
| L5–S1 fused (26) | 11 (42%) | 15 (58%) |
| L5–S1 not fused (14) | 3 (21.5%) | 11 (78.5%) |
Fischer’s test: 0.30
Table 4.
Results of the anesthetic block according to whether or not patient had a bone graft harvesting on the posterior iliac crest
| N =40 | Positive blocks | Negative blocks |
| Bone graft harvesting on the same pain side (15) | 5 (33.3%) | 10 (66.6%) |
| No iliac crest bone graft harvesting on the painful side (22) | 8 (36.4%) | 14 (63.6%) |
| Result unknown (3) | 1 | 2 |
Fischer’s test: 1.0
Table 5.
Results of the anesthetic block according to the result of bone scintigraphy
| N =40 | Positive blocks | Negative blocks |
|---|---|---|
| Bone scintigraphy + | 6 (40%) | 9 (60%) |
| Bone scintigraphy − | 8 (32%) | 17 (68%) |
Fischer’s test: 0.736
Discussion
Our study shows that, within a selected population with post-fusion low back pain, the sacroiliac joint is the likely source of pain in 35% of cases. For five patients not included in this study, the injection did not succeed either because of an extremely narrow joint or because of osteophytes covering the joint. The major limit of our results is the lack of an absolute value of the sacroiliac block, even though it is considered the gold standard for sacroiliac syndrome. According to the study of Maigne et al., among 19 low back pain patients (without a surgical history) with a positive sacroiliac screening block, only ten were confirmed with a second positive block, indicating a 47% rate of false-positive diagnoses [13]. Furthermore, pain in patients having sustained lumbar surgery is complex and multifactorial. Despite these reservations, the figure of 35% (95% CI: 20.2–49.8%) indicates that, in this context, the sacroiliac joint is a significant source of low back pain. This result can be compared with the non-surgical series of Schwartzer et al. (30% positive blocks) and Maigne et al. (35% and 18.5% of positive screening and confirmatory blocks, respectively) [13–16] and to the postoperative series of Maigne et al. in which four patients out of ten had a positive block [12]. In this latter study, a block was considered positive with a mere 50% improvement. If we had done a confirmatory block in our study, it is likely that our results would have been in line with the Maigne study [13].
As a consequence, these results drove us to perform sacroiliac injections of long-acting steroids in patients with positive blocks. We can only give an indicative figure of a 40% success rate.
Three causes of the pain can be discussed: a mechanical load transfer on the sacroiliac joint after fusion; a consequence of bone graft harvesting in the iliac crest close to the joint; and the misdiagnosing of a sacroiliac syndrome before fusion (the lumbar spine being fused erroneously). The mechanical load transfer would be due to the straightening of the fused segments. This process is known for originating an increased load transfer on the disc above the fusion [1]. The disc below the fused level is also subjected to new strains [2] related to a translational (shear) motion [17] that may lead to pain. This could also apply to the sacroiliac joint. This was the Frymoyer hypothesis [7], which is hereby confirmed. The fact that, in our study, the block was more often positive in patients with at least a 3-month interval free of pain after fusion is an argument for this hypothesis (i.e., the lumbar source of pain was successfully cured by the fusion, and it took some time before the sacroiliac joint degenerates). We also noted that patients with an L5–S1 fusion had an increased frequency of positive blocks, but this result was statistically non significant.
A history of bone graft harvesting is a potential risk factor of sacroiliac syndrome. Ebraheim et al. studied patients with donor site pain and showed the high frequency of a sacroiliac inner-table disruption resulting in an accelerated degeneration of the joint and in sacroiliac pain. In our study, it is definitely not the only cause of sacroiliac syndrome, which was present with a similar frequency in patients without bone graft harvesting.
The presence of a (misdiagnosed) sacroiliac syndrome as a cause of pre-fusion low back pain, therefore an error in the patient preoperative screening, is a theoretical third possible cause. This testifies to the difficulty in assessing these patients. We presume that it is an infrequent cause, as in our study, the absence of an interval free of pain after fusion (an absence that would be logically expected in such cases) goes against a positive block.
A bone scintigraphy with sacroiliac quantification was included in our protocol because it has a high specificity in the diagnosis of (non-postoperative) sacroiliac syndromes (however, with a very low sensitivity) [12,14–18]. This is why we thought that its use would avoid the need of a second anesthetic (confirmatory) block. Bone scintigraphy is also well known as a useful tool for the diagnosis of some lumbar post-arthrodesis complications. Despite these remarks, the lack of predictive value of this test is a surprising result. By using SPECT in our last 17 patients, we found no additional value regarding the diagnosis of a sacroiliac syndrome. It was not possible to differentiate an increased uptake of the internal part of the iliac crest from the joint itself better than when using a standard bone scintigraphy (except in one case). However, there was much more precision using SPECT for the exploration of the lumbar spine, especially concerning the dorsal arch. Thus, of the 26 patients with a negative sacroiliac block, three had major degenerative disease of the dorsal arch below the fusion level, which was clearly seen when using this technique. In the remaining 23 patients, investigations with other techniques revealed five cases of discogenic pain; in the other 17, no definite cause of the pain could be identified.
Conclusion
Our study confirms that the sacroiliac joint can play a significant role in pain persisting after lumbar fusion. Its role should be particularly evoked when the postoperative pain distribution differs from the preoperative pattern, and when post-fusion low back pain appears after a pain-free interval of at least 3 months after surgery. The anesthetic block under fluoroscopic control remains the gold standard.
References
- 1.Axelsson Spine. 1997;22:414. doi: 10.1097/00007632-199702150-00012. [DOI] [PubMed] [Google Scholar]
- 2.Balderston Spine. 1998;23:54. doi: 10.1097/00007632-199801010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Coventry J Bone Joint Surg Am. 1972;54:83. [PubMed] [Google Scholar]
- 4.Ebraheim Spine. 2000;25:2047. doi: 10.1097/00007632-200008150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Fortin Spine. 1994;19:1483. [PubMed] [Google Scholar]
- 6.Fritzell Spine. 2001;26:2521. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Frymoyer Clin Orthop. 1978;134:196. [PubMed] [Google Scholar]
- 8.Gates Clin Nucl Med. 1999;24:395. doi: 10.1097/00003072-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Heary Neurosurgery. 2002;50:510. doi: 10.1097/00006123-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kostuik JP, Esses SI (1991) Sacral destabilization and restabilization. Causes and treatment. In: Frymoyer JW (ed) The adult spine. Principles and practice. Raven, New York, pp 2159–2178
- 11.Lichtblau J Bone Joint Surg Am. 1962;44:193. [Google Scholar]
- 12.Maigne Rachis. 1995;7:324. [Google Scholar]
- 13.Maigne Spine. 1996;21:1889. doi: 10.1097/00007632-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Maigne Eur Spine J. 1998;7:328. doi: 10.1007/s005860050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onsel Clin Nucl Med. 1992;17:283. doi: 10.1097/00003072-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 16.SchwarzerSpine 199520317709277 [Google Scholar]
- 17.Shono Spine. 1998;23:1550. doi: 10.1097/00007632-199807150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Slipman Spine. 1996;21:2251. doi: 10.1097/00007632-199610010-00013. [DOI] [PubMed] [Google Scholar]
- 19.Xu Spine. 1996;2:1017. doi: 10.1097/00007632-199605010-00004. [DOI] [PubMed] [Google Scholar]


