Abstract
This case report presents a 66-year-old woman with multiple vertebral hemangiomas causing spinal cord compression at different levels with a long symptom-free interval between episodes of compression. She presented with back pain and progressive weakness and numbness in her lower limbs for 3 months. Ten years earlier, she had had a symptomatic T4 vertebral hemangioma operated successfully, and had made a full recovery. Magnetic resonance imaging (MRI) of the thoracic and lumbar spine revealed multiple thoracic and lumbar vertebral hemangiomas. Extraosseous extension of a hemangioma at T9 was causing spinal cord compression. Selective embolization was performed preoperatively, and cord decompression was achieved via anterior T9 corpectomy. The patient’s neurological status improved rapidly after surgery. After a course of radiotherapy, she was neurologically intact and could walk independently. One year later, MRI showed complete resolution of the cord edema at T9, and showed regression of the high signal intensity that had been observed at unoperated levels. These findings indicated diminished vascularity and reduced aggression of the tumor.
Keywords: Vertebral hemangioma, Cord compression, Embolization, Corpectomy
Introduction
Hemangiomas are the most common benign tumors of vertebrae, and are usually asymptomatic [2]. In very rare cases, these neoplasms can cause spinal cord compression and result in severe, progressive neurological deficits [1, 2, 6, 13]. It is proposed that very extensive vascularity signals aggressive behavior in vertebral hemangiomas, but the natural history of these lesions remains obscure [2, 4, 5, 10]. Multilevel involvement of vertebral hemangiomas has been documented [2]. Here we report a unique case in which a T4 vertebral hemangioma initially caused neurological deficit, and 10 years later the patient presented with a tumor at T9 with cord compression. This article discusses the details of this case, as well as the natural course of vertebral hemangiomas, their management, and alternative treatment.
Case Report
A 66-year-old woman presented to our hospital with back pain and progressive weakness and numbness in her lower limbs. The problems had started approximately 3 months earlier. Her legs had gradually weakened to the point where she could no longer walk. Ten years before, the patient had presented to another center with the same clinical picture of paraplegia. The details of that diagnostic work-up were unclear, but a review of hospital records revealed that she had been operated for a T4 vertebral hemangioma. Laminectomy had been performed without adjuvant radiotherapy, and the patient had made a full neurological recovery. She had been symptom-free for 10 years.
Physical examination at our center revealed signs of myelopathy, grade 3/5 muscle weakness in both lower limbs, and sensory loss above the umbilicus. The deep tendon reflexes were hyperactive, with bilateral extensor plantar responses and clonus at the ankles. The patient’s history included chronic constipation, but rectal examination showed normal tone and intact sensory innervation of the perineum and anus.
Plain radiographs of the thoracolumbar spine showed minimal degenerative changes and vertical striations in multiple vertebral bodies characteristic of hemangioma. Magnetic resonance imaging (MRI) of the thoracic and lumbar spine in axial and sagittal planes was performed immediately. These images revealed multiple thoracic (T4, T7, T9, T12) (Fig. 1a) and lumbar (L1, L5) vertebral hemangiomas. A tumor in the T9 vertebral body was observed extending into the spinal canal and causing marked cord compression (Fig. 1b). The cord at T9 level exhibited high signal intensity, indicating edema (Fig. 1a).
Fig. 1.
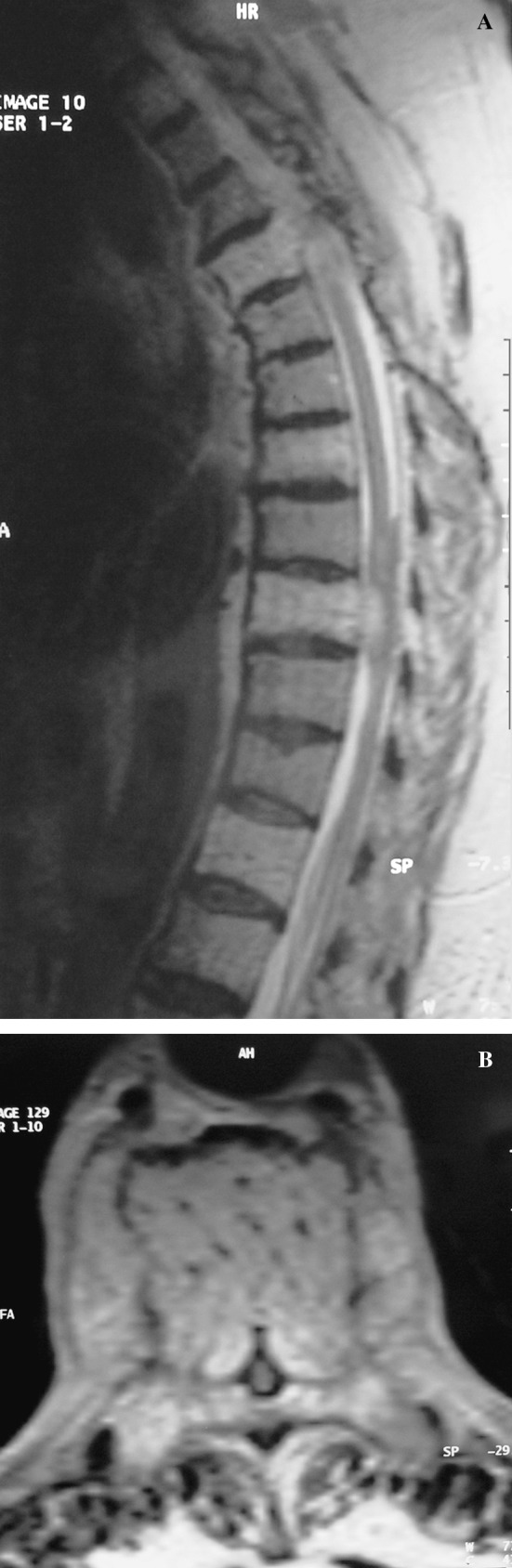
a Sagittal T2-weighted magnetic resonance image shows multiple thoracic (T4,T7,T9,T12) vertebral hemangiomas. Note the high signal intensity in the medulla of the spinal cord at T9 level, indicating edema. b An axial T2-weighted magnetic resonance image shows an expansile lesion extending into the spinal canal at T9 and causing marked cord compression
The patient was screened for a primary malignancy and metastasis using chest X-ray and serum–urine protein electrophoresis. No such lesions were found, but thoracic CT confirmed the presence of hemangiomas, with typical appearance of these lesions at the above-mentioned thoracic levels. Based on these findings, the presumptive diagnosis was multiple vertebral hemangiomas and extraosseous extension of a tumor at T9.
Preoperative angiography was performed under local anesthesia, and this showed the Adamkiewich artery originating from the left 10th intercostal artery. Both intercostal arteries at T9 level were selectively catheterized, and injection of contrast showed intense, diffuse opacification of the T9 vertebra (Fig. 2). Both these intercostal arteries were embolized with polyvinyl alcohol particles (500–700 μm) (Boston Scientific International Cedex, France) and n-butyl cyanoacrylate (Braun, Tuttlingen). This effectively embolized the T9 tumor to minimize intraoperative bleeding. The patient was then operated, and T9 anterior corpectomy and decompression were performed via a left-side thoracotomy with resection of the 8th rib. A titanium mesh cage filled with bone from the resected rib was placed in the corpectomy defect, and fixation with a rod and two screws (one in T8 and one in T10) was carried out. There was minimal bleeding during the corpectomy procedure, and no transfusions were needed intra- or postoperatively. The patient was mobilized in the early-postoperative period with a thoraco-lumbo-sacral orthesis, and she used this device for 3 months. Biopsy specimens obtained during corpectomy confirmed the diagnosis of hemangioma.
Fig. 2.
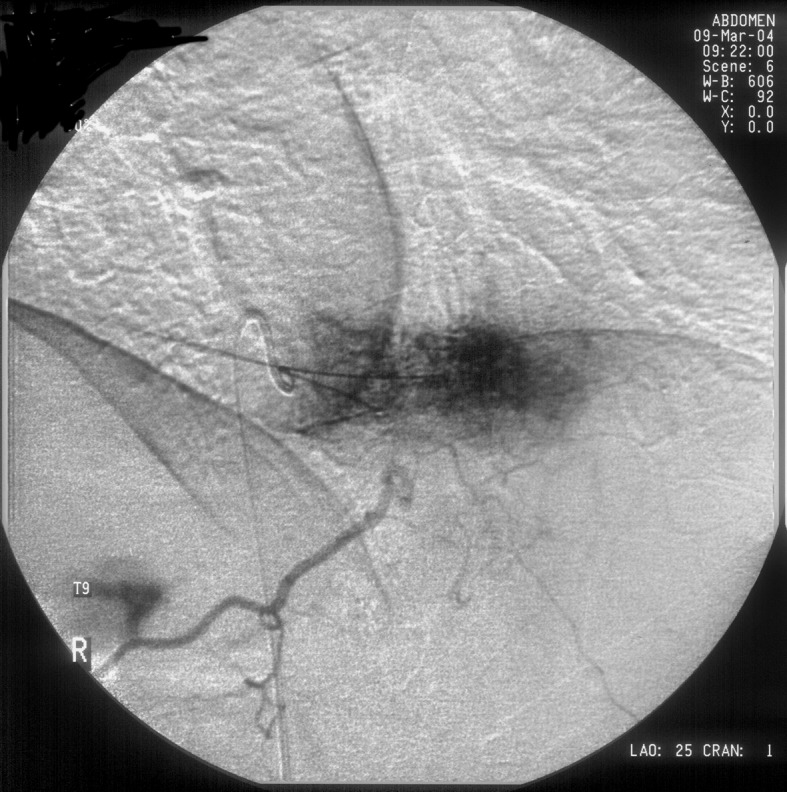
The right intercostal artery at T9 level was catheterized, and injection of contrast revealed intense, diffuse opacification of the T9 vertebra. After both intercostal artery was embolized, repeat selective angiography showed no hypervascular stain
The patient’s neurological signs and symptoms improved rapidly. Adjuvant radiotherapy was prescribed as the T9 mass was only subtotally resected during corpectomy and also for prevention of cord compression by the remaining hemangiomatous vertebrae. The total dose received was 40 Gy in 20 fractions (5 fractions per week). The patient tolerated radiotherapy well and experienced no complications. After radiotherapy was completed, a clinical check showed that the patient was neurologically intact and was able to walk independently without pain. One year later, control MRI revealed complete resolution of the signal changes previously observed in the spinal cord at T9 (Fig. 3), and showed that the high signal intensity detected at unoperated tumorous vertebrae had regressed. These findings indicated diminished vascularity and reduced aggression of the tumor (6).
Fig. 3.
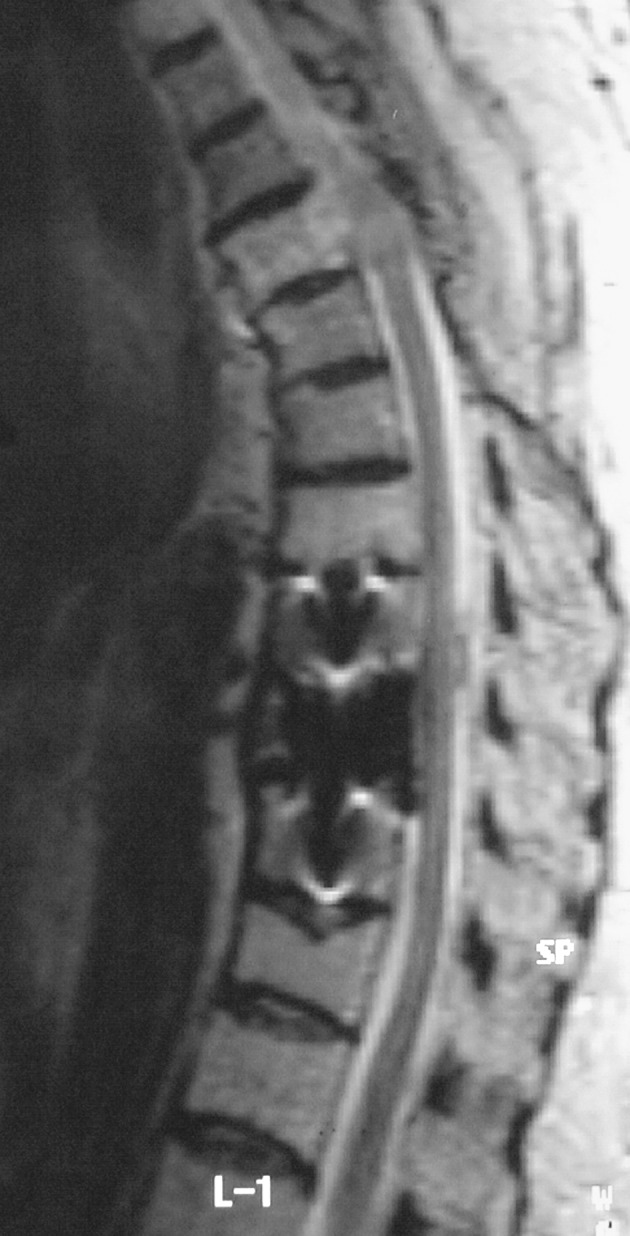
One year later, sagittal fat-supressed T2-weighted magnetic resonance imaging revealed complete resolution of the signal changes previously detected in the spinal cord at level of T9
Discussion
Little is known about the natural progress of vertebral hemangiomas. These neoplasms have a wide variety of clinical presentations, ranging from incidental inactive lesions, to symptomatic lesions with moderately aggressive behavior, to rare, active lesions that compress the spinal cord or nerve root and require aggressive treatment [2, 4, 5, 10]. Fox and Onofrio studied the natural history of 59 cases of vertebral hemangioma from the Mayo Clinic, and found that new-onset back pain followed by subacute progression of thoracic myelopathy was the most common presentation in patients with neurological deficit [2]. They recommended annual neurological and radiological examinations for cases of vertebral hemangioma associated with pain, and stated this was particularly important for young females. Four different pathophysiological mechanisms have been documented for spinal cord compression by vertebral hemangiomas [1]. Distortion of the spinal canal due to tumoral enlargement of the vertebral body is the most common, and this was responsible for the clinical picture in our case. Tumor extension into the epidural space, compression fracture, and bleeding from the mass into the epidural space are the other three mechanisms [1, 2, 7, 12].
Most vertebral hemangiomas that cause spinal cord compression are located in the thoracic spine [4]. Multilevel involvement has been reported, but the cases in the literature to date have featured spinal cord compression at one level only [2]. To our knowledge, ours is the first case of multilevel involvement in which two noncontiguous tumorous vertebrae have caused cord compression at different times.
Vertebral hemangiomas are relatively easy to diagnose. Vertical trabeculations on the vertebral body on plain radiographs (known as “jailhouse appearance”), and polka-dot appearance of the vertebral body on CT are both pathognomonic for this tumor. Magnetic resonance imaging is the technique of choice for evaluating patients with neurological symptoms suspected to be caused by hemangioma. In addition to routine scanning, determining the fat content of the hemangioma can be of prognostic value. An isointense signal on T1-weighted images and a hyperintense signal on T2-weighted images are associated with hemangiomas of low fat content and very extensive vascularity. Both these features are signs of aggressive tumor behavior and higher potential for spinal cord compression [5]. In one of the largest series of vertebral hemangiomas in the literature, Pastushyn and colleagues analyzed vertebral hemangiomas in 86 patients with a 10-year follow up period. Outcomes were assessed according to type of vertebral hemangioma, and the authors concluded that capillary-type hemangiomas are associated with better outcomes (less aggression and better neurological recovery) than cavernous-type hemangiomas after treatment [10].
Endovascular embolization can be performed preoperatively to minimize bleeding from a vertebral hemangioma during surgery, and to reduce the risk of postoperative epidural hematoma [2, 8]. Embolization alone can also be used as the sole therapy for vertebral hemangioma causing spinal cord compression. This approach can even eliminate the neurological deficit, thus reducing the need for an operation [2]. In our case, we performed presurgical embolization via both 9th intercostal arteries, and this resulted in minimal peroperative bleeding and there was no need for blood transfusion.
Painful spinal hemangioma can be treated by vertebroplasty or selective embolization, but surgical decompression is the most widely accepted therapy for progressive neurological deficit [1, 2, 3, 5, 6, 10, 13]. The specific surgical procedures most frequently used are anterior decompression via corpectomy, or complete excision or posterior laminectomy [1, 2, 10]. The choice of procedure depends on the site of cord compression and the experience of the surgeon in charge [2]. The neurological outcome after surgery for vertebral hemangioma is usually excellent [2, 10, 13]. In two cases reported by Castel et al., laminectomy was performed after intraspinal bleeding from a vertebral hemangioma, and both patients achieved complete neurological recovery [1]. Postoperative radiotherapy can be used as an adjunct to surgery, and is indicated when there is subtotal tumor excision, as in cases treated by laminectomy or corpectomy [2, 6, 13].
In our case, laminectomy was performed for the initial T4 vertebral hemangioma and no radiotherapy was prescribed. After 10 symptom-free years, the patient developed back pain and gradually progressive muscle weakness and numbness. Extraosseous extension of a hemangioma at T9 was causing these symptoms, and this was effectively treated with decompression via anterior corpectomy, and postoperative radiotherapy. Though the importance of neuromonitoring during orthopaedic spinal surgery is well recognized, as this facility was not available in our center, somatosensory or motor evoked potentials were not monitored (9, 11). Complete resolution of the signal changes previously observed in the spinal cord at T9 was observed. Regression of high signal intensity at unoperated tumorous vertebrae indicated diminished vascularity and reduced aggression of the tumor [5].
Conclusion
This case reveals that patients with multiple vertebral hemangiomas in noncontiguous vertebrae who have had an episode of cord compression at one level should be followed closely for progressive cord compression at other levels in future. Preoperative endovascular embolization of these lesions reduces intraoperative bleeding and lowers the risk of postoperative epidural hematoma. Surgical decompression and postoperative radiotherapy constitute effective treatment for vertebral hemangioma causing cord compression, and usually lead to complete neurological recovery.
References
- 1.Castel E, Lazennec JY, Chiras J, et al. Acute spinal cord compression due to intraspinal bleeding from a vertebral hemangioma: two case-reports. Eur Spine J. 1999;8(3):244–248. doi: 10.1007/s005860050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78(1):36–45. doi: 10.3171/jns.1993.78.1.0036. [DOI] [PubMed] [Google Scholar]
- 3.Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 4.Laredo JD, Reizine D, Bard M, et al. Vertebral hemangiomas: Radiologic evaluation. Radiology. 1986;161:183–189. doi: 10.1148/radiology.161.1.3763864. [DOI] [PubMed] [Google Scholar]
- 5.Laredo JD, Assouline E, Gelbert F, et al. Vertebral hemangiomas: fat content as a sign of aggressiveness. Radiology. 1990;177(2):467–472. doi: 10.1148/radiology.177.2.2217787. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Hadlow AT. Extraosseous extension of vertebral hemangioma, a rare cause of spinal cord compression. Spine. 1999;15(24(20):2111–2114. doi: 10.1097/00007632-199910150-00009. [DOI] [PubMed] [Google Scholar]
- 7.McAllister VL, Kendall BE, Bull JW. Symptomatic vertebral haemangiomas. Brain. 1975;98(1):71–80. doi: 10.1093/brain/98.1.71. [DOI] [PubMed] [Google Scholar]
- 8.Ng VW, Clifton A, Moore AJ. Preoperative endovascular embolisation of a vertebral haemangioma. J Bone Joint Surg Br. 1997;79(5):808–11. doi: 10.1302/0301-620x.79b5.7710. [DOI] [PubMed] [Google Scholar]
- 9.Niimi Y, Sala F, Deletis V, Setton A, Camargo AB, Berenstein A. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR. 2004;25(7):1131–1138. [PMC free article] [PubMed] [Google Scholar]
- 10.Pastushyn AI, Slin’ko EI, Mirzoyeva GM. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50(6):535–547. doi: 10.1016/s0090-3019(98)00007-x. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113(7):1082–1091. doi: 10.1016/s1388-2457(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 12.Schnyder P, Fankhauser H, Mansouri B. Computed tomography in spinal hemangioma with cord compression. Report of two cases. Skeletal Radiol. 1986;15(5):372–375. doi: 10.1007/BF00348865. [DOI] [PubMed] [Google Scholar]
- 13.Yazici M, Iyigun OL, Gulman B, Rakunt C, Cizmeli O. Vertebral hemangioma presenting with intermittent claudication. Eur Spine J. 1996;5(2):131–133. doi: 10.1007/BF00298394. [DOI] [PubMed] [Google Scholar]


