Abstract
The unilateral transforaminal approach for lumbar interbody fusion as an alternative to the anterior (ALIF) and traditional posterior lumbar interbody fusion (PLIF) combined with pedicle screw instrumentation is gaining in popularity. At present, a prospective study using a standardized tool for outcome measurement after the transforaminal lumber interbody fusion (TLIF) with a follow-up of at least 3 years is not available in the current literature, although there have been reports on specific complications and cost efficiency. Therefore, a study of TLIF was undertaken. Fifty-two consecutive patients with a minimum follow-up of 3 years were included, with the mean follow-up being 46 months (36–64). The indications were 22 isthmic spondylolistheses and 30 degenerative disorders of the lumbar spine. Thirty-nine cases were one-level, 11 cases were two-level, and two cases were three-level fusions. The pain and disability status was prospectively evaluated by the Oswestry disability index (ODI) and a visual analog scale (VAS). The status of bony fusion was evaluated by an independent radiologist using anterior–posterior and lateral radiographs. The operation time averaged 173 min for one-level and 238 min for multiple-level fusions. Average blood loss was 485 ml for one-level and 560 ml for multiple-level fusions. There were four serious complications registered: a deep infection, a persistent radiculopathy, a symptomatic contralateral disc herniation and a pseudarthrosis with loosening of the implants. Overall, the pain relief in the VAS and the reduction of the ODI was significant (P<0.05) at follow-up. The fusion rate was 89%. At the latest follow-up, significant differences of the ODI were neither found between isthmic spondylolistheses and degenerative diseases, nor between one- and multiple-level fusions. In conclusion, the TLIF technique has comparable results to other interbody fusions, such as the PLIF and ALIF techniques. The potential advantages of the TLIF technique include avoidance of the anterior approach and reduction of the approach related posterior trauma to the spinal canal.
Keywords: Transforaminal lumbar interbody fusion (TLIF), Low back pain, Spinal fusion, Lumbar fusion, Interbody fusion
Introduction
In 2001, the Swedish Lumbar Spine Study Group showed for the first time in a prospective and randomized study that lumbar fusion as a treatment of disabling low-back pain was significantly more effective than a conservative treatment regime [11]. The number of lumbar fusions is continuously increasing in industrial countries [33]. Various surgical techniques regarding approach, instrumentation and graft materials are being discussed [13]. Interbody arthrodesis of the lumbar spine with metallic or carbon-fiber cages filled with bone has proven to be an effective treatment of low-back pain [17, 18, 20, 29]. In combination with pedicle screw instrumentation it provides immediate structural support and a high fusion rate [13]. Furthermore, the resection of the disc as a potential pain source is crucial to eliminate discogenic back pain [2, 37].
Both the posterior (PLIF) and the anterior (ALIF) approaches for lumbar interbody fusion have been reported to be associated with specific problems. ALIF procedures require a trans- or retroperitoneal approach to the spine. This is associated with the risk of retrograde ejaculation [7, 35], injury of large vessels [1] and a longer rehabilitation when performed in two stages [12, 39]. PLIF procedures are limited to the segments L3–S1 because of the risk of spinal cord damage during necessary retraction maneuvers. Postoperative arachnopathy, peridural fibrosis and high rates of epidural blood loss are being reported [15, 28]. Furthermore, complete laminectomy may lead to instability of the upper adjacent level and it makes a posteromedial grafting impossible. Mayer [25] described the less invasive Mini-ALIF approach to the lumbar spine, which requires the use of special retraction devices. In a retrospective study the endoscopic ALIF and the Mini-ALIF were found to be comparable [16].
The unilateral transforaminal lumbar interbody fusion (TLIF) represents an alternative surgical technique avoiding both the anterior approach and the approach through the spinal canal. Although a first detailed description of the TLIF technique was given by Harms and Jeszenszky [14] as early as 1998 the number of published papers concerning its outcome and complications is limited. On the other hand a current increase in the number of papers reporting on the TLIF reflects its gaining popularity [4]. The authors started with the TLIF technique in 1997 using a specially designed titanium cage. Similar to the previously performed PLIF and ALIF the indications for the TLIF included isthmic and degenerative spondylolistheses, discogenic pain syndromes and postdiscectomy syndromes irresponsive to conservative treatment. Aim of the presented study was to evaluate whether the unilateral TLIF with one cage is comparable with established techniques regarding outcome, fusion rate and complications.
Methods
Fifty-two of 54 consecutive patients with an average follow-up of 46 months (36–64) were included into the study. Two patients were lost to follow-up. All patients were operated on by two surgeons in our department. Relevant findings concerning the case history and the current intensity and distribution of pain were prospectively evaluated by clinical examination and an interview. All patients had a preoperative magnetic resonance tomography. Additionally a diagnostic by injections of local anesthesia in order to establish the sources of pain was made use of. In nine patients a discography with distension test was needed to identify a discogenic pain as the source of low back pain and in 15 patients to clarify a suspicion of beginning degeneration of discs adjoined to the planed fusion. In 7 of these 15 patients the discography was followed by a fusion. All patients with degenerative disorders were seen by a specialized psychologist to exclude primary psychosomatic pain sources. The patients were asked to give a ratio between their back and leg pain. All patients completed the Oswestry disability index (ODI) [9, 21, 27] and a visual analog scale (VAS) (0 as no pain to 10 as maximal pain) after a brief instruction at least 24 h before the operation and returned routinely for clinical and radiographic evaluation 6, 12, 24, 36, 48 and 60 months after surgery. For radiographic examination anterior–posterior and lateral radiographs of the lumbar spine were performed. Dynamic radiographs were not done since they do not provide any substantial information concerning fusion in comparison to fibrous nonunion [30, 38]. The latest radiographs were evaluated by an independent musculoskeletal radiologist who was blinded to the clinical outcome of the patients. Criteria for bony fusion were anterior and posterior bony bridging, bony continuity between the upper and lower endplates, trabecular structure in the anterior graft and the lack of radiolucent lines around the anterior graft. He read the anterior–posterior and lateral radiographs of the lumbar spine at three times (kappa index 0.92, 0.89, 0.94) within 2 weeks and rated the results as “fused (3)” (three criteria positive), “probably fused (2)” (two criteria positive), “probably not fused (1)” (one criterion positive) and “pseudarthrosis with loosening of the implants (0)” (evidence of radiolucent lines). The average values of the three readings were calculated and used for further analysis. The fusion status was correlated with the results of the ODI and VAS. Intraoperative blood loss and operation time as well as complications were registered. A P value of 0.05 and a correlation quotient of R>0.5 were considered to be statistically significant.
The male to female ratio of the patients was 29–23. The age at the time of surgery averaged 48.6 years (19–69). Thirty-nine patients received a one-level fusion, 11 patients a two-level fusion and two patients a three-level fusion. In 43 cases L5/S1 was included into the fusion.
Twenty-two patients had a isthmic spondylolisthesis grade I or II and 30 patients suffered from degenerative disorders of the lumbar spine. Of the latter patients eight had undergone previous open discectomy and one patient had been treated by a percutaneous facet joint denervation. Preoperatively, 38 of the 52 patients suffered from both back and leg pain. On average, the patients reported a ratio of back pain and leg pain of 68–32%. Thirteen of the patients with leg pain had radicular pain or neurological symptoms and received additionally to the fusion a decompression of the affected neurological structures. Fourteen patients had back pain without any leg pain. In all patients the symptoms were more than 12 months irresponsive to conservative therapy.
All patients were ambulated on the first postoperative day, usually without any external support. In 14 cases, however, a lumbar corset was prescribed for 6 months due to a reduced bone quality. This decision was individually made by the surgeon depending on the fixation strength of the pedicle screws.
Surgical technique
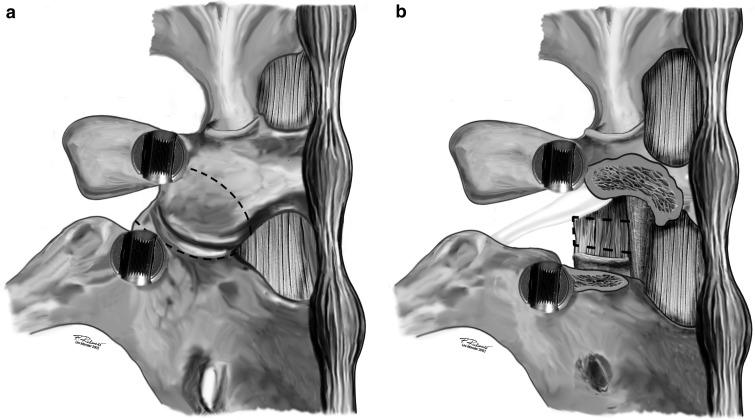
In general anesthesia the patient is placed in a prone position. The posterior elements of the spine are exposed to the bases of the transverse processes. After pedicle screw insertion the superior and inferior articular processes of one facet joint are resected (Fig. 1a) and the disc is exposed in the neuroforamen (Fig. 1b). Care should be taken to coagulate the epidural veins running superior to the pedicle into the neuroforamen before incising the disc. The disc is subtotally resected using rongeurs, shavers and curettes. While proceeding with the disc removal the disc space is progressively distracted via the contralaterally inserted rod. After scraping of the endplates the anterior part of the disc space is packed with autologous bone chips taken from the iliac crest through the same skin incision. A curved cage specially designed for the TLIF technique (De Puy AG, Switzerland) (Fig. 2a) is filled with bone chips and inserted into the posterior or central part of the disc space. The shape of the cage and the 40° angle of the introducer (Fig. 2b) enable a controlled cage positioning. Finally, both rods are mounted under slight compression. The remaining posterior elements are decorticated and bone ships are placed to achieve a posteromedial spondylodesis. If a decompression of the spinal canal is required this is done before resecting the disc. In cases of unilateral nerve root compression the resection of the facet joint is done on this side. In cases of isthmic spondylolisthesis with bilateral nerve root compression and mobile lamina a complete laminectomy is performed to allow sufficient nerve root decompression and to use the lamina as bone graft. In these cases a posterior grafting is not performed (Fig. 3a–c). Details of the TLIF technique were previously published by Harms and Jeszenszky [14] in 1998, Humphreys and co-workers [15] in 2001, Lowe et al. [22] in 2002 and Moskowitz [26] in 2002; however, all these authors used two mesh cages per segment.
Fig. 1.
a In order to approach the neuroforamen and the posterolateral part of the disc it is necessary to remove the facet joint. The osteotomies are marked by the broken lines. b The disc can be removed from the gap between the nerve root of the upper segment and the dura after bipolar coagulation of epidural veins. The nerve root should not be retracted to minimize the risk of postoperative radicular pain
Fig. 2.
a The specially designed curved cage for the transforaminal interbody fusion (micomed Ortho, Switzerland) is available in different sizes and has a lordotic profile of 5°. b The introducer is connected in a 40° angle to enable cage positioning using the transforaminal approach to the disc space
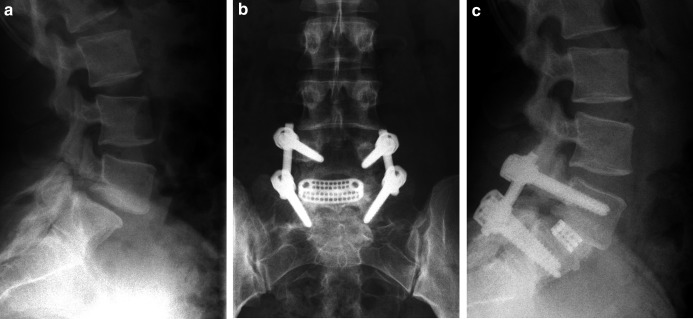
Fig. 3.
a Lateral preoperative radiograph of a patient with an isthmic spondylolisthesis L5/S1 grade I. b Anterior–posterior and c lateral radiographs 2 years after pedicle screw instrumentation and TLIF L5/S1. Please note the bony fusion in the anterior part of the disc space
Results
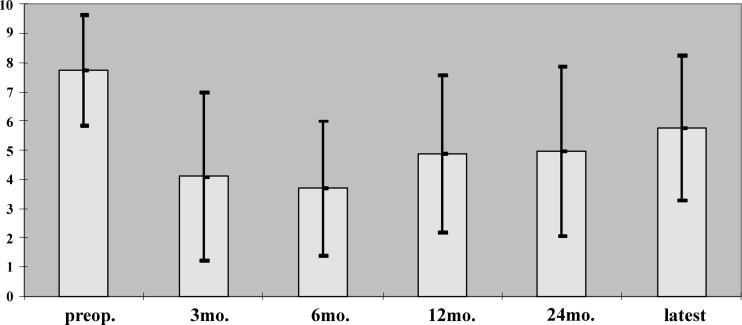
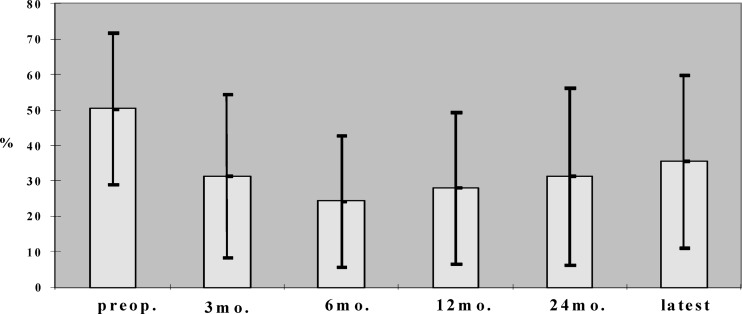
Overall postoperative pain relief measured by the VAS was significant both after 6 months (P<0.001) and at the latest follow-up (P<0.05) despite a gradual but not significant pain deterioration during the follow-up period (Fig. 4). The postoperative ODI showed a significant reduction of disability during the whole period of follow-up (P<0.001). A significant deterioration of disability was measured only between the 24-month follow-up and the latest follow-up (P<0.05) (Fig. 5).
Fig. 4.
Results on pain evaluation by VAS between 0 (no pain) and 10 (maximal pain), preoperatively (preop.) and during the follow-up period (latest follow-up between 36 and 64 months (mo. months postoperatively). All postoperative values are significantly lower compared with preoperative (P<0.05)
Fig. 5.
Results on functional outcome measurement by ODI (%) preoperatively (preop.) and during follow-up (latest latest follow-up between 36 and 64 months (mo. months postoperatively). All postoperative values are significantly lower compared with preoperative (P<0.05)
The 22 mainly younger patients operated on for isthmic spondylolisthesis were compared with the group of 30 patients with degenerative disorders. In the spondylolisthesis group (P<0.001) and the degenerative group (P<0.05) the improvement of ODI was significant at the time of latest follow-up. Preoperatively the ODI of patients with degenerative disorders was significantly higher (P<0.05). No significant differences were found between the groups at any time of the postoperative evaluation (Table 1).
Table 1.
Pre- and postoperative mean ODI and VAS values of 22 patients with isthmic spondylolisthesis versus 30 patients with degenerative diseases; standard deviations in parentheses
| Preoperative | 6 months | 12 months | 24 months | Latest | |
|---|---|---|---|---|---|
| ODI spondylolisthesis (%) | 41.6 (21.7) | 17.9 (14.2) | 20.3 (20.2) | 23.6 (19.8) | 31.6 (24.9) |
| VAS spondylolisthesis | 7.6 (2.3) | 3.4 (2.4) | 3.2 (1.8) | 3.3 (2.3) | 4.1 (2.2) |
| ODI degenerative (%) | 58.4 (18.4) | 31.5 (20.5) | 37.5 (19.3) | 37 (24.8) | 39 (21) |
| VAS degenerative | 8.3 (2.6) | 4.4 (2.2) | 4.5 (2.3) | 4.8 (2.8) | 5.8 (2.5) |
The comparison of the ODI of one-level and multiple-level fusions showed a significant postoperative improvement of disability in both groups (P<0.05), although the group of 13 patients with multiple-level fusions was small. No significant differences were found between the groups at any time of the evaluation (Table 2).
Table 2.
Pre- and postoperative mean ODI and VAS values of 39 patients with one-level fusions versus 13 patients with multiple-level fusions; standard deviations in parentheses
| Preoperative | 6 months | 12 months | 24 months | Latest | |
|---|---|---|---|---|---|
| ODI one-level (%) | 48.5 (22.8) | 20.3 (16.6) | 26.2 (21.2) | 30.3 (25.2) | 33.3 (24.2) |
| VAS one-level | 7.8 (2.6) | 3.9 (2.3) | 3.6 (2.1) | 4.3 (2.3) | 5.3 (2.5) |
| ODI multiple-level (%) | 55.1 (18.2) | 33.1 (19.8) | 33.7 (22.5) | 31.8 (25.6) | 40 (18.7) |
| VAS multiple-level | 8.2 (2.4) | 4.0 (2.4) | 3.8 (2.0) | 4.8 (2.2) | 5.5 (2.7) |
In the group of nine previously operated patients a statistically significant improvement of ODI could not be found at the latest follow-up. However, these results should be interpreted with caution due to the small number of patients in this subgroup. In previously not operated patients the improvement of disability was significant during the whole period of follow-up (P<0.05).
The blood loss of the one-level fusions averaged 485 ml (220–860) and that of the multiple level fusions 560 ml (430–1140). The operation time for one-level fusions averaged 173 min (135–220) and for multiple-level fusions 238 min (190–255).
The radiographic evaluation showed an overall fusion rate in 89% of the patients. Three patients were rated as probably not fused and one patient had a pedicle screw loosening in S1. The correlation of the status of fusion with both the ODI and the VAS at the latest follow-up was not significant (R=0.39 and R=0.34).
Four serious complications (8%) were registered. In one case, a deep infection occurred 2 weeks postoperatively. Removal of the implants, including the cage, settled the infection and 4 weeks later a pedicle screw reinstrumentation and an ALIF procedure was done with an uneventful postoperative course. The second complication was a L5 radiculopathy with pain and dysesthesia which occurred immediately postoperatively on the side of the transforaminal approach after a TLIF L5/S1. Despite early revision, which did not reveal any signs of compression or nerve root injury, the symptoms persisted. The third complication was a symptomatic compression of the contralateral nerve root resulting from herniated disc material which must have been pushed to the contralateral side during the cage insertion. Open sequestrotomy performed two weeks later led to resolution of the symptoms. The fourth complication was an evident pseudarthrosis 3 years after TLIF L4–S1. The patient suffers from rheumatoid arthritis and had an osteoporosis due to corticoid therapy. So far he has rejected the reoperation in spite of persisting pain.
Discussion
The TLIF procedure was pioneered by Harms and Jeszenszky [14]. As early as 1998 they published results on 191 patients operated on between 1993 and 1996. The indications for the procedure were isthmic and degenerative spondylolistheses, de novo scoliosis, spinal stenosis and postdiscectomy syndromes. They reported excellent results in isthmic and degenerative spondylolisthesis. The results in postdiscectomy syndromes and degenerative scolioses were moderate. A standardized questionnaire, however, was not used for patient evaluation. Thirty-eight intra- and postoperative complications were described. The most common were 12 cases of pseudarthrosis with loosening of the implants, nine dura leaks, three cases of damage to a nerve root and four infections. Lowe and co-workers [23] reported on the two-year results of 29 patients with degenerative disc disease and 11 with isthmic spondylolisthesis using the same technique. Twenty-five patients had an excellent outcome, six were rated good, and two had a poor result. However, the authors did not apply a standardized outcome measurement tool. The radiographical interpretation showed a 90% evidence of fusion at the latest follow-up. Only one case of postoperative neurapraxia was registered as a serious complication. Humphreys and co-workers [15] compared 34 PLIF procedures with 40 TLIF procedures in respect of blood loss, operation time and complications. They found no significant differences in terms of these parameters for one-level fusions. However, significantly less blood loss occurred in the TLIF when two-level procedures were compared. No serious complications were registered with the TLIF, whereas PLIF resulted in multiple complications. Whitecloud and co-workers [39] compared the blood loss, operation time and the costs of TLIF and ALIF with additional pedicle screw instrumentation and found blood loss, as well as operation time and the costs, to be lower in the TLIF group.
Results after PLIF and ALIF combined with pedicle screw instrumentation reported by other authors [3, 8, 10, 19, 36] appear to be better than our results; however, a standardized questionnaire comparable to the ODI was not applied in most of the cited studies. To discuss the results of this study it appears essential to discuss studies in which the outcome of lumbar fusion was evaluated by means of the ODI regardless of the applied surgical technique. Mandan and Boeree [24] found a postoperative ODI of 31% after PLIF in 23 cases of grade I and II isthmic spondylolistheses. Schofferman and co-workers [31] reported a reduction of the ODI from 57.5 to 38.2% after circumferential fusions. The improvement of ODI was significant. Tiusanen and co-workers [34] reported a reduction from 48.8% to 30.5% after ALIF in 83 patients with severe low-back pain. Buttermann and co-workers [5] found an improvement of the ODI from 63% to 33% in 35 cases of degenerative disc disease 3 years after lumbar fusion. All these data correspond to the outcome results of this study (Fig. 5).
Fritzell and co-workers [11] measured an improvement of the ODI from 47.3% to 35.7% with a two-year follow-up of 201 lumbar fusions. All patients had chronic low-back pain and were surgically treated using different fusion techniques. They demonstrated a gradual deterioration of the disability during the follow-up period, which is in accordance with our observations. Therefore the authors of this study do not attribute the demonstrated deterioration of the ODI at follow-up to specific disadvantages of the TLIF technique.
Studies dealing with the outcome of lumbar fusions of previously operated patients report a satisfactory outcome in two thirds of the evaluated cases [6, 32]. The subgroup of previously operated patients in this study was too small to allow a relevant conclusion; however, in this group the improvement of the ODI was not significant at the latest follow-up. Therefore the authors recommend careful patient selection.
The fusion rate of 89% in this study is comparable with reports on other fusion techniques combining pedicle screw instrumentation and anterior interbody cages. The reported fusion rates in these studies range between 90 and 100% [3, 6, 8, 10, 17–19, 22, 31, 39] and there was no correlation between the evaluated status of bony fusion and the clinical outcome which is in accordance to the presented study.
Conclusion
The clinical outcome of the TLIF appears to be comparable with that reported in the literature for interbody fusions using the PLIF or ALIF technique. The postoperative improvement of both the ODI and the VAS values were significant at follow-up. The radiographic fusion rate was 89%. The potential advantages of the TLIF technique include avoidance of the anterior approach and reduction of the approach related posterior trauma to the spinal canal.
Key points:
The TLIF requires an unilateral resection of a facet joint but neither laminectomy nor an anterior approach is necessary to enable interbody fusion with cages.
The clinical outcome of TLIF appears to be comparable to other interbody fusion techniques.
Overall, the pain relief in the VAS and the improvement of the ODI were significant (P<0.05) at follow-up.
The overall radiographic fusion rate was 89%. There was one case with a definite pseudarthrosis and loosening of the pedicle screws.
The complication rate, blood loss and operation time were comparable to those of the PLIF and the ALIF techniques.
References
- 1.Baker JK, Reardon PR, Reardon MJ, Heggeness MH. Vascular injury in anterior lumbar spine surgery. Spine. 1993;18:2227–2230. doi: 10.1097/00007632-199311000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Barrick WT, Schoffermann JA, Reynolds JB, Goldthwaite ND, Mc Keehen M, Keanay D, White AH. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25:853–857. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine. 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 4.Brislin B, Vaccaro AR. Advances in posterior lumbar interbody fusion. Orthop Clin North Am. 2002;33:367–374. doi: 10.1016/s0030-5898(01)00013-x. [DOI] [PubMed] [Google Scholar]
- 5.Buttermann G, Garvey T, Hunt A, Transfeld E, Bradford D, Boachie-Adjei O, Ogilvie J. Lumbar fusion results related to diagnosis. Spine. 1998;23:116–127. doi: 10.1097/00007632-199801010-00024. [DOI] [PubMed] [Google Scholar]
- 6.Chitnavis B, Barbagallo G, Selway R, Dardis R, Hussain A, Gullan R. Posterior lumbar interbody fusion for revision disc surgery: review of 50 cases in which carbon fiber cages were implanted. J Neurosurg. 2001;95:190–195. doi: 10.3171/spi.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 7.Christensen FB, Bunger CE. Retrograde ejaculation after retroperitoneal lower lumbar interbody fusion. Int Orthop. 1997;21:176–180. doi: 10.1007/s002640050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enker P, Steffee AD. Interbody fusion and instrumentation. Clin Orthop. 1994;300:90–101. [PubMed] [Google Scholar]
- 9.Fairbanks JE, Couper JC, Davies JB. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 10.Freeman BJ, Licina P, Mehdian SH. Posterior lumbar interbody fusion combined with instrumented postero-lateral fusion: 5-year results in 60 patients. Eur Spine J. 2000;9:42–46. doi: 10.1007/s005860050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritzell Spine. 2001;26:2521. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hacker RJ. Comparison of interbody fusion approaches for disabling low back pain. Spine. 1997;22:660–666. doi: 10.1097/00007632-199703150-00017. [DOI] [PubMed] [Google Scholar]
- 13.Hanley EN, David SM. Current concepts review—lumbar arthrodesis for the treatment of back pain. JBJS. 1999;5:716–730. doi: 10.2106/00004623-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle Fusion in unilateraler transforaminaler Technik. Orthop Traumatol. 1998;10:90–102. doi: 10.1007/s00064-006-0112-7. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine. 2001;26:567–571. doi: 10.1097/00007632-200103010-00023. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser MG, Haid RW, Jr, Subach BR, Miller JS, Smith CD, Rodts GE., Jr Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51:97–103. doi: 10.1097/00006123-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Kozak JA, O‘Brien JP. Simultaneous combined anterior and posterior fusion. An independent analysis of a treatment for the disabled low-back pain patient. Spine. 1990;15:322–328. doi: 10.1097/00007632-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Kuslich S, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, technique, and 2-year follow-up results of a United States prospective, multicenter trail. Spine. 1998;23:1267–1279. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 19.Leufven C, Nordwall A. Management of chronic disabling low back pain with 360 degrees fusion. Results from pain provocation test and concurrent posterior lumbar interbody fusion, posterolateral fusion, and pedicle screw instrumentation in patients with chronic disabling low back pain. Spine. 1999;24:2042–2045. doi: 10.1097/00007632-199910010-00014. [DOI] [PubMed] [Google Scholar]
- 20.Linson MA, Williams H. Anterior and combined anteroposterior fusion for lumbar disc pain. A preliminary study. Spine. 1991;16:143–145. [PubMed] [Google Scholar]
- 21.Little DG, Mac Donald D. The use of the percentage change in Oswestry disability index score as an outcome measure in lumbar spinal surgery. Spine. 1994;19:2139–2143. doi: 10.1097/00007632-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Lowe TG, Tahernia AD, O‘Brien MF, Smith DA. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique and two year results. J Spinal Disord Tech. 2002;15:31–38. doi: 10.1097/00024720-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lowe TG, Tahernia AD. Unilateral transforaminal posterior lumbar interbody fusion. Clin Orthop. 2002;394:64–72. doi: 10.1097/00003086-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mandan S, Boeree NR. Outcome of posterior interbody fusion versus posterolateral fusion for spondylolythic spondylolisthesis. Spine. 2002;27:1536–1542. doi: 10.1097/00007632-200207150-00011. [DOI] [PubMed] [Google Scholar]
- 25.Mayer HM. The ALIF concept. Eur Spine J. 2000;9:35–43. doi: 10.1007/PL00010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz A. Transforaminal lumbar interbody fusion. Orthop Clin North Am. 2002;33:359–366. doi: 10.1016/s0030-5898(01)00008-6. [DOI] [PubMed] [Google Scholar]
- 27.Niskanen RO. The Oswestry low back pain disability questionnaire. A two-year follow-up of spine surgery patients. Scand J Surg. 2002;91:208–211. doi: 10.1177/145749690209100214. [DOI] [PubMed] [Google Scholar]
- 28.Okuyama K, Abe E, Suzuki T, Tamura Y, Chiba M, Sato K. Posterior lumbar interbody fusion: a retrospective study of complications after facet joint excision and pedicle screw fixation in 148 cases. Acta Orthop Scand. 1999;70:329–334. doi: 10.3109/17453679908997819. [DOI] [PubMed] [Google Scholar]
- 29.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine. 1997;22:667–680. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 30.Santos ERG, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interboy fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/00007632-200305150-00007. [DOI] [PubMed] [Google Scholar]
- 31.Schofferman J, Slosar P, Reynolds J, Golthwaite N, Koestler M. A prospective randomized comparison of 270 degrees fusion to 360 degrees fusion (circumferential fusions) Spine. 2001;26:E207–E212. doi: 10.1097/00007632-200105150-00019. [DOI] [PubMed] [Google Scholar]
- 32.Steward G, Sachs BL. Patient outcome after reoperation on the lumbar spine. J Bone Joint Surg Am. 1996;78:706–711. doi: 10.2106/00004623-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low back pain hospitalisation. Recent United States trends and regional variations. Spine. 1994;19:1207–1213. doi: 10.1097/00007632-199405310-00002. [DOI] [PubMed] [Google Scholar]
- 34.Tiusanen H, Seitsalo S, Osterman K, Soini J. Anterior interbody lumbar fusion in severe low back pain. Clin Orthop. 1996;324:153–163. doi: 10.1097/00003086-199603000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Tiusanen H, Seitsalo S, Ostermann K, Soini J. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J. 1995;4:339–342. doi: 10.1007/BF00300293. [DOI] [PubMed] [Google Scholar]
- 36.Wang JM, Kim DJ, Yun YH. Posterior pedicular screw instrumentation and anterior interbody fusion in adult lumbar spondylolisthesis or grade one spondylolisthesis with segmental instability. J Spinal Disord. 1996;9:83–88. [PubMed] [Google Scholar]
- 37.Weatherley CR, Pricked CF, O‘Brien JP. Discogenic pain persisting despite solid posterior fusion. JBJS. 1986;68B:142–143. doi: 10.1302/0301-620X.68B1.2934399. [DOI] [PubMed] [Google Scholar]
- 38.Weiner BK, Fraser RD. Spine update lumbar interbody cages. Spine. 1998;23:634–640. doi: 10.1097/00007632-199803010-00020. [DOI] [PubMed] [Google Scholar]
- 39.Whitecloud TS, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior–posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord. 2001;14:100–102. doi: 10.1097/00002517-200104000-00002. [DOI] [PubMed] [Google Scholar]