Abstract
This biomechanical study was performed to test the primary segmental in vitro stabilising effect of a standard and large footprint radiolucent poly-ether-ether-ketone (PEEK) box cage versus a titanium box cage for anterior lumbar interbody fusion. Eighteen L2-L3 and sixteen L4-L5 cadaveric motion segments were divided into three groups and received a titanium cage or a radiolucent PEEK cage with standard or large footprint. All specimens were tested in three testing conditions: intact, stand-alone anterior cage and finally with supplemental translaminar screw fixation. Full range of motion and neutral zone measurements were determined and anterior cage pull out force was tested. The titanium design was significantly more effective in reducing the range of motion only in axial rotation. The larger footprint radiolucent cage did not increase stability as compared to the standard footprint. The titanium cage pull out force was significantly (P=0.0002) higher compared to both radiolucent cage constructs. Clinical relevance: Supplemental posterior fixation is strongly recommended to increase initial stability of any anterior interbody fusion cage construct. Although the biomechanical stability necessary to achieve spinal fusion is not defined, the radiolucent designs tested in this study, with a standard footprint as well as with a larger footprint, may be insufficiently stabilised with translaminar screws as compared to the titanium implant. Supplemental pedicle screw fixation may be required to obtain adequate stabilisation in the clinical setting.
Keywords: Poly-ether-ether-ketone, Cage, Anterior lumbar interbody fusion, Biomechanical testing, Segmental stability
Introduction
Current methods to achieve anterior lumbar interbody fusion for degenerative disc disease include the use of cages [38]. Cages are spacers filled with bone graft, which maintain spinal alignment and segmental stability while facilitating bony fusion. Titanium cages have become popular [17, 18, 30], but two disadvantages are the unreliable radiological assessment of the fusion mass [22], and the stiffness of the cage construct leading to subsidence into the adjacent vertebrae. Assessment of lumbar spine fusion with instrumentation is difficult in either conventional radiography [1, 14] or computed tomography [11]. Shah [33] recently reported on the need for high-quality thin-slice computed tomography to evaluate interbody fusion through titanium cages. Cages made of radiolucent biomaterials [2, 3, 6] may facilitate fusion assessment in lumbar interbody fusion, although there may be persistent need of computed tomography [32].
Cage subsidence has been reported [15, 16, 21, 23, 38] and this may be related to cage design, density of underlying cancellous bone [27, 28] and surgical technique. The footprint size of a cage and the position related to the vertebral endplate surface are important [27], because the central area is the weakest part of the endplate [9, 12, 31]. From the study by Steffen [36] it is known that a wider implant, supported in the periphery of the endplate, is more effective in providing segmental stability, and also has a higher axial strength to resist implant subsidence compared to a narrower implant. Therefore, a titanium box cage with a large footprint was developed. Clinical experience has been good, but fusion assessment remains a problem [26, 35].
Furthermore, another problem of titanium cages may be the resultant stiffness of the spinal motion segment. Kanayama et al [13] studied the stress shielding effects by cage devices in an in vitro biomechanical study. The material of the cage itself may lead to stress shielding, migration and non-union. The effect of cage stiffness on fusion rate was studied in an in vivo investigation evaluating a poly-L-lactic acid cage versus a titanium cage of similar design [8, 34]. Increased interbody fusion was observed with the less stiff poly-L-lactic acid cages as compared with titanium cages after 6 months.
Considering the radiological problems with fusion assessment, and the possible advantages of cage material less stiff than titanium, a radiolucent poly-ether-ether-ketone (PEEK) cage, of similar design as the titanium box cage, with standard and larger footprint was developed (Fig. 1). The aim of this study was to determine, if this particular box shaped radiolucent PEEK cage would render similar biomechanical results when compared to a clinically proven design of a titanium implant with similar geometry. Of concern was the primary segmental stability of the radiolucent cage designs compared to the titanium design and the anchorage [7] of the teeth in the endplates, because the radiolucent design teeth are somewhat less sharp compared to the titanium implant.
Fig. 1.
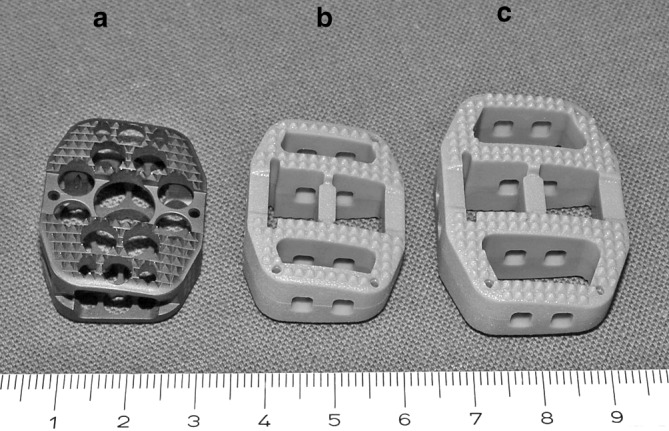
The anterior lumbar interbody fusion implants (Synthes, Oberdorf, Switzerland) used in the study: a SynCage titanium b SynCage LR standard footprint c SynCage LR large footprint
Materials and methods
Eighteen (14 male and 4 female) human lumbar spine donor specimens, average age 60.8 years (range 33–86), harvested from routine autopsies were used for this study. Antero-posterior (AP) and lateral plain radiographs were used to exclude specimens with severe disc degeneration associated with complete disc collapse, bony anomalies or severe osteoporosis. A total of eighteen L2–3 and sixteen L4–5 levels were included in the study. Measurements for AP and lateral disc width, as well as central disc height were obtained from radiographs. A 10% magnification error was corrected for. All spines underwent bone mineral density (BMD) measurements in a lateral dual-energy radiograph absorptiometry (DPX-L, Lunar Radiation Corporation, Madison, WI). AP DEXA was not used as it gives a higher value influenced by the posterior spinal elements. The average BMD for L2 and L3, or L4 and L5 was calculated and used for stratification of the motion segments into three experimental groups with a similar BMD distribution and segmental level distribution. Specimens with a larger diameter were preferably assigned to the experimental group used for the wider footprint PEEK cages similar to the clinical setting.
Three experimental implant (Synthes, Oberdorf, Switzerland) groups were to be instrumented with either a SynCage titanium (n=11), or a Syncage Lumbar Radiolucent (LR) (n=23) made of PEEK (a linear and semi-cristalline (35%) thermoplastic polymer). The SynCage LR was available in a standard (24×30 mm) footprint (n=12), identical to the SynCage titanium and a larger (28×38 mm) footprint (n=11) (Fig. 2). The cages of each group were available in different heights (13.5, 15,17 and 19 (mm)) to adapt to each individual specimen. The wedge angle of all cages is 12 ° and this angle remains the same for increasing cage size. It is was not possible to adapt this angle to individual specimens. Both the titanium and the radiolucent design have biconvex surfaces supplied with teeth. The titanium cage teeth are somewhat sharper compared to the PEEK cage teeth due to manufacturing limitations of PEEK.
Fig. 2.
Comparison of SynCage LR standard and large footprints
The specimens were kept in sealed plastic bags at −20° C until the day of testing. Prior to testing the specimens were fully thawed slowly at 4°C for 24 h and once thawed kept moist with saline soaked gauzes. Once thawed the entire testing cyclus was performed. The individual motion segments were prepared by removing excessive soft tissue, but all ligaments were kept intact. The ends were embedded in cylindrical polymethyl-metacrylate (PMMA) blocks of 12.5 cm diameter. Short screws introduced in the distant endplates improved anchoring of the specimens in the PMMA blocks. A rectangular anterior annulotomy was performed centrally just wide enough to allow insertion of the cage. After discectomy and careful endplate preparation to subchondral bone the optimal cage height was determined using trial spacers, and the selected cage was inserted under enough distraction to create adequate tension over the annulus by an experienced spine surgeon (MS). Additional translaminar transfacet screws (TLS) were introduced as described by Magerl [20].
The relative AP and lateral endplate diameter occupied by the cage compared to the entire endplate dimensions was expressed in normalized AP and lateral disc space diameter. Normalized AP and lateral disc space diameters were calculated for each specimen in each group. The overall specimen height was measured using a standard platform at each step of the preparation (intact, stand-alone cage, cage with TLS) at five standardized positions (left, right, front, back, center) on the top surface of the cranial PMMA block, while the caudal block was resting on a flat surface. Distraction height at the center of the endplate and change in sagittal angulation of the segment were then calculated for the stand-alone cage and cage with TLS instrumentation relative to the intact segment’s parameters.
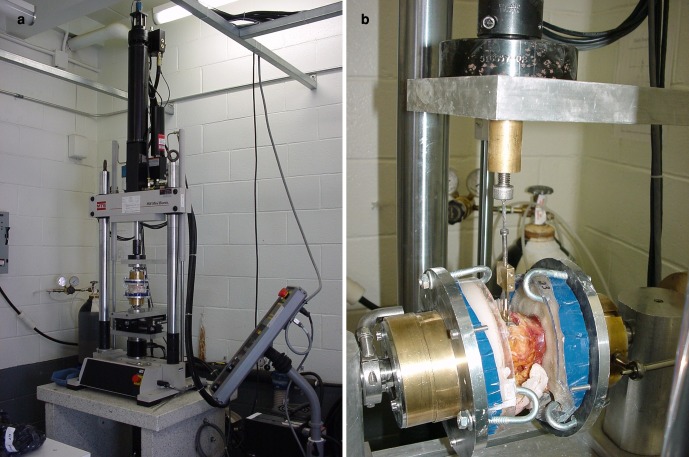
All specimens were tested sequentially in a biaxial MTS 858 Mini Bionix Test System (MTS Systems, Eden Prairie, MN) in three testing conditions. First intact, then with stand-alone anterior cage, and finally with cage and TLS instrumentation. The system was designed with a custom set-up for unconstrained application of moments. In flexion and extension tests a pneumatic cylinder was used to apply a constant preload (Fig. 3a).
Fig. 3.
a Set-up of the vertebral specimen in the test machine for flexion-extension motion application b Pull-out test set-up
The intact specimen was first preconditioned with 200 N axial load for 10 min. The instrumented specimens underwent five cyclic load cycles from 0 N to 600 N to lock the cage in place. The actual protocol was identical for all testing conditions, applying a total of three complete cycles from −6 Nm to +6 Nm stepwise loading in 2 Nm increments in axial rotation, flexion-extension and lateral bending, while a constant axial preload of 200 N was maintained. The data of only the third cycle were plotted with load versus displacement. Full range of motion (ROM) and neutral zone (NZ) measurements were determined. NZ represents the intervertebral motion segment laxity and is defined as the displacement from the neutral position to the zero-load point of the third loading cycle. The ROM was listed separately for flexion and extension, all other motion directions were listed as full ROM. The mechanical testing was concluded with an anterior cage pull-out test (TLS fixation left in place) under 600 N constant axial load and the actuator pulling at a 1 mm/s rate on the implant. All specimens were completely fixed in the testing machine (Fig. 3b) during pull-out testing. Maximal recorded pull-out force was used for further analysis.
For both instrumented conditions the relative change in ROM and NZ normalized to the intact condition were calculated for each motion direction. Analysis of variance (all P-values set at 0.05) was used to compare mean values between experimental groups and between instrumentation conditions. For comparing the pull-out force the BMD value was used as a covariable.
Results
There were no significant differences between implant groups for BMD and all segmental parameters studied (Table 1). In the titanium cage group the anterior heights of the inserted cages were 13.5 mm (2), 15 mm (6) and 17 mm (3). The heights of the inserted standard footprint LR cages were 13.5 mm (2), 15 mm (6) and 17 mm (4). The large footprint LR cages inserted had heights of 13.5 mm (1), 15 mm (8) and 17 mm (2).
Table 1.
Mean values and standard deviations of BMD, normalized disc space AP- and lateral diameter, segmental height and lordosis angle for each cage group. No significant differences
| Titanium (n=11) | LR standard (n=12) | LR large (n=11) | |
|---|---|---|---|
| Lateral BMD (g/cm2) | 0.80±0.19 | 0.82±0.18 | 0.71±0.14 |
| Normalized AP diameter ratio | 0.67±0.06 | 0.63±0.05 | 0.67±0.06 |
| Normalized lat diameter ratio | 0.62±0.07 | 0.60±0.07 | 0.65±0.05 |
| Distraction height (mm) | 2.9±1.2 | 2.8±1.2 | 2.5±1.6 |
| Lordosis angle (degrees) | 3.3±2.3 | 2.7±2.4 | 3.2±3.0 |
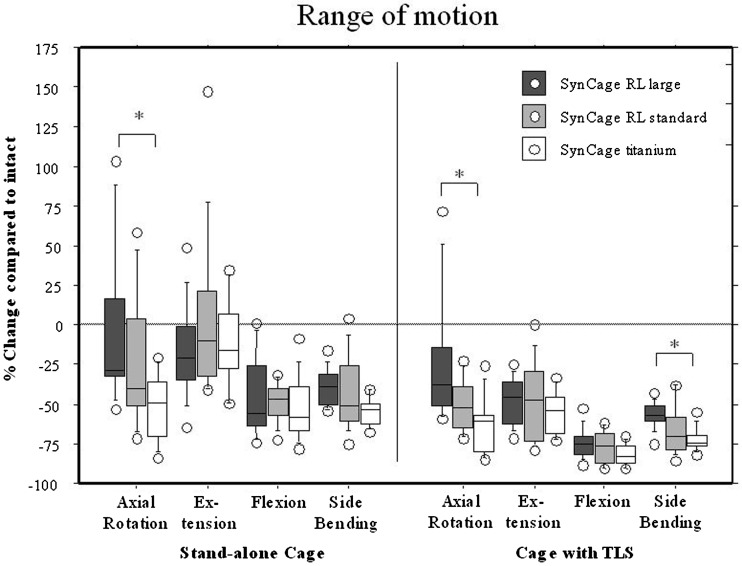
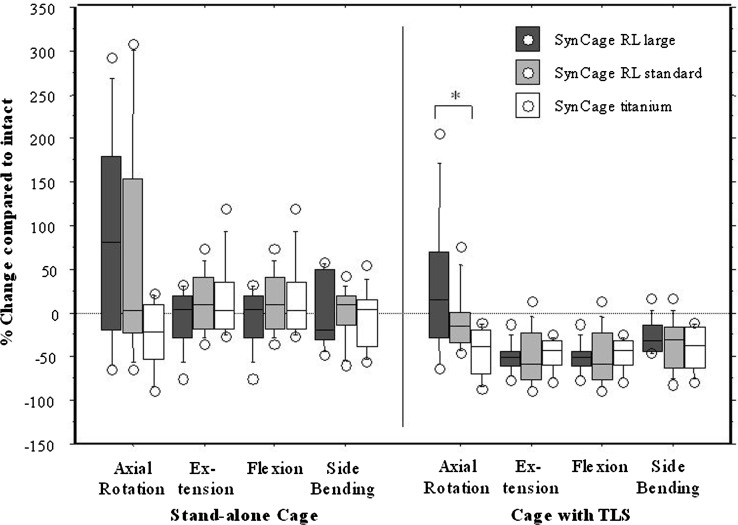
The changes in ROM and NZ for each group in each motion direction and for each instrumentation condition are shown in box-stem plots (Figs. 4, 5). The variation in reduction of ROM among the specimens with the radiolucent cages is generally greater than that of the titanium cage. No stand-alone cage was able to reduce the segment movement for extension and there was a significant difference between the reduction in ROM during axial rotation between the radiolucent large footprint cage and the titanium cage. All stand-alone cages were clearly effective in flexion and side bending. While the augmentation with TLS fixation further reduced the ROM in all motion directions, there remained a significant difference between the large footprint radiolucent cage and the titanium cage during axial rotation. All stand-alone cages failed to reduce the NZ during motion in any direction. Only after augmentation with TLS was the NZ reduced, and again there was a significant difference between the large footprint radiolucent cage and the standard titanium cage during axial rotation.
Fig. 4.
Box-stem plot comparing the three cages for ranges of motion in each loading direction and instrumented testing condition
Fig. 5.
Box-stem plot showing neutral zones for all implant groups and instrumented testing conditions
The anterior cage pull-out force was 693±157 N for the titanium cage, 498±139 N for the radiolucent standard footprint cage and 433±94 N for the radiolucent large footprint cage. The BMD did not have a significant influence on the pull-out force. The titanium cage pull-out force was significantly higher (P=0.0002) compared to both radiolucent cages.
Discussion
In this in vitro study the initial implant stability for a standard titanium box cage versus a radiolucent PEEK box cage of two footprint sizes with otherwise similar design features to the standard titanium cage was determined. The results indicate that the titanium design is generally more effective in stabilising the vertebral motion segment than the radiolucent designs. The sharpness of the teeth of the titanium cage is a matter of interest, which is illustrated by the significantly higher pull-out force necessary to release the titanium cage out of the construct with translaminar screws. The titanium cage is firmly anchored in the vertebral endplates, because of the sharpness of the teeth. The teeth of the radiolucent designs are less sharp due to manufacturing limitations and supplemental translaminar screw fixation does not result in segmental stability comparable with the titanium reference cage construct.
Anterior interbody stand-alone fusion constructs with the Bagby and Kuslich (BAK) cages (Sulzer Spine-Tech, Minneapolis, MN) have been reported to have satisfactory 4 year clinical results [17]. Worse 3 to 6 year results of this particular stand-alone construct have been presented [5] and Nibu [24] advised to avoid extension motions after stand-alone BAK constructs. Experimental data [10, 13, 19, 25, 29, 37] suggest that stand-alone cage constructs are not stable enough to create a safe environment for fusion to occur. The critical issue here is the stand-alone concept, not the cage itself. This is particularly true for motion in axial rotation and extension, as we again demonstrated in the current study. Additional posterior fixation with TLS or pedicle screws will solve this problem and increases segmental in vitro stability [10, 13, 25, 29].
In this study the different radiolucent cage material might be responsible for the relatively less effective adjuvant stability of the supplemental posterior TLS instrumentation. PEEK was chosen, because it is radiolucent, biocompatible, [4] avoids the adverse reactions of carbon fiber and may improve fusion rate because of its reduced stiffness compared to titanium[8, 34]. Although in the current study the PEEK design has similar features to the titanium cage such as a wedge shape, convex surfaces and teeth, the PEEK material cage clearly provides a less stable construct, even with translaminar screws. The larger footprint, designed and meant to provide more effective segmental stability surprisingly does not succeed in doing this, as the design was not as effective in decreasing ROM compared to the small footprint and certainly not compared to the titanium cage design. This may be because the larger footprint makes a wider annulotomy necessary, which in fact may decrease the preload caused by distraction and reduce stabilizing potential in axial rotation and extension. The PEEK cage teeth are less sharp than the titanium cage teeth, which may also account for the difference in stabilising potential.
The in vitro stabilizing effect of the PEEK cages tested in this study is less compared to the titanium cage of similar design even with translaminar screws. Although not tested in this study, and although the stability necessary to provide a solid spinal fusion is still not defined, pedicle screws may be required to create a more stable environment. Obviously, pedicle screws would be less attractive than translaminar screws as one encounters the need for more extensive muscle stripping, a longer operative procedure, a longer recovery period and higher costs. Anterior lumbar interbody fusion allows for better preparation of fusion surfaces, and can be performed with no muscle stripping of the muscles of the spine. If pedicle screw instrumentation is required to create a sufficiently stable situation, as may be the case with this radiolucent PEEK cage, then the advantages of anterior lumbar interbody fusion over other posterior procedures are lost.
Conclusion
Anterior interbody fusion constructs using box-shaped cages have an inherent weakness to provide stability in extension and axial rotation. Addition of TLS fixation is highly effective to increase any construct’s initial stability, and therefore is strongly recommended.
The radiolucent PEEK cages used in this study appear to provide a lower primary fixation and stability when compared to titanium cages of equal dimensions. This observation was most obvious in axial rotation, and for the overall cage stability as determined by an anterior implant pull-out test. The potential benefits of radiolucency and less stiffness of PEEK compared to titanium as a material for cage manufacturing are counteracted by the risk of failed fusion as a result of reduced initial segmental stability. In this study the use of a larger footprint did not have a beneficial effect on the constructs initial stability. Although the biomechanical stability necessary to provide solid spinal fusion is not defined, perhaps these PEEK cages should be used only with pedicular screw fixation, which is expected to provide better overall stability than TLS fixation.
Acknowledgements
The study was financially supported by Mathys Medical Ltd, Bettlach, Switzerland. The experiments comply with the current laws of Canada, in which country the experiments were performed.
References
- 1.Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients. Spine. 1993;18:1186–1189. doi: 10.1097/00007632-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion: two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2117. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 3.Brantigan JW, Steffee AD, Lewis ML, Quinn ML, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system. Two-year results from a food and drug administration investigational device exemption clinical trial. Spine. 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 4.Brantigan JW, McAfee PC, Cunningham BW, Wang H, Orbegoso CM. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone: an investigational study in the Spanish goat. Spine. 1994;19:1436–1444. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Button G, Gupta M, Barrett C, Barrett C, Cammack P, Benson D (2003) 3 to 6 year follow-up of stand-alone BAK cages. (Poster presentation). Scoliosis Research Society 38th annual meeting Quebec City, Canada
- 6.Christensen FB, Hansen ES, Eiskjaer SP, Høy K, Helmig P, Neumann P, Niedermann B, Bünger CE. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation. A prospective, randomized clinical study of 146 patients. Spine. 2002;27:2674–2683. doi: 10.1097/00007632-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Dietl RH, Krammer M, Kettler A, Wilke HJ, Claes L, Lumenta CB. Pull out test with three lumbar interbody fusion cages. Spine. 2002;27:1029–1036. doi: 10.1097/00007632-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dijk van M, Smit TH, Sugihara S, Burger EH, Wuisman PIJM. The effect of cage stiffness on the rate of lumbar interbody fusion. An in vivo model using poly (L-Lactic Acid) and titanium cages. Spine. 2002;27:682–688. doi: 10.1097/00007632-200204010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Edwards WT, Zheng Y, Ferrara LA, Yuan HA. Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine. 2001;26:218–225. doi: 10.1097/00007632-200101150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Fidler MW. Spinal fusion: a combined anterior and supplementary interspinous technique. Eur Spine J. 1997;6:214–218. doi: 10.1007/BF01301441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert TJ, Heithoff KB, Mullin WJ. Radiographic assessment of cage-assisted interbody fusions in the lumbar spine. Seminars in Spine Surgery. 2001;13:311–315. [Google Scholar]
- 12.Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine. 2001;26:889–896. doi: 10.1097/00007632-200104150-00012. [DOI] [PubMed] [Google Scholar]
- 13.Kanayama M, Cunningham BW, Haggerty CJ, Abumi K, Kaneda K, McAfee PC. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg. 2000;93(suppl 2):259–265. doi: 10.3171/spi.2000.93.2.0259. [DOI] [PubMed] [Google Scholar]
- 14.Kant AP, Daum WJ, Dean M, Uchida T. Evaluation of lumbar spine fusion: plain radiographs versus direct surgical exploration and observation. Spine. 1995;20:2313–2317. [PubMed] [Google Scholar]
- 15.Kozak JA, Heilman AE, O‘Brien JP. Anterior lumbar fusion options: technique and graft materials. Clin Orthop. 1994;300:45–51. [PubMed] [Google Scholar]
- 16.Krammer M, Dietl R, Lumenta CB, Kettler A, Wilke HJ, Buttner A, Claes L. Resistance of the lumbar spine against axial compression forces after implantation of three different posterior lumbar interbody cages. Acta Neurochir (Wien) 2001;143:1217–1222. doi: 10.1007/s007010100017. [DOI] [PubMed] [Google Scholar]
- 17.Kuslich SD, Danielson G, Dowdle JD, Sherman J, Frederickson B, Yuan H, Griffith SL. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine. 2000;25:2656–2662. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 18.Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques and 2-year follow-up results of a United States prospective multicenter trial. Spine. 1998;23:1267–1278. doi: 10.1097/00007632-199806010-00019. [DOI] [PubMed] [Google Scholar]
- 19.Lund T, Oxland TR, Jost B, Cripton P, Grassmann S, Etter C, Nolte LP. Interbody cage stabilisation in the lumbar spine. Biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80:351–359. doi: 10.1302/0301-620X.80B2.7693. [DOI] [PubMed] [Google Scholar]
- 20.Magerl F. Stabilization techniques: thoracolumbar spine. In: Aebi M, Thalgott JS, Webb JK, editors. AO ASIF principles in spine surgery. BerlinHeidelberg, New york: Springer; 1998. pp. 101–102. [Google Scholar]
- 21.McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 22.McAfee PC, Boden SD, Brantigan JW, Fraser RD, Kuslich SD, Oxland TR, Panjabi MM, Ray CD, Zdeblick TA. Symposium: a critical discrepancy: a criteria of successful arthrodesis following interbody spinal fusions. Spine. 2001;26:320–334. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 23.McAfee PC, Cunningham BW, Lee GA, Orbegoso CM, Haggerty CJ, Fedder IL, Griffith SL. Revision strategies for salvaging or improving failed cylindrical cages. Spine. 1999;24:2147–2153. doi: 10.1097/00007632-199910150-00015. [DOI] [PubMed] [Google Scholar]
- 24.Nibu K, Panjabi MM, Oxland T, Cholewicki J. Multidirectional stabilizing potential of BAK interbody spinal fusion system for anterior surgery. J Spinal Disord. 1997;10:357–362. [PubMed] [Google Scholar]
- 25.Oxland TR, Hoffer Z, Nydegger T, Rathonyi GC, Nolte LP. A comparative biomechanical investigation of anterior lumbar interbody cages: central and bilateral approaches. J Bone Joint Surg Am. 2000;82:383–393. doi: 10.1302/0301-620X.82B3.9887. [DOI] [PubMed] [Google Scholar]
- 26.Pavlov PW, Meijers H, Limbeek van J, Jacobs WCH, Lemmens JAM, Obradov M, Kleuver de M. Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine. 2004;29:1893–1900. doi: 10.1097/01.brs.0000137067.68630.70. [DOI] [PubMed] [Google Scholar]
- 27.Polikeit A, Ferguson SJ, Nolte LP, Orr TE. Factors influencing stresses in the lumbar spine after the insertion of intervertebral cages: finite element analysis. Eur Spine J. 2003;12:413–420. doi: 10.1007/s00586-002-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polikeit A, Freguson SJ, Nolte LP, Orr TE. The importance of the endplate for interbody cages in the lumbar spine. Eur Spine J. 2003;12:556–561. doi: 10.1007/s00586-003-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathonyi GC, Oxland TR, Gerich U, Grassmann S, Nolte LP. The role of supplemental translaminar screws in anterior lumbar interbody fixation: a biomechanical study. Eur Spine J. 1998;7:400–407. doi: 10.1007/s005860050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine. 1997;22:667–679. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 31.Roberts S, McCall I, Menage J, Haddaway M, Eisenstein S. Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc. Eur Spine J. 1997;6:385–389. doi: 10.1007/BF01834064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/00007632-200305150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Shah RR, Mohammed S, Saifuddin A, Taylor BA. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12:378–385. doi: 10.1007/s00586-002-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smit TH, Müller R, Dijk van M, Wuisman PIJM. Changes in bone architecture during spinal fusion: three years follow-up and the role of cage stiffness. Spine. 2003;28:1802–1809. doi: 10.1097/01.BRS.0000083285.09184.7A. [DOI] [PubMed] [Google Scholar]
- 35.Spruit M, Pavlov PW, Leitao J, Kleuver M, Anderson PG, Boer den F. Posterior reduction and anterior lumbar interbody fusion in symptomatic low-grade isthmic spondylolisthesis: short-term radiological and functional outcome. Eur Spine J. 2002;11:428–433. doi: 10.1007/s00586-002-0450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine. 2000;25:1077–1084. doi: 10.1097/00007632-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 37.Tsantrizos A, Andreou A, Aebi M, Steffen T. Biomechanical stability of five stand-alone anterior lumbar interbody fusion constructs. Eur Spine J. 2000;9:14–22. doi: 10.1007/s005860050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner BK, Fraser RD. Lumbar interbody cages. Spine. 1998;23:634–640. doi: 10.1097/00007632-199803010-00020. [DOI] [PubMed] [Google Scholar]