Abstract
The literature reports on the safety and efficacy of titanium cages (TCs) with additional posterior fixation for anterior lumbar interbody fusion. However, these papers are limited to prospective cohort studies. The introduction of TCs for spinal fusion has resulted in increased costs, without evidence of superiority over the established practice. There are currently no prospective controlled trials comparing TCs to femoral ring allografts (FRAs) for circumferential fusion in the literature. In this prospective, randomised controlled trial, our objective was to compare the clinical outcome following the use of FRA (current practice) to the use of TC in circumferential lumbar spinal fusion. Full ethical committee approval and institutional research and development departmental approval were obtained. Power calculations estimated a total of 80 patients (40 in each arm) would be required to detect clinically relevant differences in functional outcome. Eighty-three patients were recruited for the study fulfilling strict entry requirements (>6 months chronic discogenic low back pain, failure of conservative treatment, one- or two-level discographically proven discogenic low back pain). The patients completed the Oswestry Disability Index (ODI), Visual Analogue Score (VAS) for back and leg pain and the Short-Form 36 (SF-36) preoperatively and also postoperatively at 6, 12 and 24 months, respectively. The results were available for all the 83 patients with a mean follow-up of 28 months (range 24–75 months). Five patients were excluded on the basis of technical infringements (unable to insert TC in four patients and FRA in one patient due to the narrowing of the disc space). From the remaining 78 patients randomised, 37 received the FRA and 41 received the TC. Posterior stabilisation was achieved with translaminar or pedicle screws. Baseline demographic data (age, sex, smoking history, number of operated levels and preoperative outcome measures) showed no statistical difference between groups (p<0.05) other than for the vitality domain of the SF-36. For patients who received the FRA, mean VAS (back pain) improved by 2.0 points (p<0.01), mean ODI improved by 15 points (p=<0.01) and mean SF-36 scores improved by >11 points in all domains (p<0.03) except that of general health and emotional role. For patients who received the TC, mean VAS improved by 1.1 points (p=0.004), mean ODI improved by 6 points (p=0.01) and SF-36 improved significantly in only two of the eight domains (bodily pain and physical function). Revision procedures and complications were similar in both groups. In conclusion, this prospective, randomised controlled clinical trial shows the use of FRA in circumferential lumbar fusion to be associated with superior clinical outcomes when compared to those observed following the use of TCs. The use of TCs for circumferential lumbar spinal fusion is not justified on the basis of inferior clinical outcome and the tenfold increase in cost.
Keywords: Fusion, Lumbar spine, Randomised controlled trial, Femoral ring allograft, Titanium cage
Introduction
Anterior lumbar interbody fusion has been an established surgical treatment for degenerative low back pain since its first description by Lane and Moore [18]. Simultaneous combined anterior and posterior fusion was first carried out by O’Brien in 1960 [26]. Supplementation with posterior fixation affords greater stability and a more favourable environment for fusion [11, 29, 31], and is now well established in modern practice with excellent clinical and radiological outcomes. Evidence for the intervertebral disc as the pain generator in low back pain is increasing [2, 6, 7, 9, 32], suggesting a need for removal of the disc for successful treatment of discogenic low back pain.
Following the introduction of interbody cages, their use has become widespread [38] with authors citing impressive clinical results in prospective cohort studies [28]. Femoral ring allograft (FRA) used for interbody fusion has also proven to be successful in the surgical goals set for the treatment for degenerative low back pain [11, 13, 19, 33], but there has been criticism of its use [28]. One prospective, randomised controlled trial of anterior lumbar interbody fusion compares a titanium cylindrical threaded fusion device to FRA [35]. In this study, stand-alone titanium cylindrical threaded interbody fusion cages were reported to have a higher fusion rate when compared to a stand-alone anterior FRA; however, improvements in clinical outcome were similar in both groups. The cost of titanium cages (TCs), however, is rarely mentioned in papers written by the proponents of their use, even though, the implant cost of a TC may be tenfold that of an FRA.
We were interested to know, in the setting of a prospective, randomised controlled trial, whether there was an observed difference in clinical outcome following circumferential lumbar fusion with the femoral ring (current practice) (Fig. 1) or the TC (Fig. 2). The null hypothesis, therefore, stated that there was no difference in the clinical outcome between these two methods of circumferential fusion. Also, if there was an observed difference in favour of the TC, was the additional cost of the TC justified?
Fig. 1.
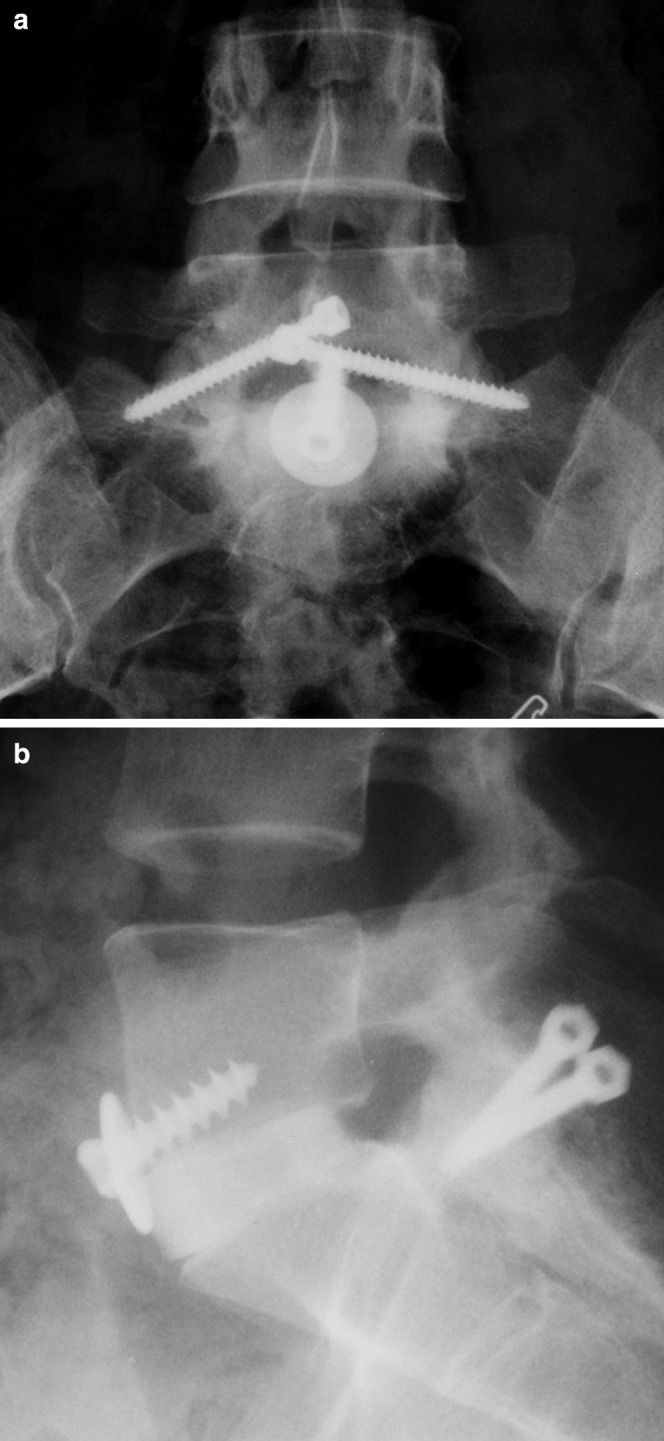
Anterior–posterior radiograph (a) and lateral radiograph (b). Circumferential fusion with femoral ring allograft/translaminar screws at L5/S1
Fig. 2.
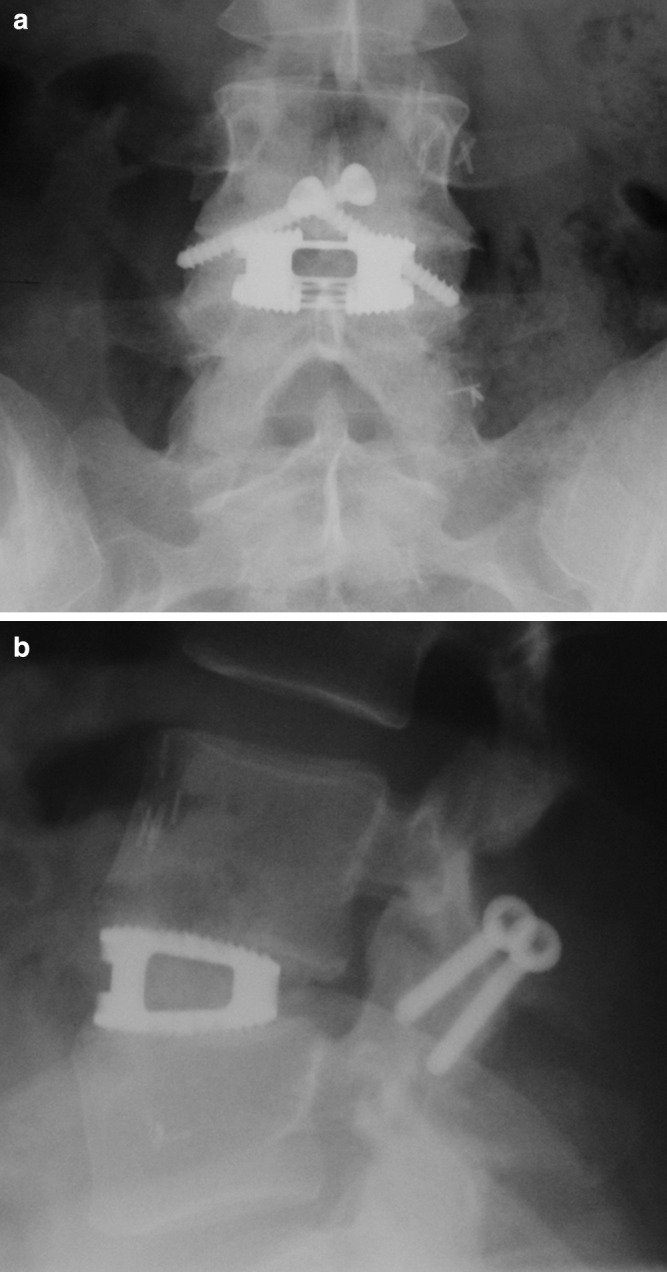
Anterior–posterior radiograph (a) and lateral radiograph (b). Circumferential fusion with titanium cage/translaminar screws at L4/5
Materials and methods
Study design
Local ethical committee and institutional research and development departmental approval were obtained for the study. A single-centre multi-surgeon prospective, randomised controlled trial was conducted. The inclusion criteria (Table 1) were degenerative disc disease between L3 and S1 with a maximum of two consecutive motion segments to be instrumented, pain or functional deficit present preoperatively for a minimum period of 6 months and the patient failing to respond to conservative treatment modalities for at least 3 months. The diagnostic criteria for inclusion required the radiographic evidence of sclerosis, osteophyte formation, degenerative changes of facet joints or greater than 50% collapse of the interspace (as determined by the measurement across the middle of the endplate on the lateral radiograph); 3.5 mm or more movement on flexion/extension radiographs; MRI evidence of dehydration of the lumbar disc with or without reactive sclerosis of the adjacent vertebral body; and discographic evidence of abnormal disc morphology with concordant pain reproduction on provocation. The exclusion criteria (Table 2) listed skeletal immaturity or patients over 70 years, more than two vertebral levels involved, previous spinal fusion, Meyerding grade II or greater spondylolisthesis, active or systemic infection, osteoporosis or the presence of active malignancy. After a full informed consent, randomisation was by sealed envelope with a 1:1 ratio opened just prior to surgery.
Table 1.
Inclusion criteria
| Clinical criteria | Radiographic criteria | MRI criteria | Provocative discography |
|---|---|---|---|
| Degenerative disc disease from L 3 to S1 | Osteophyte formation | Disc dehydration ± reactive changes in vertebral body endplate | Abnormal morphology at target level |
| Maximum of two consecutive motion segments to be fused | Sclerosis | Concordant pain reproduction at target level | |
| Pain/functional deficit present preoperatively for 6 months | Facet joint degeneration | ||
| Failure to respond to conservative measures for 3 months or more | ≥50% loss of intervertebral height | ||
| 3.5 mm movement on flexion extension radiographs |
Table 2.
Exclusion criteria
| Age < 18 years Age > 70 years Previous spinal fusion ≥Grade II spondylolisthesis >Two motion segments to be fused Systemic infection Active malignancy Known osteoporosis |
Implants
The titanium implant used in this study was the SynCage (Synthes, Oberdorf, Switzerland). It is wedge shaped with convex toothed surfaces to match the vertebral body and plates and has a built-in lordosis. The central area of the cage has a web-like structure allowing the autograft to be packed within, offering a large surface area centrally for graft incorporation whilst maintaining structural support peripherally. It is inserted after distraction of the intervertebral space and sizing. The cost of the Syncage is presently approximately £1,200 (€1,724).
The FRA was obtained from the National Blood Service (Edgware, England). It is a cortical femoral ring sterilised with ethylene oxide. Donors are tested for hepatitis B surface antigen and antibodies to hepatitis C, HIV 1 and 2. The FRA is shaped with a high-speed burr at the time of surgery to fit the disc space and incorporate lordosis. The cost of the femoral ring is presently approximately £120 (€172).
Surgical technique
The surgical technique followed was the one that was described by Kumar et al. [17], with the posterior procedure being carried out first. In this study, we did not use the graft harvested from the vertebral body. Two techniques of posterior fixation were performed in the study, namely, translaminar screw fixation or pedicle screw fixation (ClickX, Synthes). Translaminar screws are placed via stab incisions as described by Montesano [24]. Subperiosteal dissection is carried out through a midline posterior approach to expose the facet joints, which are decorticated and packed with bone graft harvested from the posterior iliac crest. Further harvested bone graft is kept sterile, while the patient is turned supine and re-draped. The lumbar spine was exposed anteriorly via a retroperitoneal approach and the visualisation of the disc was aided by the Steinmann pins or Synframe retractors (Synthes). The surgical level was identified using intraoperative radiographs and a complete discectomy was carried out. The vertebral body endplates were prepared by curetting until point bleeding was seen. Trial implants were used for sizing the TC. Measuring calipers and a depth gauge were used to size the FRA. An allograft larger than the measured disc space was chosen and burred down to the correct size. The previously harvested autograft was then packed into the implant before insertion. The FRA was secured with a 6.5 mm large fragment cancellous screw and washer inserted into the superior vertebral body at each fused level to act as a buttress to prevent anterior migration of the graft. This measure was not necessary for the TC due to its design. Three doses of intravenous antibiotics were given, one preoperatively and two further doses postoperatively at 8 and 16 h, respectively. TED stockings were worn for 6 weeks postoperatively, with mobilisation commencing the day after the surgery. We did not use lumbar orthoses.
Outcome measures
The Oswestry Disability Index (ODI) questionnaire, Visual Analogue Score (VAS) for back and leg pain (maximum 10 points) and the Short-Form 36 (SF-36) questionnaire were completed preoperatively and postoperatively at 6, 12 and 24 months, respectively. The minimum clinically important differences for outcome measures were established from previously published data; ODI 10 points, VAS 2 points [10], SF-36 seven points in each domain [27]. Radiographs of the lumbar spine were taken at the same time intervals and are the subject of a separate study.
Power of the study
The number of participants sufficient to detect a clinically relevant difference in functional outcome was calculated using the following formula, as used in previous studies [3]:
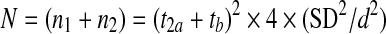 |
where, N is the total number of participants in the two groups, and n1 and n2 are the number of participants in each group. The risk of a Type I error (t2a) was set to 5% (t2a=1.96) and the risk of a Type II error (tb) was set to 20% (tb=0.842). The standard deviation (SD) of the observation, that is the SD of the ODI, was 16 and was derived from more than 100 patients from a previous database. The symbol ‘d’ indicates the clinically relevant difference (10 points) in the ODI.
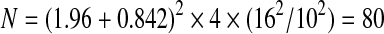 |
This equates to 40 patients in each arm of the trial.
Statistical analysis
Statistical analysis was carried out using SPSS (Version 11). Independent t-test, paired t-test and Pearson chi-squared test were used to establish differences between the groups.
Results
Between February 1998 and October 2002, 83 patients were recruited for the trial: 45 were randomised to receive the TC and 38 to receive the FRA. Entry and exit data were available on all 83 patients with a mean follow-up of 28 months (range 21–75).
Technical infringements
Four female patients requiring two-level fusion and randomised to the SynCage had a disc space that was too narrow to take the smallest size implant at one level; an intraoperative decision was made to insert FRA instead of the SynCage. These four patients, therefore, had one of each implant. One patient was found to have a disc space so narrow that we were unable to insert an FRA; this patient had autologous bone graft chips placed in the disc space. These patients were excluded from analysis, thereby leaving 41 patients in the TC group and 37 in the FRA group.
Patient demographics are set out in Table 3 and showed no significant differences between the groups with regards to sex, smoking history or level of degenerative disc disease. Age at operation, although not statistically significant, was on average 3.6 years less in the femoral ring group. In view of the difference in the age found between the two groups, an analysis of the correlation coefficient for each outcome measure and the age of patient at operation was carried out, but no significant correlation was found.
Table 3.
Patient demographics
| Femoral ring allograft | Titanium cage | p-value | |
|---|---|---|---|
| No. of patients | 37 | 41 | |
| Gender (female/male) | 20/17 | 23/18 | 0.858* |
| Age in years (range) | 39.0 (24–53) | 41.4 (29–65) | 0.210** |
| Surgical levels: | 0.606* | ||
| One level (TLS/Pedicle) | 22 (20/2) | 22 (22/0) | |
| Two levels (TLS/Pedicle) | 15 (13/2) | 19 (13/6) | |
| Previous discectomy/decompression | 4 | 6 | 0.728* |
| Smoking | 10 | 10 | 0.614* |
*Pearson chi-squared
**Independent t-test
TLS translaminar screws, Pedicle screw fixation
Translaminar screws were used in 68 patients (94 levels) and pedicle screws in 10 patients (18 levels).
Clinical outcome
Analysis of preoperative outcome measures showed no statistical difference between the two groups (Table 4) except in the vitality domain of the SF-36, which was higher in the TC group.
Table 4.
Mean preoperative outcome measures (standard deviation)
| Outcome measure | Femoral ring allograft | Titanium cage | p-value |
|---|---|---|---|
| Oswestry Disability Index | 57 (14) | 54 (14) | 0.394 |
| VAS back pain | 7.2 (1.7) | 7.1 (1.9) | 0.846 |
| VAS leg pain | 3.8 (2.7) | 4.3 (3.2) | 0.424 |
| SF-36 domains | |||
| - Physical function | 27 (15) | 34 (19) | 0.086 |
| - Physical role | 13 (27) | 15 (32) | 0.700 |
| - Bodily pain | 22 (14) | 24 (16) | 0.365 |
| - General health | 54 (24) | 54 (22) | 0.991 |
| - Vitality | 27 (18) | 35 (18) | 0.038* |
| - Social function | 35 (22) | 40 (28) | 0.396 |
| - Emotional role | 47 (49) | 41 (47) | 0.608 |
| - Mental health | 50 (19) | 56 (20) | 0.179 |
*Statistically significant
Oswestry Disability Index
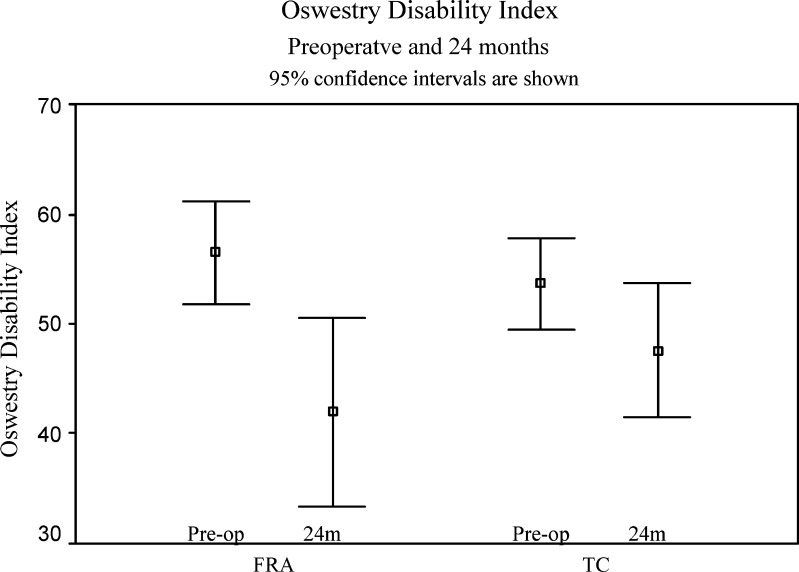
Two years postoperatively both groups had significantly improved their ODI, with a greater improvement seen in the femoral ring group (mean 15 points, SD 20 points) compared to a mean of 6 points (SD 15 points) for the TC group (Table 5 and Fig. 3). Comparing the change in ODI, there is a significantly greater improvement in the FRA group when compared to the TC group (p=0.027). The FRA group reached the mean clinically important difference (MCID) for ODI, whilst the TC group did not.
Table 5.
Mean scores for ODI, VAS back pain and VAS for leg pain
| Preoperative | 6 months | 12 months | 24 months | Change (P-value) | |
|---|---|---|---|---|---|
| Mean ODI | |||||
| FRA | 57 | 44 | 39 | 42 | 15 (0.000) |
| TC | 54 | 46 | 49 | 48 | 6 (0.011) |
| Mean VAS back pain | |||||
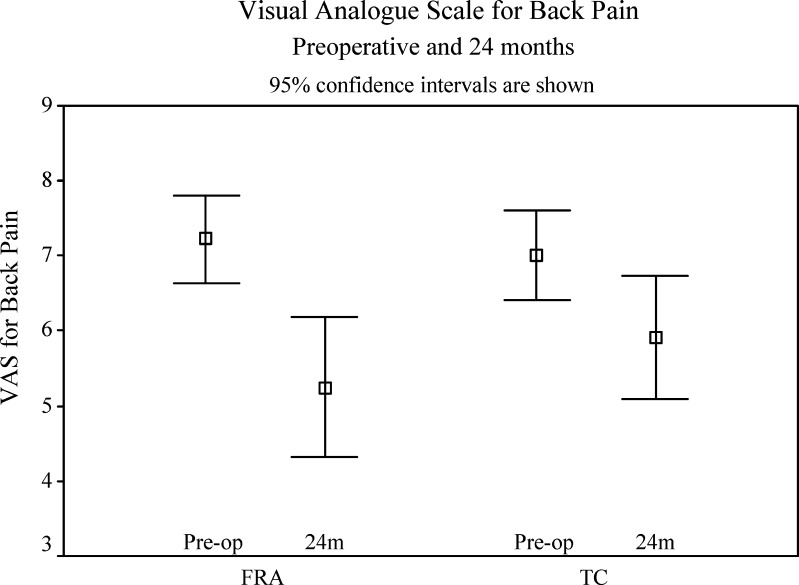
| FRA | 7.2 | 5.0 | 4.8 | 5.2 | 1.9 (0.000) |
| TC | 7.1 | 5.8 | 6.4 | 6.0 | 1.1 (0.004) |
| Mean VAS leg pain | |||||
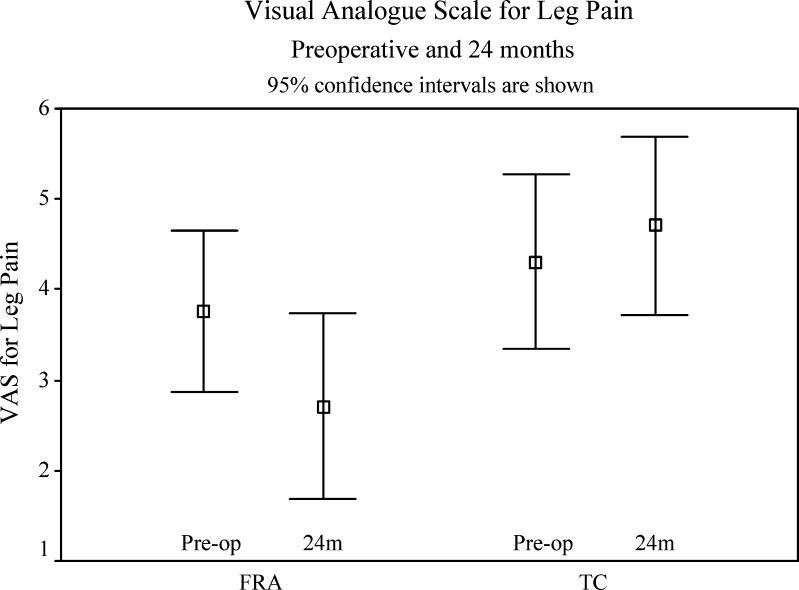
| FRA | 3.8 | 2.3 | 2.8 | 2.5 | 1.3 (0.008) |
| TC | 4.3 | 3.0 | 4.6 | 4.7 | 0.4 (0.498) |
FRA femoral ring allograft, TC titanium cage
Fig. 3.
Oswestry Disability Index preoperatively and at 24 months for femoral ring allograft (FRA) group and titanium cage (TC) group
Visual Analogue Score for back pain
Both groups again showed a significant improvement in mean VAS for back pain with the FRA group improving by 2.0 points (SD 2.8) and the TC group by 1.1 points (SD 2.2) (Table 5 and Fig. 4). Again, the FRA group reached the MCID for ODI, whilst the TC group did not. There was, however, no significant difference in change of VAS for back pain between the two groups (p=0.188).
Fig. 4.
Visual analogue score (back pain) preoperatively and at 24 months for FRA group and TC group
Visual Analogue Score for leg pain
The TC group had worse leg pain on the VAS than preoperatively, increasing by 0.4 points (SD 3.1) (Table 5 and Fig. 5). The FRA patients had a decrease in leg pain by 1.1 points (SD 2.5). The difference between the changes seen in this outcome measure in the two groups was significant (p=0.029).
Fig. 5.
Visual analogue score (leg pain) preoperatively and at 24 months for FRA and TC groups
Short Form-36
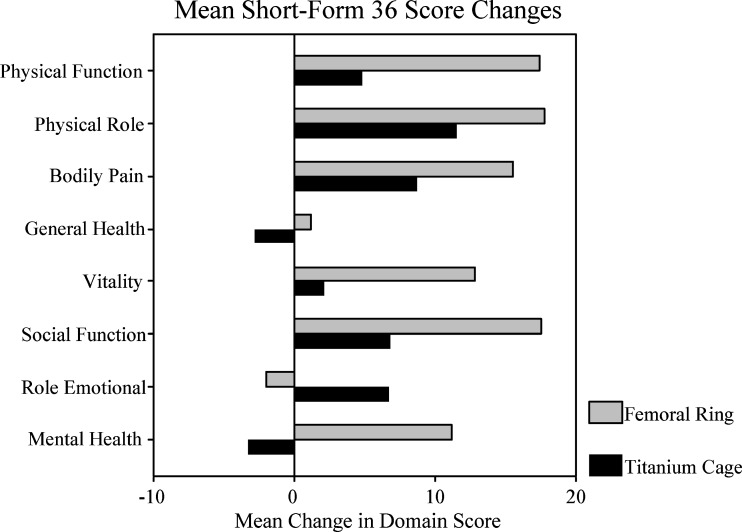
Table 6 shows the scores for each domain preoperatively and at 2 years for both the groups. Figure 6 shows the mean score changes for each domain preoperatively and at 2 years for both the groups. The FRA patients made clinically important and significant improvements in six of the eight domains (general health and emotional role did not reach >7.0 point improvement). The TC patients had consistently lower score improvements compared to the FRA group (except for the emotional role domain). Only two of the eight domains (physical function and bodily pain) reached statistically significant improvement in the TC group.
Table 6.
Mean Short Form-36 scores
| FRA | Titanium cage | |||||
|---|---|---|---|---|---|---|
| Preoperative | 24 months | Change (p) | Preoperative | 24 months | Change (p) | |
| Physical function | 27 | 44 | 17 (0.002)* | 34 | 39 | 5 (0.030)* |
| Physical role | 13 | 31 | 18 (0.028)* | 15 | 26 | 11 (0.167) |
| Bodily pain | 22 | 37 | 16 (0.003)* | 24 | 33 | 9 (0.006)* |
| General health | 54 | 55 | 1 (0.694)) | 54 | 53 | (1 (0.268) |
| Vitality | 27 | 40 | 13 (0.015)* | 35 | 38 | 3 (0.468) |
| Social function | 35 | 52 | 17 (0.003)* | 40 | 47 | 7 (0.097) |
| Emotional role | 47 | 45 | (2 (0.826) | 41 | 50 | 9 (0.420) |
| Mental health | 50 | 61 | 11 (0.008)* | 56 | 52 | (4 (0.349) |
*Change reaching significance
An increase in score indicates improvement
Fig. 6.
Mean score changes for each domain of the SF-36. Improvement of +7.0 points for each domain is considered to be a clinically significant improvement. Any negative change indicates deterioration
Smokers did not have worse preoperative scores (p=0.641) than non-smokers, but as a whole, smokers had poorer outcomes at 2 years (p=0.030). With respect to the change in ODI, smokers did significantly worse than non-smokers within the TC group (p=0.014), but there was no significant relationship between the outcome and smoking status in the femoral ring group (p=0.292).
The presence of previous discectomy or decompression had no relationship with either the preoperative ODI or the postoperative change in ODI (p=0.879).
Adverse events
The complications encountered are outlined in Table 7. There was no difference in the complication rate between the two groups (p=0.316). No deep infection was seen in any patient in the trial. Superficial infection occurred in 1/37 patients in the Femoral Ring group and 1/41 patients in the TC group. Vascular injuries to the common iliac vein occurred in five cases (2/37 FRA group and 3/41 TC group) and were primarily repaired without further complication. Retrograde ejaculation was seen in 1/37 (2.7%) patients in the femoral ring group and 1/41 (2.4%) patients in the TC group. There were four dural tears noted during the insertion of translaminar screws, none of which were explored or repaired primarily. Four patients implanted with the TC subsequently developed breakage of the translaminar screws, which required revision with pedicle screw fixation. Two of these patients had single-level fusions and two had two-level fusions. There were no cases of broken translaminar screws in the femoral ring group. One FRA fractured subsequently, which required revision with posterior pedicle screw fixation.
Table 7.
Adverse events
| Complication | FRA | TC | Number (%) |
|---|---|---|---|
| Superficial infection | 1 | 1 | 2 (7) |
| Transient radiculopathy | 2 | 3 | 5 (6) |
| Retrograde ejaculation | 1 | 1 | 2 (3) |
| Donor site pain | 1 | 1 | 2 (2) |
| Vascular injury | 2 | 3 | 5 (6) |
| Dural tear | 2 | 2 | 4 (5) |
| Bowel perforation | 1 | 1 (1) | |
| Wound haematoma | 1 | 1 (1) | |
| Incisional hernia | 1 | 1 | 2 (2) |
| Patient complication rate | 11/37 (29%) | 13/41 (31%) | 24/78 (30%) |
Discussion
This prospective, randomised controlled trial shows superior clinical outcome when a FRA is used compared to when a TC is used for circumferential lumbar fusion. This is the first prospective, randomised controlled study to compare these two implants.
A detailed radiological analysis looking at intervertebral height, lordosis and evidence for fusion will be the subject of a further study. Controversy still exists over the relationship between fusion and clinical outcome.
Our results show that the femoral ring group achieved a mean of 15 points improvement in ODI, a mean of 2.0 points on the VAS for back pain and greater than 7.0 points in six of the eight domains of the SF-36 2 years following surgery. For this group, the majority of patients achieved the MCID that authors have previously defined [10, 27].
By contrast the TC group achieved a mean of 6 points improvement in ODI, a mean of 1.1 points on the VAS for back pain and greater than 7.0 points in only two of the eight domains of the SF-36 two years following surgery. For this group, the majority of patients did not achieve the MCID previously described [10, 27]. The VAS for leg pain actually worsened in this group by a mean of +0.4.
Femoral ring allograft patients who improved their ODI score by 10 points or more had a significantly lower preoperative ODI than those that failed to make MCID (p=0.044). The same did not hold true for the TC group (p=0.427).
The standard deviation of the preoperative ODI in both our groups was 14 points. The pre-trial power calculation was based on database information showing a standard deviation of 16 points. Therefore, the trial has a power of well in excess of 80% with the numbers we recruited. In view of the difference in age found between the two groups, an analysis of the correlation coefficient for each outcome measure and the age of patient at operation was performed, but no significant correlation was found.
The use of FRA in circumferential fusion is well established and was the subject of previous retrospective studies [11, 13, 19, 26]. Liljenqvist et al. [19] retrospectively reviewed 41 patients with circumferential fusion using FRA and reported a fusion rate of 95%, with 83% of the patients satisfied or highly satisfied with the outcome of the surgery. Sarwat et al. [33] reported fusion rates of 100% for one level and 93% for two levels using this technique, but by using allograft chips instead of cancellous autograft with FRA. Sasso et al. [35], in their randomised trial of a threaded TC versus FRA, observed a significantly higher fusion rate in their cage patients (97 vs 40%), but similar clinical outcomes in both the groups. This trial [35] used the femoral ring as a stand-alone implant without posterior fixation, which is known to result in a lower fusion rate as shown by Holte et al. [11], a study in which the fusion rate was increased from 75 to 98% with the addition of translaminar screws to anterior lumbar interbody fusion.
Our clinical results are similar to those previously reported in prospective cohort studies of lumbar spine fusion for discogenic back pain [20]. Pavlov et al. published a prospective cohort study of the same TC used in our trial [28]. Clinical results were extremely promising, however, it is readily accepted that randomised controlled trials seldom show such impressive results when compared to prospective cohort studies. Age or previous operation status had no influence on outcome in this trial—which is at discrepancy with other studies [4, 33, 36].
Posterior fixation techniques (translaminar vs pedicle screws) have been compared with circumferential fusion previously [12]. The authors showed no difference in the rate of fusion, but reported a higher incidence of myofascial pain with pedicle screws. Our study failed to show any difference between the translaminar screws and the pedicle screws with respect to change in ODI (p=0.286). Injury to the left common iliac vein occurred in 2/37 (5.4%) cases in the femoral ring group and 3/41 (7.3%) cases in the TC group—which is comparable with previous studies [14, 21]. We found no cases of postoperative deep vein thrombosis. The breakage of translaminar screws was seen more frequently in the TC group, although this did not reach a statistical significance (p=0.178). One patient in each group (2.5%) reported retrograde ejaculation, again in keeping with previously reported incidences with a retroperitoneal approach [34, 37].
Although smoking has been shown to influence fusion rates, its effect on the functional outcome is not always seen [1]; smokers in our study had poorer outcomes.
There are several theories that we believe may explain the difference in clinical outcomes between these two groups. While accepting the central role played by the degenerate disc in producing back pain, a more mechanistic concept is proposed by Mulholland and Sengupta [25] and McNally et al. [23]. Their view is that the altered degenerate disc no longer acts as an isotropic structure, and hence transfers loads abnormally, producing high areas of focal load on the endplate and supporting cancellous bone. The pattern of loading is affected by position in the normal disc, which would not be the case if the disc was isotropic. So, for example, when the spine is flexed, the anterior endplate and vertebrae are loaded excessively, and hence the pain on bending, which is a common feature in patients with back pain. Using the finite element analysis, it has also been shown that loads below a cage, which is load bearing, maybe 500% higher than loads below a normal disc [16, 30]. The conclusion by McAfee [22] that pain relief following cage fusions seems to be little different from other methods of fusion, despite much better rates of fusion, may be a reflection of loading problems below some cages that remain weight bearing. If the mechanistic concept suggested by Mulholland and Sengupta is accepted, then the explanation of the different results may be that the femoral rings allow the development of organised weight-bearing bone blending with the bone of the femoral ring, which transfers load in an increasingly normal pattern as the bone remodels according to Wolff’s law. Young’s modulus of titanium is ten times higher than that of cortical bone and may lead to point loading of the endplate. The cage, whilst integrated and providing a ‘union’ in so far as there is no movement, still transfers the load through the metal, producing high loads in a small area as opposed to the dispersal of load produced by the developing ‘ray’ of bone from the femoral ring.
It is suggested that the disc material itself (nucleus) and inner annulus are important pain generators in low back pain. However, in both types of fusion, the discs are excised identically, yet the fact that there was a difference in pain relief must cast doubt on the importance of these structures as pain generators.
FRA undergoes creeping substitution over time and the initial disc space distraction gained during anterior interbody fusion by 1 year may be lost over time [8, 15]. Studies have reported that interbody fusion with FRA may take up to 18 months to fuse [5]—the time for fusion for the TC used in this trial has yet to be reported. The remodelling that can occur in a fusion achieved with FRA in combination with endplate settling may result in better sagittal alignment of the lumbar spine and a more normal pattern of loading. The restoration of normal lumbar lordosis may improve the clinical outcome. To achieve this goal, O’Brien et al. [26] changed his surgical technique to perform the anterior surgery before posterior fixation, but there are no results comparing the clinical outcomes of these groups.
The insertion of the TC can be performed without fear of damage to the cage itself during the insertion process. FRA is potentially prone to fracture during insertion and, therefore, may persuade the surgeon to ‘undersize’ the implant reducing disc distraction; this may explain the increased leg symptoms seen in the ‘potentially over-distracted’ TC group. Radiological analysis may clarify this concern.
In conclusion, we have found the clinical results of FRA to be superior to TCs when used as interbody spacers in circumferential fusion of the lumbar spine 2 years after surgery. The TC is ten times more expensive than the FRA and its use appears not to be justified on the basis of clinical outcome.
Acknowledgements
We would like to thank Mr. James Hegarty and Mrs. Ellie Bevan-Davies for their assistance in data collection and database management.
References
- 1.Andersen T, Christensen FB, Larsen M, Hoy K, Hansen ES, Bunger C. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001;26:2623–2628. doi: 10.1097/00007632-200112010-00018. [DOI] [PubMed] [Google Scholar]
- 2.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of pro-inflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620X.84B2.12511. [DOI] [PubMed] [Google Scholar]
- 3.Christensen FB, Hansen ES, Escaper SP, Hoy K, Helming P, Neumann P, Tiedemann B, Bunger CE. Circumferential lumbar spinal fusion with Bartizan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine. 2002;27:2674–2683. doi: 10.1097/00007632-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Christensen FB, Karlsmose B, Hansen ES, Bunger CE. Radiological and functional outcome after anterior lumbar interbody spinal fusion. Eur Spine J. 1996;5:293–298. doi: 10.1007/BF00304343. [DOI] [PubMed] [Google Scholar]
- 5.Cohen DB, Chotivichit A, Fujita T, Wong TH, Huckell CB, Sieber AN, Kostuik JP, Lawson HC. Pseudarthrosis repair. Autogenous iliac crest versus femoral ring allograft. Clin Orthop Relat Res. 2000;371:46–55. [PubMed] [Google Scholar]
- 6.Colhoun E, McCall IW, Williams L, Cassar PV. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg Br. 1988;70:267–271. doi: 10.1302/0301-620X.70B2.2964449. [DOI] [PubMed] [Google Scholar]
- 7.Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of “painful” lumbar discs. Spine. 1997;22:2342–2349. doi: 10.1097/00007632-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dennis S, Watkins R, Landaker S, Dillin W, Springer D. Comparison of disc space heights after anterior lumbar interbody fusion. Spine. 1989;14:876–878. doi: 10.1097/00007632-198908000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Derby R, Howard MW, Grant JM, Lettice JJ, Peteghem PK, Ryan DP. The ability of pressure-controlled discography to predict surgical and non-surgical outcomes. Spine. 1999;24:364–371. doi: 10.1097/00007632-199902150-00014. [DOI] [PubMed] [Google Scholar]
- 10.Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 11.Holte DC, O’Brien JP, Renton P. Anterior lumbar fusion using a hybrid interbody graft. A preliminary radiographic report. Eur Spine J. 1994;3:32–38. doi: 10.1007/BF02428314. [DOI] [PubMed] [Google Scholar]
- 12.Janssen ME, Lam C, Beckham R. Outcomes of allogenic cages in anterior and posterior lumbar interbody fusion. Eur Spine J. 2001;10(Suppl 2):S158–S168. doi: 10.1007/s005860100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozak JA, O’Brien JP. Simultaneous combined anterior and posterior fusion An independent analysis of a treatment for the disabled low-back pain patient. Spine. 1990;15:322–328. doi: 10.1097/00007632-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni SS, Lowery GL, Ross RE, Ravi SK, Lykomitros V. Arterial complications following anterior lumbar interbody fusion: report of eight cases. Eur Spine J. 2003;12:48–54. doi: 10.1007/s00586-002-0460-4. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Kozak JA, Doherty BJ, Dickson JH. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine. 1993;18:2393–2400. doi: 10.1097/00007632-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Meakin JR, Kumar A, Mishra V, Mulholland RC. Analysis of Stress in Lumbar interbody Fusion. Spine. 2005;30:1713–1735. doi: 10.1097/01.brs.0000172160.78207.49. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N, Wild A, Webb JK, Aebi M. Hybrid computer-guided and minimally open surgery: anterior lumbar interbody fusion and translaminar screw fixation. Eur Spine J. 2000;9(Suppl 1):S71–S77. doi: 10.1007/PL00010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane JD, Moore ES. Transperitoneal approach to the intervertebral disc in the lumbar area. Ann Surg. 1948;127:537. doi: 10.1097/00000658-194803000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liljenqvist U, O’Brien JP, Renton P. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J. 1998;7:125–131. doi: 10.1007/s005860050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linson MA, Williams H. Anterior and combined anteroposterior fusion for lumbar disc pain. A preliminary study. Spine. 1991;16:143–145. [PubMed] [Google Scholar]
- 21.Mayer HM. Comment to “Arterial complications following anterior lumbar interbody fusion: report of eight cases”, by S.S. Kulkarni et al. Eur Spine J. 2003;12:55–56. doi: 10.1007/s00586-002-0461-3. [DOI] [PubMed] [Google Scholar]
- 22.McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 23.McNally DS, Shackleford IM, Goodship AE, Mulholland RC. In vivo stress measurement can predict pain on discography. Spine. 1996;21:2580–2587. doi: 10.1097/00007632-199611150-00007. [DOI] [PubMed] [Google Scholar]
- 24.Montesano PX, Magerl F, Jacobs RR, Jackson RP, Rauschning W. Translaminar facet joint screws. Orthopedics. 1988;11:1393–1397. doi: 10.3928/0147-7447-19881001-08. [DOI] [PubMed] [Google Scholar]
- 25.Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002;11(Suppl 2):S198–S205. doi: 10.1007/s00586-002-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien JP, Dawson MH, Heard CW, Momberger G, Speck G, Weatherly CR. Simultaneous combined anterior and posterior fusion. A surgical solution for failed spinal surgery with a brief review of the first 150 patients. Clin Orthop Relat Res. 1986;203:191–195. [PubMed] [Google Scholar]
- 27.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov PW, Meijers H, Limbeek J, Jacobs WC, Lemmens JA, Obradov-Rajic M, Kleuver M. Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine. 2004;29:1893–1899. doi: 10.1097/01.brs.0000137067.68630.70. [DOI] [PubMed] [Google Scholar]
- 29.Phillips FM, Cunningham B, Carandang G, Ghanayem AJ, Voronov L, Havey RM, Patwardhan AG. Effect of supplemental translaminar facet screw fixation on the stability of stand-alone anterior lumbar interbody fusion cages under physiologic compressive preloads. Spine. 2004;29:1731–1736. doi: 10.1097/01.BRS.0000134570.08901.30. [DOI] [PubMed] [Google Scholar]
- 30.Polikeit A, Ferguson SJ, Nolte LP, Orr TE. Factors influencing stresses in the lumbar spine after the insertion of intervertebral cages: finite element analysis. Eur Spine J. 2003;12:413–420. doi: 10.1007/s00586-002-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathonyi GC, Oxland TR, Gerich U, Grassmann S, Nolte LP. The role of supplemental translaminar screws in anterior lumbar interbody fixation: a biomechanical study. Eur Spine J. 1998;7:400–407. doi: 10.1007/s005860050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine. 1995;20:2645–2651. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Sarwat AM, O’Brien JP, Renton P, Sutcliffe JC. The use of allograft (and avoidance of autograft) in anterior lumbar interbody fusion: a critical analysis. Eur Spine J. 2001;10:237–241. doi: 10.1007/s005860000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasso RC, Kenneth BJ, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine. 2003;28:1023–1026. doi: 10.1097/00007632-200305150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Sasso RC, Kitchel SH, Dawson EG. A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine. 2004;29:113–122. doi: 10.1097/01.BRS.0000107007.31714.77. [DOI] [PubMed] [Google Scholar]
- 36.Tiusanen H, Hurri H, Seitsalo S, Osterman K, Harju R. Functional and clinical results after anterior interbody lumbar fusion. Eur Spine J. 1996;5:288–292. doi: 10.1007/BF00304342. [DOI] [PubMed] [Google Scholar]
- 37.Tiusanen H, Seitsalo S, Osterman K, Soini J. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J. 1995;4:339–342. doi: 10.1007/BF00300293. [DOI] [PubMed] [Google Scholar]
- 38.Weiner Fraser BK RD. Spine update lumbar interbody cages. Spine. 1998;23:634–640. doi: 10.1097/00007632-199803010-00020. [DOI] [PubMed] [Google Scholar]