Abstract
BACKGROUND
Sublingual buprenorphine is an effective maintenance treatment for opioid dependence, yet intravenous buprenorphine misuse occurs. A buprenorphine/naloxone formulation was developed to mitigate this misuse risk. This randomized, double-blind, crossover study was conducted to assess the intravenous abuse potential of buprenorphine/naloxone compared with buprenorphine in buprenorphine-maintained injection drug users (IDUs).
METHODS
Intravenous heroin users (n=12) lived in the hospital for 8–9 weeks and were maintained on each of 3 different sublingual buprenorphine doses (2 mg, 8 mg, 24 mg). Under each maintenance dose, participants completed laboratory sessions during which the reinforcing and subjective effects of intravenous placebo, naloxone, heroin, and low and high doses of buprenorphine and buprenorphine/naloxone were examined. Every participant received each test dose under the 3 buprenorphine maintenance dose conditions.
RESULTS
Intravenous buprenorphine/naloxone was self-administered less frequently than buprenorphine or heroin (P < 0.0005). Participants were most likely to self-administer drug intravenously when maintained on the lowest sublingual buprenorphine dose. Subjective ratings of “drug liking” and “desire to take the drug again” were lower for buprenorphine/naloxone than for buprenorphine or heroin (P = 0.0001). Participants reported that they would pay significantly less money for buprenorphine/naloxone than for buprenorphine or heroin (P < 0.05). Seven adverse events were reported; most were mild and transient.
CONCLUSIONS
These data suggest that although the buprenorphine/naloxone combination has intravenous abuse potential, it is lower than for buprenorphine alone, particularly when participants received higher maintenance dosages and lower buprenorphine/naloxone challenge doses. Buprenorphine/naloxone may be a reasonable option for managing the risk for buprenorphine misuse during opioid dependence treatment.
Keywords: buprenorphine/naloxone, abuse liability, opioid dependence
INTRODUCTION
Illicit use of heroin and other opioids is a serious international health problem affecting an estimated 16 million persons worldwide [1–3]. Treatments for opioid dependence, such as methadone and buprenorphine, substantially reduce the morbidity and mortality associated with this disease and play a critical role in addressing the needs of opioid-dependent patients [2,4–7]. As with all mu opioid agonist-based medications, these treatments may be associated with intravenous misuse that can compromise patient care and access to opioid treatment [1,8].
Buprenorphine (Subutex) is available in more than 40 countries worldwide. Despite its widespread success as a maintenance therapy for opioid dependence [1,9], buprenorphine misuse [11–14] and diversion to the black market have been reported. The use of a combination of buprenorphine plus the opioid antagonist naloxone in a fixed 4:1 ratio (Suboxone) theoretically would reduce misuse and diversion. When taken sublingually as prescribed, its therapeutic efficacy and safety are similar to those of buprenorphine alone [15]. However, laboratory studies have shown that the naloxone component precipitates withdrawal in most opioid-dependent persons and attenuates the euphoric effects of buprenorphine when the combination is administered intravenously [17–19]. Buprenorphine/naloxone’s real-life use as a strategy to reduce buprenorphine abuse is also supported by retrospective surveys documenting lower misuse rates after buprenorphine/naloxone was introduced in areas known to have high rates of buprenorphine injection [11,14].
Although these survey data are encouraging, no controlled laboratory studies have directly evaluated the abuse liability of buprenorphine/naloxone in buprenorphine-maintained intravenous drug users (IDUs). Because high doses of naloxone are required to displace buprenorphine from the opioid receptor [20], the extent to which the combination medication will mitigate misuse by buprenorphine-dependent IDUs remains unclear. The purpose of this study was to quantitatively assess the intravenous abuse potential of buprenorphine/naloxone compared with buprenorphine using a controlled laboratory intravenous self-administration paradigm. IDUs maintained on buprenorphine and willing to self-administer drug by injection were enlisted to simulate a population of buprenorphine-dependent injectors.
METHODS
Participants
Healthy men and women between ages 21 and 45 years who met the diagnostic criteria for opioid dependence according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [21] and were not seeking treatment for drug use were eligible to participate in this study (see Supplementary Appendix for exclusion criteria). All participants were enrolled between September 10, 2007, and August 13, 2008. Participants were required to reside at the clinical study center for the duration of the study and provide informed consent. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute and was conducted in accordance with the tenets of the Declaration of Helsinki.
Study Design
The study consisted of an initial 2-week qualification phase and three 2-week experimental phases (see Supplementary Appendix). During the qualification phase, participants were maintained on 2 mg sublingual buprenorphine and treated for emergent withdrawal symptoms until these symptoms no longer manifested. Generally 5–7 days elapsed between admission to the hospital and conduct of the first laboratory session. This dose-stabilization period ensured that participants would not experience withdrawal symptoms during experimental phases. After the initial period of stabilization, participants completed sampling and choice sessions to establish eligibility for the experimental phase. During this part of the qualification phase, participants received ascending doses of intravenous buprenorphine (2, 4, 8, 16 mg) in the morning; a choice session followed in the afternoon to confirm that buprenorphine was well tolerated and intravenously self-administered at or above 4 mg. To maintain blind dosing, a saline placebo and a second 2-mg dose were given on random days during the qualification phase instead of the intended buprenorphine dose. Participants who did not intravenously self-administer at least 4 mg buprenorphine more frequently than placebo during this period were discontinued from the study.
Each experimental phase consisted of a sublingual buprenorphine stabilization period (first week) and a double-blind test period (second week). During the stabilization period, participants were randomized to 1 of 3 sublingual buprenorphine maintenance doses (2, 8, 24 mg). The 2 mg maintenance dose condition was used as an equivalent to a low-dose control condition. On each test period day, participants were randomized to receive intravenous doses of buprenorphine/naloxone, buprenorphine, or control. Placebo, naloxone, and heroin (25 mg) were neutral, negative, and positive controls, respectively. Intravenous buprenorphine doses used for testing were chosen for each participant based on his or her responses during the qualification phase. For participants who self-administered a maximum of 8 mg buprenorphine during the qualification phase (n = 2), 4 mg and 8 mg were the low and high doses of buprenorphine in each formulation, respectively. For participants who self-administered a maximum of 16 mg buprenorphine during this phase (n = 10), 8 mg and 16 mg were the low and high doses of buprenorphine administered in each formulation, respectively. All buprenorphine/naloxone formulations were administered at a 4:1 ratio. The dose of the naloxone control was based on the highest naloxone dose participants received in the buprenorphine/naloxone formulation. Intravenous doses were administered to participants by a staff physician.
On each test period morning, participants completed a sample session during which they received a full dose of that day’s study drug and USD$20. Sample doses were administered at approximately 11 AM. Physiological, subjective, and performance measures were assessed before and repeatedly after dose administration. On the afternoon of each test period day participants completed a choice session during which they were informed they could work for all or part of the test drug or money they received in the morning. The work assignment involved a 40-minute, computerized, self-administration task; responses consisted of finger presses on a computer mouse. Participants were given 10 opportunities under an independent progressive ratio schedule to choose between one-tenth of the test drug dose or one-tenth of the money (USD$2) they received during the morning’s sample session. At the end of the task, participants received the amount of drug and money they chose during the task. Choice session doses were administered at approximately 4 PM. Over the course of the study, each participant received all the sublingual buprenorphine maintenance doses and all the intravenous test doses. Given that all participants received their buprenorphine maintenance doses at 8 PM, sessions were conducted near the nadir of the maintenance dose levels.
This study was designed and performed by the academic authors. All authors participated in the analysis of the data and/or contributed to writing the manuscript. All authors also had full access to the primary data and to the analysis, and all vouch for the accuracy and completeness of the reported data.
Efficacy and Safety Measures
The primary objective of the study was to compare the reinforcing effects of intravenous buprenorphine/naloxone and buprenorphine in buprenorphine-maintained IDUs using a drug-versus-money choice procedure. Drugs were considered to have reinforcing effects (abuse liability) if they were self-administered by injection more than placebo. Reinforcing effects were quantified with a progressive ratio schedule during the choice session. After each choice was made for one option (50 clicks of the mouse), the ratio increased progressively (100, 200, 400, 800, 1,200, 1,600, 2,000, 2,400, and 2,800 clicks) each time that option was selected. The progressive ratio breakpoint was defined as the amount of work (highest ratio completed) a participant was willing to perform to obtain drug or money; test drugs with greater reinforcing effects would have correspondingly higher drug breakpoints. Ratio values were chosen based on previous research [22]. Percentages of trials completed for drug, amount of drug self-administered by injection (in mg), progressive ratio breakpoint values for money, and amount of money self-administered (in USD$) were also measured.
Secondary objectives were to compare subjective, physiological, and performance effects of intravenous buprenorphine/naloxone, buprenorphine, placebo, naloxone, and heroin in buprenorphine-maintained IDUs. Subjective effects were assessed at 4, 10, 40, and 60 minutes after drug administration during the sample session using 4 questionnaires: a 6-item Drug Effects Questionnaire; a 26-item, 100-mm visual analog scale (VAS); a 13-item Opioid Symptom Checklist; and a 16-item Subjective Opioid Withdrawal Scale (SOWS) [22]. Photographs of the right pupil and vital sign measurements were used to assess physiological effects. Performance measures were assessed with 3-minute digit-symbol substitution [23] and 10-minute divided attention [24] tasks. Routine safety and tolerability were evaluated from the results of reported signs and symptoms of opioid withdrawal and other adverse events.
Statistical Analysis
Primary and secondary efficacy variables were analyzed using a within-subjects repeated-measures ANOVA model, with sublingual buprenorphine maintenance dose and intravenous test dose as factors. Planned comparisons were made among placebo and each active dose of drug, heroin and each of the other drug conditions, and low- and high-dose buprenorphine/naloxone and buprenorphine. P ≤ 0.05 was considered significant.
Based on results from previous research, a 12-participant study would provide 80% power to detect a 380-click difference in the breakpoint (assuming SD of 472 and between-level correlation of 0.60) [22]. VAS ratings of “good effect” were representative of the magnitude of most of the significant subjective effects found in previous research [22]. Based on these data, a 12-participant study would provide 80% power to a difference of 12.5 points for this VAS item.
RESULTS
Participant Disposition and Demographics
Forty-four participants signed the screening consent form and were screened for this study. Nineteen participants signed the study consent form. Of those, 12 completed the study and were included in the final analysis. Reasons for not completing the study included domestic issues, chronic pain, failure to pass the qualification phase, behavioral problems, excessive methadone use (1 participant each), and failure to arrive on admission day (2 participants). Of the 12 participants who completed the study, low- and high-doses for the buprenorphine formulations administered were 4 and 8 mg, respectively, for 2 participants, and 8 and 16 mg, respectively, for 10 participants.
Demographic data for participants completing the study are shown in Table 1. Mean age of this population was 36.2 years; most participants were male (67%; 8/12) and white (58%; 7/12). All participants reported daily intravenous heroin use. Mean daily amounts spent on heroin was $67.03 (range, $30–$145), and mean duration of use was 11.3 years (range, 2–32 years).
Table 1.
Baseline demographic data for participants completing the study (n=12).
| Demographic Characteristic | Total, n (%) |
|---|---|
| Age — yr | |
| Mean±SD | 36.2±6.2 |
| Min – Max | 25 – 46* |
| Sex | |
| Male | 8 (67) |
| Ethnicity | |
| African American | 2 (17) |
| White | 7 (58) |
| Hispanic | 3 (25) |
| Heroin use | |
| Every day per week | 12 (100) |
| Intravenous use | 12 (100) |
| Daily amount spent on heroin — USD$ | |
| Mean±SD | 67.03±33.74 |
| Min – Max | 30 – 145 |
| History of use — yr | |
| Mean±SD | 11.3±9.1 |
| Min – Max | 2 – 32 |
Because the eligibility criteria limited participant age to 45 years, an exception was requested and approved by the Institutional Review Board for the 46-year-old participant.
Reinforcing Effects
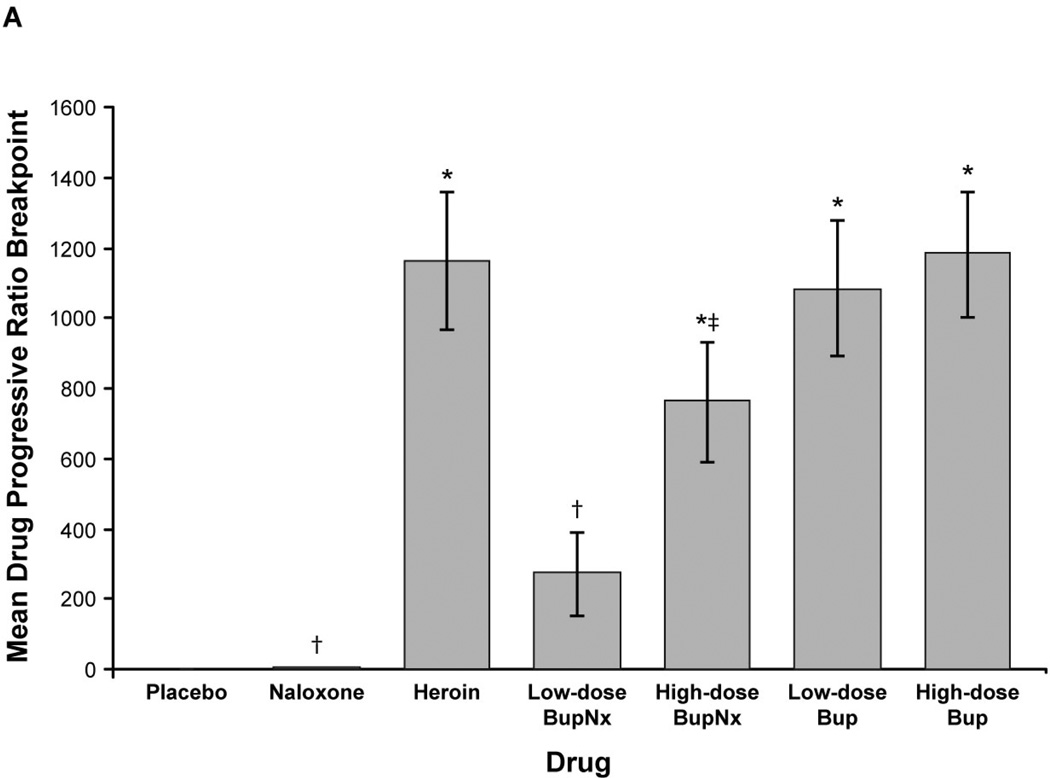
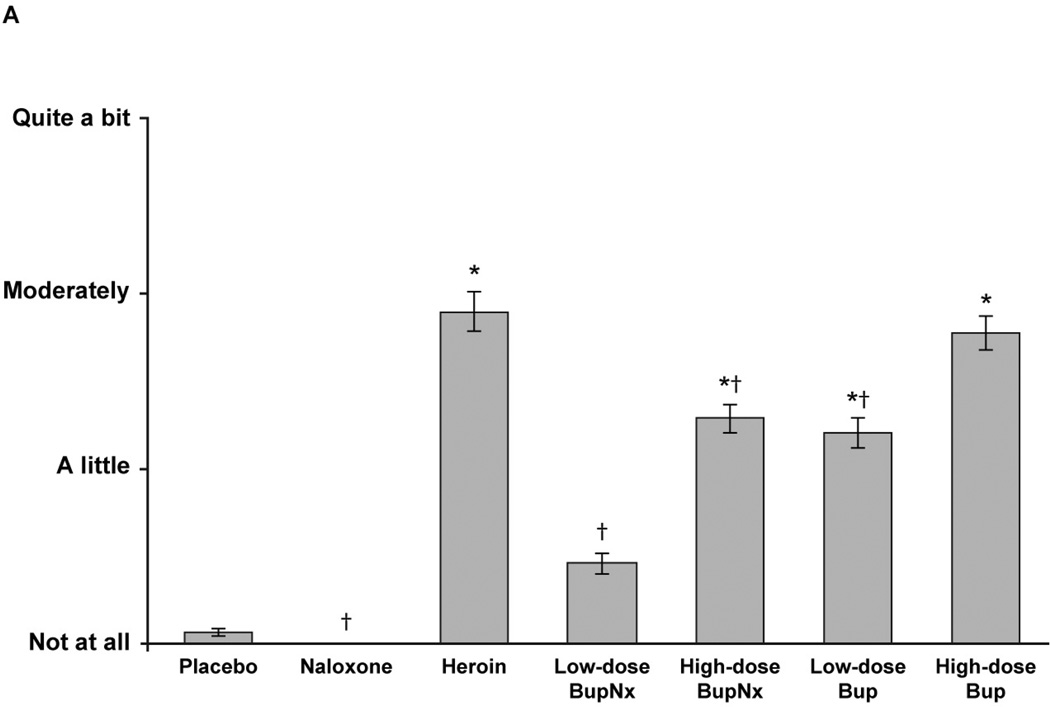
Based on mean drug progressive ratio breakpoints, reinforcing effects for heroin, high-dose buprenorphine/naloxone, low-dose buprenorphine, and high-dose buprenorphine were greater than for placebo across all sublingual buprenorphine maintenance doses (all P < 0.0005; Figure 1A). Low-dose buprenorphine/naloxone demonstrated reinforcing effects that were lower than for heroin (P = 0.0001); high-dose buprenorphine/naloxone also demonstrated a trend toward lower reinforcing effects (P = 0.055; Figure 1A). Drug breakpoint values for low- and high-dose buprenorphine alone did not differ from those for heroin. When individual doses were compared, participants opted to intravenously self-administer high-dose buprenorphine/naloxone less than high-dose buprenorphine (P < 0.05) and low-dose buprenorphine/naloxone less than low-dose buprenorphine (P = 0.0002). The drug breakpoint value for low-dose buprenorphine/naloxone was lower than for high-dose buprenorphine/naloxone (P = 0.02), but low-dose buprenorphine alone was not significantly different from high-dose buprenorphine alone.
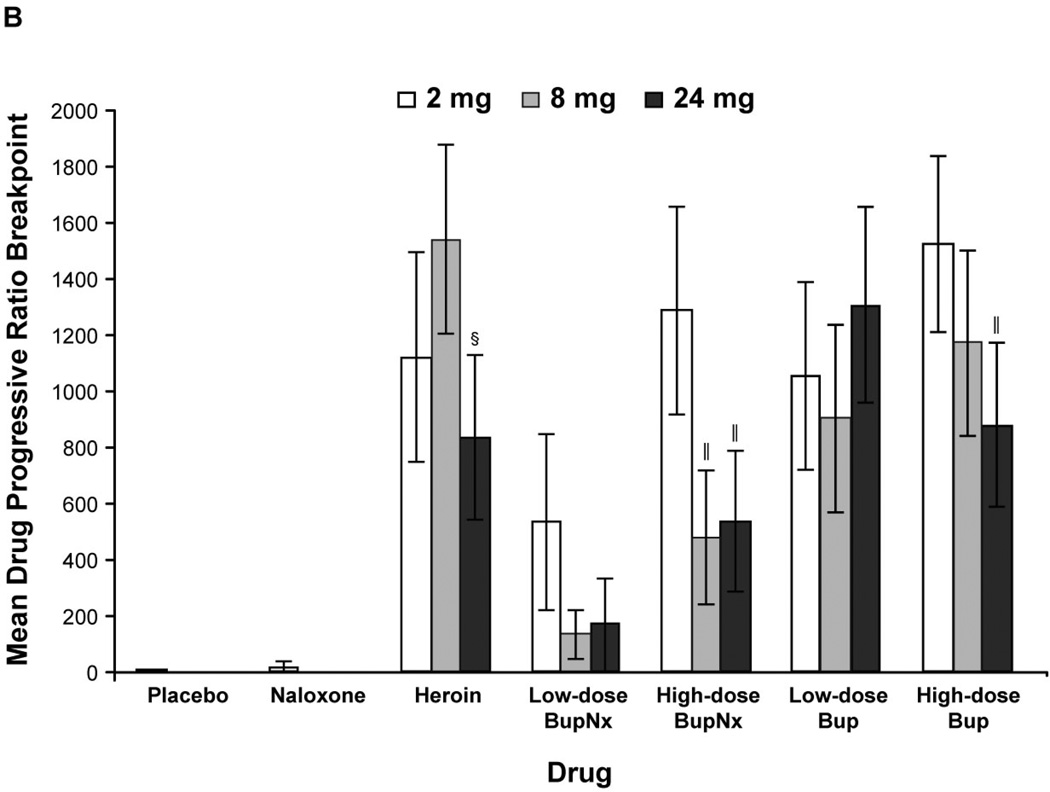
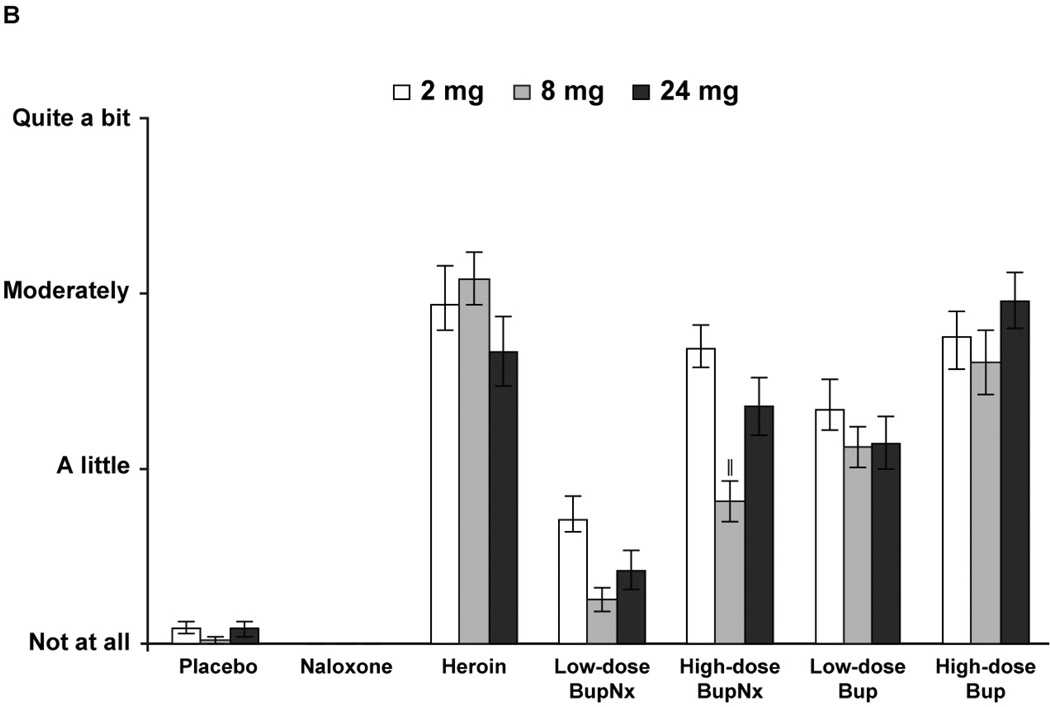
Figure 1.
(A) Mean drug progressive ratio breakpoints for intravenous test doses across all buprenorphine maintenance doses. (B) Mean progressive ratio breakpoints for test drug stratified by buprenorphine maintenance dose. BupNx, buprenorphine/naloxone; Bup, buprenorphine. * P < 0.01 compared with placebo. †P < 0.01 compared with heroin. ‡P ≤ 0.055 compared with heroin. §P < 0.03 compared with 8 mg buprenorphine maintenance dose. ‖P < 0.05 compared with 2 mg buprenorphine maintenance dose.
Participants maintained on 2 mg buprenorphine were more likely to intravenously self-administer drug than those maintained on 8 or 24 mg sublingual buprenorphine (Figure 1B). This effect was most pronounced when participants intravenously self-administered high-dose buprenorphine/naloxone. The percentage of total available dose intravenously self-administered was lower for buprenorphine/naloxone than for heroin and buprenorphine alone (all P < 0.03). In all cases, participants never intravenously self-administered more than 50% of available drug.
Subjective, Performance, and Physiological Effects
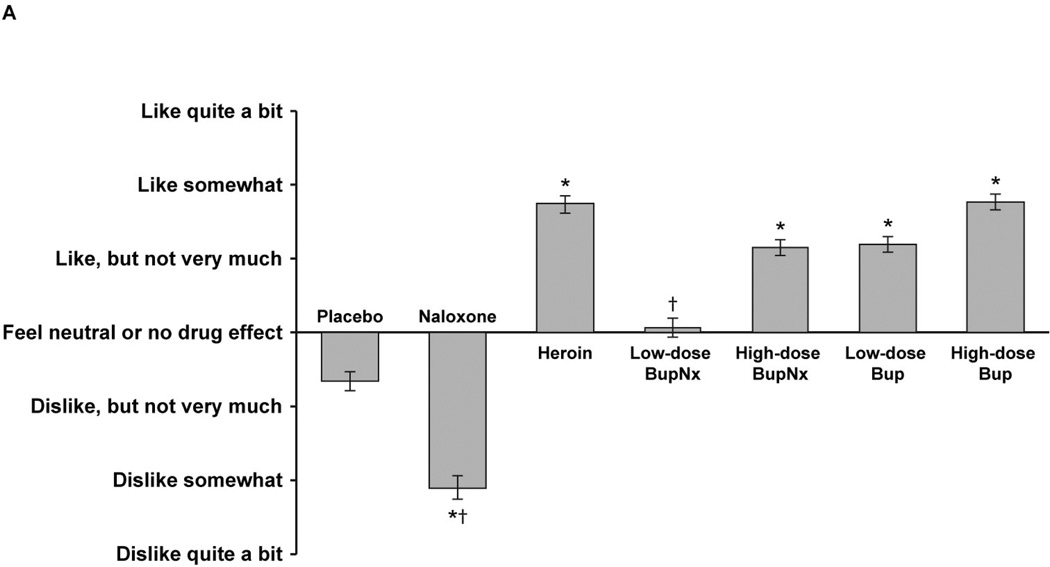
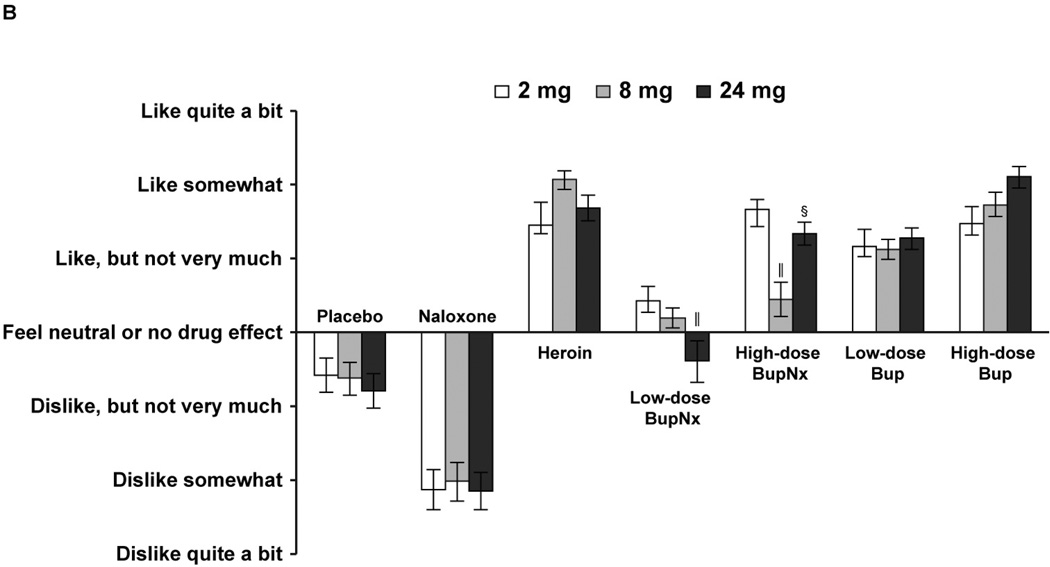
Based on responses to the Drug Effects Questionnaire, participants across all maintenance doses reported higher mean “drug liking” measures for heroin, high-dose buprenorphine/naloxone, and low- and high-dose buprenorphine than for placebo (all P < 0.0001; Figure 2A). In addition, participants across all maintenance doses reported lower mean “drug liking” measures for naloxone and low-dose buprenorphine/naloxone than for heroin (both P = 0.0001). At the 24-mg maintenance dose, participants challenged with low-dose buprenorphine/naloxone reported significantly less “drug liking” compared with the 2-mg maintenance dose (P <0.05); this difference also included a change from participants reporting a positive “liking” effect to a negative “liking” effect (Figure 2B). For all buprenorphine formulations and doses, participants reported significantly greater willingness to take the drug again compared with placebo (Figure 3A) and significantly less willingness to take low- and high-dose buprenorphine/naloxone again compared with heroin. There were no trends toward a common response when participants were stratified by maintenance dose and test drug (Figure 3B). Only naloxone produced higher ratings of “bad drug effect” compared with placebo.
Figure 2.
(A) Mean “drug liking” responses from the Drug Effects Questionnaire across all buprenorphine maintenance doses. (B) Mean “drug liking” responses from the Drug Effects Questionnaire stratified by buprenorphine maintenance dose. BupNx, buprenorphine/naloxone; Bup, buprenorphine. * P < 0.001 compared with placebo. †P < 0.005 compared with heroin. §P < 0.03 compared with 8 mg buprenorphine maintenance dose. ‖P < 0.05 compared with 2 mg buprenorphine maintenance dose.
Figure 3.
(A) Mean “willing to take the drug again” responses from the Drug Effects Questionnaire across all buprenorphine maintenance doses. (B) Mean “willing to take the drug again” responses from the Drug Effects Questionnaire stratified by buprenorphine maintenance dose. BupNx, buprenorphine/naloxone; Bup, buprenorphine. * P < 0.001 compared with placebo. †P < 0.005 compared with heroin. ‖P < 0.05 compared with 2 mg buprenorphine maintenance dose.
Low-dose buprenorphine/naloxone was significantly different from all other buprenorphine formulations and doses on a number of VAS measures across all buprenorphine maintenance doses. Specifically, only low-dose buprenorphine/naloxone produced ratings similar to those of placebo in the following positive subjective-effects categories: good effects, feeling high, liking drug, feeling mellow, potency and quality of drug effects, feeling sedated, and amount of money participants would pay for drug (Table 2). Compared with heroin, mean peak VAS scores were significantly lower for low- and high-dose buprenorphine/naloxone on measures of drug liking and amount of money participants would pay for drug. For all other positive subjective-effects measures except feeling mellow, participants reported significantly lower VAS scores for low-dose buprenorphine/naloxone than for heroin; no buprenorphine doses were significantly different from heroin for these measures. Neither of the buprenorphine formulations was significantly different from placebo in negative subjective effects such as feeling anxious, bad effects, and feeling irritable (Table 2). When stratified by maintenance dose, patients receiving 2 mg sublingual buprenorphine reported significantly greater good effects, high, potency, and amount of money they would pay for drug compared with 8 mg (but not 24 mg) buprenorphine. There were no significant differences between any of the maintenance groups in the negative subjective effects categories. There were also no trends toward a common response when participants were stratified by maintenance dose and test drug.
Table 2.
| VAS Measure (mean±SE) |
Placebo | Naloxone | Heroin | Low-Dose Buprenorphine/ Naloxone |
High-Dose Buprenorphine/ Naloxone |
Low-Dose Buprenorphine |
High-Dose Buprenorphine |
|
|---|---|---|---|---|---|---|---|---|
| Peak Positive | ||||||||
| Good effects | 1.75±0.90 | 4.83±3.11 | 41.11±5.24 | 12.67±3.07 | 30.61±4.20 | 29.58±4.62 | 40.53±4.76 | |
| High | 2.06±1.08 | 0.00±0.00 | 36.81±5.04 | 12.19±2.94 | 31.56±3.65 | 27.19±4.24 | 40.83±5.07 | |
| Liked drug choice | 1.19±0.62 | 3.03±2.78 | 41.56±5.42 | 10.50±2.44 | 27.28±3.97 | 29.83±4.35 | 42.50±4.90 | |
| Mellow | 18.11±4.74 | 17.81±3.98 | 28.81±4.11 | 26.17±4.92 | 34.86±4.98 | 32.72±4.23 | 36.06±4.87 | |
| Potency of drug | 0.19±0.17 | 16.25±5.74 | 34.50±5.60 | 9.94±2.47 | 25.72±3.86 | 24.50±4.69 | 35.11±4.69 | |
| Quality of drug | 1.58±1.23 | 1.39±1.28 | 35.89±5.23 | 8.81±2.32 | 25.28±3.58 | 28.69±4.24 | 35.89±4.47 | |
| Sedated | 1.47±1.07 | 4.00±2.52 | 20.44±4.62 | 6.39±2.25 | 19.58±3.87 | 14.42±3.32 | 23.31±4.64 | |
| Would pay | 0.25±0.13 | 0.00±0.00 | 8.50±1.10 | 1.72±0.51 | 5.81±0.91 | 5.97±0.91 | 8.36±1.06 | |
| Peak Negative | ||||||||
| Anxious | 6.56±2.39 | 27.56±5.33 | 5.69±2.24 | 6.83±2.81 | 8.11±2.60 | 6.92±2.18 | 8.47±2.53 | |
| Bad effects | 3.14±1.47 | 56.50±7.17 | 0.00±0.00 | 4.00±2.14 | 5.03±3.49 | 1.67±1.35 | 0.56±0.56 | |
| Depressed | 4.61±2.16 | 23.19±5.60 | 0.86±0.78 | 4.86±2.63 | 1.78±1.28 | 2.61±2.56 | 3.61±2.47 | |
| Gooseflesh | 4.47±1.90 | 59.39±6.25 | 0.25±0.18 | 0.06±0.06 | 4.00±2.10 | 3.75±2.12 | 1.14±0.61 | |
| Irritable | 14.97±4.71 | 50.86±6.82 | 7.89±3.36 | 6.42±2.55 | 7.14±2.65 | 8.92±4.07 | 8.78± 3.31 | |
| Muscle pain | 2.03±1.32 | 30.44±7.05 | 2.58±1.39 | 5.14±2.45 | 5.56±3.07 | 3.78±1.69 | 6.33±2.48 | |
| Nauseated | 3.28±1.76 | 31.06±6.16 | 3.25±2.25 | 1.19±0.71 | 2.86±2.29 | 2.33±1.76 | 3.75±2.25 | |
| Restless | 3.36±1.73 | 33.83±5.52 | 5.69±1.71 | 2.22±0.99 | 2.19±0.98 | 7.39±2.69 | 3.69±1.52 | |
Scales ranged from 0 (“Not at all”) to 100 (“Extremely”).
Values in bold are significantly different from placebo (P < 0.05).
Values in italics are significantly different from heroin (P < 0.05).
Compared with placebo or heroin, no significant differences were observed in mean subjective opioid withdrawal symptoms using the SOWS for any of the buprenorphine formulations. Additional subjective, performance, and physiological effects are reported in the Supplementary Appendix.
Safety
Seven adverse events were reported in 4 participants (3 participants, 2 adverse events; 1 participant, 1 adverse event). Adverse events not associated with study drug included vasovagal response to intravenous injection (2 events, same participant) and nausea. Adverse events possibly or probably related to study drug (and drug received) included urticaria (buprenorphine/naloxone), vomiting (heroin), dizziness (buprenorphine), and chest discomfort/mild withdrawal (naloxone). Most adverse events were mild, transient, and resolved without treatment.
DISCUSSION
This study identified conditions under which buprenorphine/naloxone had less potential to be abused intravenously compared with buprenorphine alone or heroin among buprenorphine-maintained IDUs, namely when participants were (a) receiving higher buprenorphine maintenance doses and (b) receiving the lower dose of the buprenorphine/naloxone formulation. In addition, participants were willing to pay significantly less for buprenorphine/naloxone than for buprenorphine or heroin. Although other studies have demonstrated reduced abuse liability with intravenous buprenorphine/naloxone [17–19], this is the first study in buprenorphine-maintained IDUs to quantitatively and prospectively demonstrate conditions under which the abuse liability for buprenorphine/naloxone is less than that of buprenorphine alone. To date, only retrospective surveys of IDUs or patients switched from buprenorphine to buprenorphine/naloxone have reported a difference in abuse potential [11, 14]. For example, results from a questionnaire administered to 145 attendees of a needle exchange program indicated that few (8%) abused buprenorphine/naloxone regularly; in comparison, most (82%) reported daily intravenous buprenorphine misuse. These attendees also reported they would pay substantially less for the combination formulation than for buprenorphine alone [11], consistent with the findings of the current report.
Reduced self-administration by injection was greater for participants maintained on 8 mg and 24 mg sublingual buprenorphine than for those maintained on 2 mg, suggesting that a higher buprenorphine maintenance dose is an important factor in curtailing misuse. Indeed, the abuse liability of high-dose intravenous buprenorphine/naloxone was comparable to that of heroin or high-dose buprenorphine at the 2-mg maintenance dose but not at higher sublingual buprenorphine maintenance doses. Additionally, participants receiving the 2 mg buprenorphine maintenance dose also reported similar “drug liking” and “willingness to take drug again” measures for high-dose buprenorphine/naloxone compared with participants receiving high-dose buprenorphine or heroin at any of the maintenance doses. These observations are supported by others [28–30] and by many direct evaluations of dose-response effects of opioid medications [31,32]; in addition, these observations highlight the importance of the underlying maintenance dose in controlling injection behavior. In this study, the 8- and 24-mg maintenance doses were more reflective of clinically relevant buprenorphine maintenance levels. The 2-mg maintenance dose was intended as a low-dose control condition. It is important to note that the sample sessions were performed approximately 15 hours after the participant’s last sublingual buprenorphine maintenance dose. This latency period approaches the trough concentration of the participant’s maintenance dose. It is possible that the reinforcing responses might have be less pronounced if the maintenance dose was administered closer to when the experimental tasks were performed. Because trends in subjective effect changes were not apparent as a function of sublingual buprenorphine maintenance dose using this study design, it is probable that there would also not be different subjective effects if dosing was performed closer to the experimental session.
The higher progressive ratio breakpoint for high-dose buprenorphine/naloxone—which is similar to that of low-dose buprenorphine, particularly at the 2 mg buprenorphine maintenance dose—suggested that, in buprenorphine-dependent IDUs, the naloxone component did not precipitate withdrawal symptoms but instead attenuated the euphoric effect of buprenorphine. Participant’s subjective responses also supported this response-blunting effect. Specifically, negative subjective responses and SOWS scores were not significantly different between the buprenorphine/naloxone formulation and placebo, suggesting that the naloxone component did not strongly precipitate withdrawal. In contrast, positive subjective responses were lower when participants were challenged with buprenorphine/naloxone compared with buprenorphine but were greater than placebo (particularly for high-dose buprenorphine/naloxone), which indicates a diminished—but not absent—euphoric effect. In separate studies with morphine [18], hydromorphone [17], and methadone-maintained [19] individuals, significant negative subjective effects were noted when participants received intravenous buprenorphine/naloxone. The effect of the naloxone component in the buprenorphine/naloxone formulation may be partially dependent on opioid receptor occupancy. In particular, the high affinity and slow dissociation of buprenorphine from μ-opioid receptors may partially prevent naloxone-induced withdrawal, whereas the shorter receptor occupancy of other opioids allows greater access of naloxone to the receptor and its concomitant negative effects [25]. These findings indicate that, in individuals receiving higher dosages of buprenorphine/naloxone, an attenuated abuse potential still remains, and that this abuse potential is subject to the individual’s recent opioid exposure.
This study also indicated that the reinforcing effects of buprenorphine alone were similar to those of heroin in buprenorphine-maintained IDUs. In addition, many of the positive subjective effects for buprenorphine alone, as well as high-dose buprenorphine/naloxone, were comparable to heroin, with the high-dose buprenorphine/naloxone values numerically similar to those of low-dose buprenorphine. These findings, when considered with those of other studies, suggest that the abuse liability of buprenorphine partially depends on recent opioid exposure. For example, in non-opioid–maintained persons, the intravenous abuse liability of buprenorphine was similar to that of the full opioid agonist methadone [26]. However, in morphine-maintained persons, buprenorphine behaved like a partial opioid agonist, precipitating withdrawal and, consequently, having lower abuse liability [27]. These findings also further support the attenuation of the euphoric effect by naloxone.
This study had some limitations. The stringent criteria for enrollment, qualification, and retention in this trial were highly selective for a certain subpopulation of opioid-dependent persons. This resulted in a small, specific population of participants that may be responsible for some of the anomalous results observed in this study, for example, the lack of a trend across the 3 maintenance doses in subjective effects or the variability in response at different maintenance doses. Although this subpopulation might not be representative of the entire opioid-dependent population, participants who completed the trial were likely to be more homogeneous in their susceptibility to the positive and negative reinforcing effects of the study drugs. Another limitation was the long duration of buprenorphine’s effects, which might have allowed the effects of the intravenous buprenorphine test doses to persist between days. However, the randomized, crossover design of this trial should have served as a partial control for any potential carryover effects.
In conclusion, this study provides empirical support for the lower potential of buprenorphine/naloxone misuse by injection. Findings from this study are also supported by international experience with buprenorphine/naloxone. For example, in the United States, where buprenorphine/naloxone is available by prescription from qualified physicians, reports of misuse have been limited [33]. In Australia [36–38] and Finland [11], where buprenorphine misuse was relatively common, the introduction of buprenorphine/naloxone has been associated with lower rates of misuse, even for patients receiving the medication without close monitoring [11,14,39,40]. The results from our quantitative approach complement those observed in these surveys, and further identified conditions under which the abuse liability of buprenorphine/naloxone was minimized (i.e., higher underlying maintenance doses and lower buprenorphine/naloxone dosage). Coupled with efficacy, safety, and tolerability data from other studies, buprenorphine/naloxone’s reduced potential for abuse makes it an option for first-line opioid dependence treatment, including take-home therapy.
Supplementary Material
Acknowledgements
We thank Joseph Lazar, BS, for technical support and Janet Murray, RN, Ronnie Shapiro, RN, Benjamin Bryan, MD, Soteri Polydorou, MD, and Jennifer Hanner, MD, for medical support during the study. We also thank Beth Kamp, PharmD, and Brian Falcone, PhD, in association with ApotheCom, for providing editorial services for the manuscript.
Dr. Comer has served as a consultant to Alpharma Pharmaceuticals LLC, BioDelivery Sciences Inc., Cephalon Pharmaceuticals, King Pharmaceuticals, Neuromed Pharmaceuticals, Pain Therapeutics, and Purdue Pharmaceuticals, manufacturers of opioid medications. She has also consulted for Analgesic Research and Inflexxion, Inc. In addition, she has received research funding from Reckitt-Benckiser Pharmaceuticals, Endo, and Johnson & Johnson. Dr. Comer received honoraria from the Rehabilitation Institute of Chicago for presenting her research at a conference entitled “Examining Critical Issues in Opioid Management,” Yale University for presenting her research on a depot formulation of naltrexone, and Wake Forest University and the University of Michigan, Ann Arbor, for presenting her research on opioid abuse liability. Dr. Vosburg received partial salary support from and served as a consultant to Grunenthal USA, Inc. Dr. Vosburg also served as a consultant to Analgesic Research and Purdue Pharmaceuticals. Dr. Kleber served as a consultant to Abbott, Alkermes, Grunenthal, Cephalon, Purdue, and US World Med. He also received lecture fees from Pri Cara and was awarded an unrestricted educational grant from Reckitt Benckiser. Dr. Amass is an employee of Schering-Plough Corporation and reports having equity interest in the company.
This study was supported by Schering-Plough Corporation. Buprenorphine tablets for sublingual use, obtainable from Reckitt Benckiser Pharmaceuticals Inc. (Richmond, VA), were provided by the National Institute on Drug Abuse (NIDA). Buprenorphine HCl for injection (4 mg/mL), obtainable from Murty Pharmaceuticals (Lexington, KY), was provided by NIDA. Heroin HCl, obtainable from Macfarlan Smith Limited (Edinburgh, Scotland), was provided by NIDA. Naloxone HCl for injection (1 mg/mL; Narcan) was obtained from International Medication System Limited Amphastar (South Elmonte, CA).
Footnotes
Conflicts of Interest
The other authors have no conflicts to declare.
References
- 1.Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43:S197–S215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- 2.Teesson M, Mills K, Ross J, Darke S, Williamson A, Havard A. The impact of treatment on 3 years' outcome for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS) Addiction. 2008;103:80–88. doi: 10.1111/j.1360-0443.2007.02029.x. [DOI] [PubMed] [Google Scholar]
- 3.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 4.Gossop M, Marsden J, Stewart D, Treacy S. Reduced injection risk and sexual risk behaviours after drug misuse treatment: results from the National Treatment Outcome Research Study. AIDS Care. 2002;14:77–93. doi: 10.1080/09540120220097955. [DOI] [PubMed] [Google Scholar]
- 5.Gossop M, Marsden J, Stewart D, Kidd T. The National Treatment Outcome Research Study (NTORS): 4–5 year follow-up results. Addiction. 2003;98:291–303. doi: 10.1046/j.1360-0443.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard RL, Craddock SG, Anderson J. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS) J Subst Abuse Treat. 2003;25:125–134. doi: 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 7.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD002207.pub3. CD002207. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: the role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100:150–158. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 9.Fatseas M, Auriacombe M. Why buprenorphine is so successful in treating opiate addiction in France. Curr Psychiatry Rep. 2007;9:358–364. doi: 10.1007/s11920-007-0046-2. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 11.Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee CE. Tackling Subutex abuse in Singapore. Singapore Med J. 2006;47:919–921. [PubMed] [Google Scholar]
- 13.Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti J-P. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96:267–272. doi: 10.1046/j.1360-0443.2001.96226710.x. [DOI] [PubMed] [Google Scholar]
- 14.Simojoki K, Vorma H, Alho H. A retrospective evaluation of patients switched from buprenorphine (Subutex) to the buprenorphine/naloxone combination (Suboxone) Subst Abuse Treatment Prevention Policy. 2008;3:16. doi: 10.1186/1747-597X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 16.Kamien JB, Branstetter SA, Amass L. Buprenorphine-naloxone (8/2 mg and 16/4 mg) versus methadone (45 mg and 90 mg) maintenance therapy: a randomised double-blind trial with opioid dependent patients. Heroin Addiction Related Clinical Problems. In press. [Google Scholar]
- 17.Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl) 2001;154:230–242. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- 18.Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN. Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend. 1998;50:1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Preston KL, Bigelow GE, Liebson IA. Buprenorphine and naloxone alone and in combination in opioid-dependent humans. Psychopharmacology (Berl) 1988;94:484–490. doi: 10.1007/BF00212842. [DOI] [PubMed] [Google Scholar]
- 20.Gal TJ. Naloxone reversal of buprenorphine-induced respiratory depression. Clin Pharmacol Ther. 1989;45:66–71. doi: 10.1038/clpt.1989.10. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 22.Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology (Berl) 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- 23.McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the Digit Symbol Substitution Test (DSST) Behav Res Methods Instrumentation. 1982;14:463–466. [Google Scholar]
- 24.Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology. 1988;19:90–96. doi: 10.1159/000118441. [DOI] [PubMed] [Google Scholar]
- 25.Eissenberg T, Johnson RE, Bigelow GE, et al. Controlled opioid withdrawal evaluation during 72 h dose omission in buprenorphine-maintained patients. Drug Alcohol Depend. 1997;45:81–91. doi: 10.1016/s0376-8716(97)01347-1. [DOI] [PubMed] [Google Scholar]
- 26.Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–1330. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roux P, Villes V, Blanche J, et al. Buprenorphine in primary care: risk factors for treatment injection and implications for clinical management. Drug Alcohol Depend. 2008;97:105–113. doi: 10.1016/j.drugalcdep.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Gerra G, Borella F, Zaimovic A, et al. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004;75:37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend. 2003;70:S49–S57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 31.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose-response effects of methadone in the treatment of opioid dependence. Ann Intern Med. 1993;119:23–27. doi: 10.7326/0003-4819-119-1-199307010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Methadone dose and treatment outcome. Drug Alcohol Depend. 1993;33:105–117. doi: 10.1016/0376-8716(93)90052-r. [DOI] [PubMed] [Google Scholar]
- 33.JBS International, Inc. Silver Spring, MD: Substance Abuse and Mental Health Services Administration Center for Substance Abuse Treatment; 2006. Diversion and abuse of buprenorphine: a brief assessment of emerging indicators: final report. Available at: http://buprenorphine.samhsa.gov/Buprenorphine_FinalReport_12.6.06.pdf. [Google Scholar]
- 34.Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict. 2008;17:116–20. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- 35.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 36.Lintzeris N, Ritter A, Panjari M, Clark N, Kutin J, Bammer G. Implementing buprenorphine treatment in community settings in Australia: experiences from the Buprenorphine Implementation Trial. Am J Addict. 2004;13:S29–S41. doi: 10.1080/10550490490440799. [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- 38.Aitken CK, Higgs PG, Hellard ME. Buprenorphine injection in Melbourne, Australia—an update. Drug Alcohol Rev. 2008;27:197–199. doi: 10.1080/09595230701829553. [DOI] [PubMed] [Google Scholar]
- 39.Bell J, Shanahan M, Mutch C, et al. A randomized trial of effectiveness and cost-effectiveness of observed versus unobserved administration of buprenorphine-naloxone for heroin dependence. Addiction. 2007;102:1899–1907. doi: 10.1111/j.1360-0443.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- 40.Bell JR, Ryan A, Mutch C, Batey R, Rea F. Optimising the benefits of unobserved dose administration for stable opioid maintenance patients: follow-up of a randomised trial. Drug Alcohol Depend. 2008;96:183–186. doi: 10.1016/j.drugalcdep.2008.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.