Summary
Background
Different environmental stimuli, including exposure to dauer pheromone, food deprivation, and high temperature, can induce C. elegans larvae to enter the dauer stage, a developmentally arrested diapause state. Although molecular and cellular pathways responsible for detecting dauer pheromone and temperature have been defined in part, other sensory inputs are poorly understood, as are the mechanisms by which these diverse sensory inputs are integrated to achieve a consistent developmental outcome.
Results
In this paper, we analyze a wild C. elegans strain isolated from a desert oasis. Unlike wild-type laboratory strains, the desert strain fails to respond to dauer pheromone at 25°C, but it does respond at higher temperatures, suggesting a unique adaptation to the hot desert environment. We map this defect in dauer response to a mutation in the scd-2 gene, which, we show, encodes the nematode anaplastic lymphoma kinase (ALK) homolog, a proto-oncogene receptor tyrosine kinase. scd-2 acts in a genetic pathway shown here to include the HEN-1 ligand, the RTK adaptor SOC-1, and the MAP kinase SMA-5. The SCD-2 pathway modulates TGF-β signaling, which mediates the response to dauer pheromone, but SCD-2 might mediate a nonpheromone sensory input, such as food.
Conclusions
Our studies identify a new sensory pathway controlling dauer formation and shed light on ALK signaling, integration of signaling pathways, and adaptation to extreme environmental conditions.
Introduction
To feed, reproduce, and navigate successfully within their surroundings, animals must accurately sense and interpret a variety of environmental stimuli. These different sensory inputs must be integrated correctly to elicit appropriate behavioral or developmental responses. Substantial work has identified various sensory systems in experimental organisms [1, 2] but has not revealed how multiple sensory signals are integrated to achieve the correct outcomes.
In C. elegans sensory processing controls entry into the developmentally arrested L3 diapause state called dauer [3]. Dauer formation is promoted by harsh environmental conditions, including lack of food, high temperature, and high concentration of dauer pheromone [4], a constitutively secreted fatty-acid derivative [5, 6] that communicates population density. Dauers are resistant to diverse environmental stresses, including food deprivation [7].
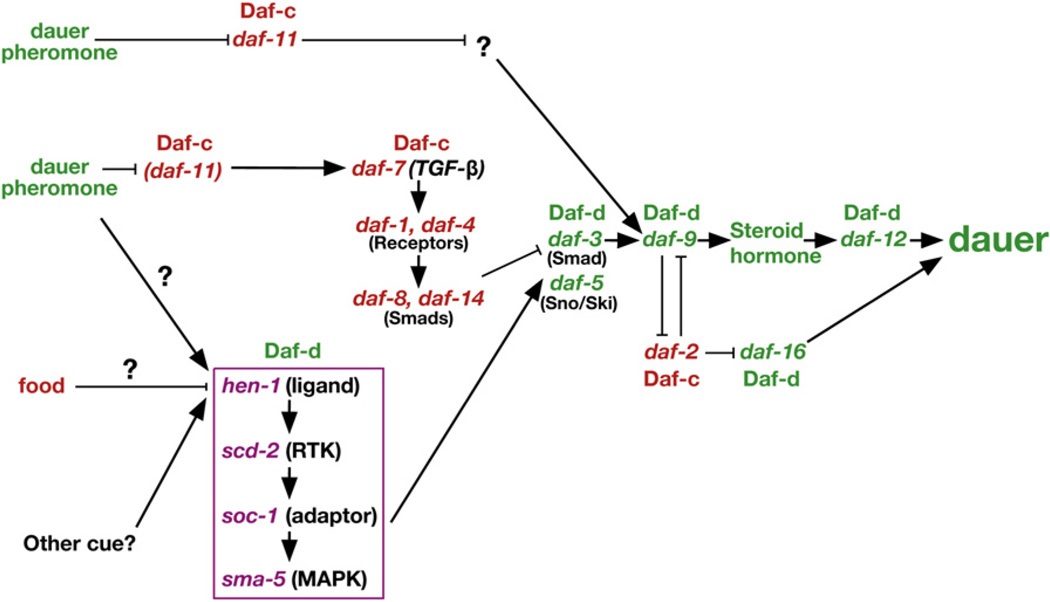
Environmental cues regulating dauer formation are detected by amphid sensory neurons, whose endings are exposed to the environment [8, 9], and the various inputs—chemosensory, thermosensory and metabolic—are integrated by a complex signaling network [7, 10–14] (Figure 1). Mutations that affect this signaling network are classified by their effect on dauer formation, either dauer constitutive (Daf-c) or dauer defective (Daf-d).
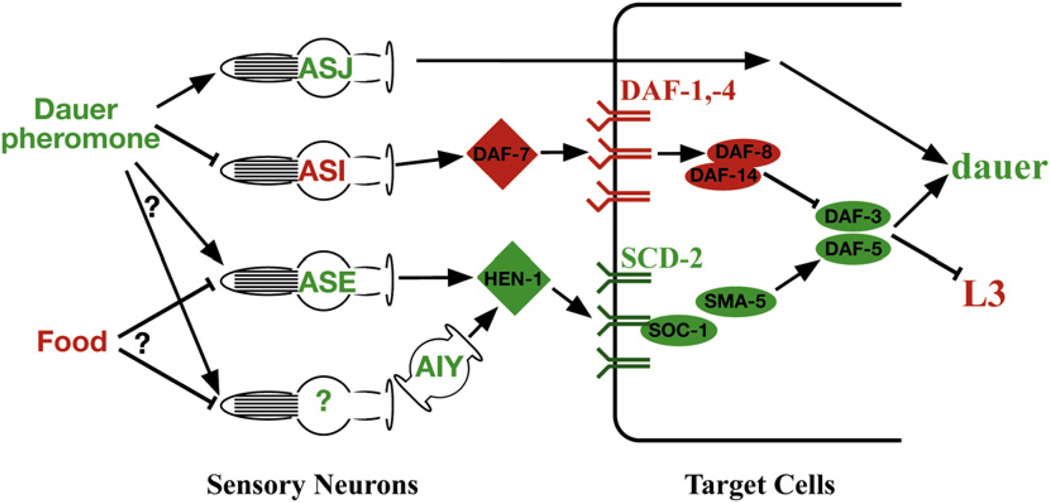
Figure 1. A Revised Genetic Model of Dauer Formation, Incorporating the Newly Defined scd-2 Branch.
The genes in the scd-2 branch are enclosed in a purple box. Sensory stimuli and groups of genes are color-coded by wild-type function; i.e., green for genes/stimuli that promote dauer formation and red for those that inhibit dauer formation. Arrows indicate a positive genetic interaction, and bars indicate an inhibitory genetic interaction. Several DAF-2 insulin-branch components have been omitted.
Two pairs of sensory neurons, ASJ and ASI, mediate detection of dauer pheromone by signaling through a branched genetic pathway that reflects the branched cellular pathway [7] (Figure 1). Dauer pheromone activates the ASJ sensory neurons to promote dauer formation using the DAF-11 guanylyl cyclase in a negative regulatory cascade [9, 15]. In contrast, dauer pheromone inhibits the ASI sensory neurons. When active, the ASI neurons inhibit dauer formation by secreting DAF-7 transforming growth factor-β (TGF-β) [8, 9].
The mechanism of temperature sensation is only partially understood. The AFD sensory-neuron pair mediates temperature sensation for thermotaxis behavior, and signaling to its synaptic partner, the AIY interneuron, controls thermal-based behaviors. Genetic disruption of AIY via mutations in the ttx-3 transcription factor abolishes most of the temperature input into recovery from the dauer state [11, 16]. A genetic program for temperature regulation of dauer induction is strongly implicated [17], but the mechanism of this input is unknown. Lack of food also promotes dauer formation [4], but the nature or mechanism of this input is entirely unknown.
Pheromone, food, and temperature signals are integrated through unknown mechanisms and are thought to control the secretion of redundant insulin-like ligands [18–21], whose signals are transduced via the insulin receptor DAF-2 [22]. Ultimately, the combination of these insulin signals is likely to regulate the secretion of “dafachronic acid” steroid hormones that bind and activate the DAF-12 nuclear hormone receptor in the most-downstream portion of the dauer-regulatory network [23].
Here, we describe a new sensory pathway that regulates dauer formation by interacting with the TGF-β branch of the dauer pathway. Genetic studies in C. elegans combined with molecular work in mammalian systems, suggest the following model of TGF-β function in dauer formation. DAF-7/TGF-β binding induces multimerization and activation of the DAF-1/ DAF-4 receptor serine/threonine kinase. Subsequent phosphorylation of the DAF-8 and DAF-14 Smads sequesters DAF-3/Smad and blocks dauer formation. Given that DAF-3 mediates all of the known functions of DAF-7/TGF-β, DAF-3 probably functions as the main downstream transcriptional effector of the DAF-7/TGF-β branch [24] (Figure 1).
A previous screen identified several regulators of the TGF-β pathway, including the suppressor of constitutive dauer genes scd-1, scd-2, and scd-3 25]. Mutations in these genes suppress the Daf-c phenotypes caused by disruptions of the dauer TGF-β pathway.
In this study, we describe a wild C. elegans strain, isolated from an oasis in a southern California desert, which is dauer defective and carries a mutation in the gene scd-2. Our analysis of this strain suggests that perturbation of dauer formation could represent an adaptation to harsh climates. We show that scd-2 encodes the nematode homolog of the anaplastic lymphoma kinase (ALK) receptor tyrosine kinase (RTK), a central player in the development of non-Hodgkin’s lymphoma in humans. Along with identification of the SCD-2 receptor, we have identified a ligand, an RTK adaptor protein, and a MAP kinase that function in the same pathway as SCD-2, defining a new signal transduction pathway regulating dauer formation. Using GFP reporters that reflect DAF-3/Smad transcriptional activity, we find that the SCD-2 branch is required for maximal DAF-3 activity, suggesting that the SCD-2 RTK signal is integrated with the TGF-β dauer pheromone signal by directly modulating the activity of the TGF-β pathway. Together, our results suggest that a sensory cue promotes DAF-3/Smad transcriptional activity through an ALK receptor tyrosine kinase/MAP kinase signaling cascade, thus regulating dauer formation. We discuss the possibility that the SCD-2 sensory pathway integrates the gustatory input of food into the dauer pathway.
Results
A Wild C. elegans Strain Isolated from the Desert is Dauer-Defective
C. elegans has been found throughout the non-arctic zones of the world. The size of local populations is thought to grow exponentially when an abundant source of bacteria becomes available, then crash when the food supply is exhausted. High population density and low food induce young larvae to adopt the dauer developmental program instead of normal L3 development, thus allowing them to survive starvation and to disperse in search of new food sources. We reasoned that dauer formation might be under active selection in different environments, leading to natural variants in the process.
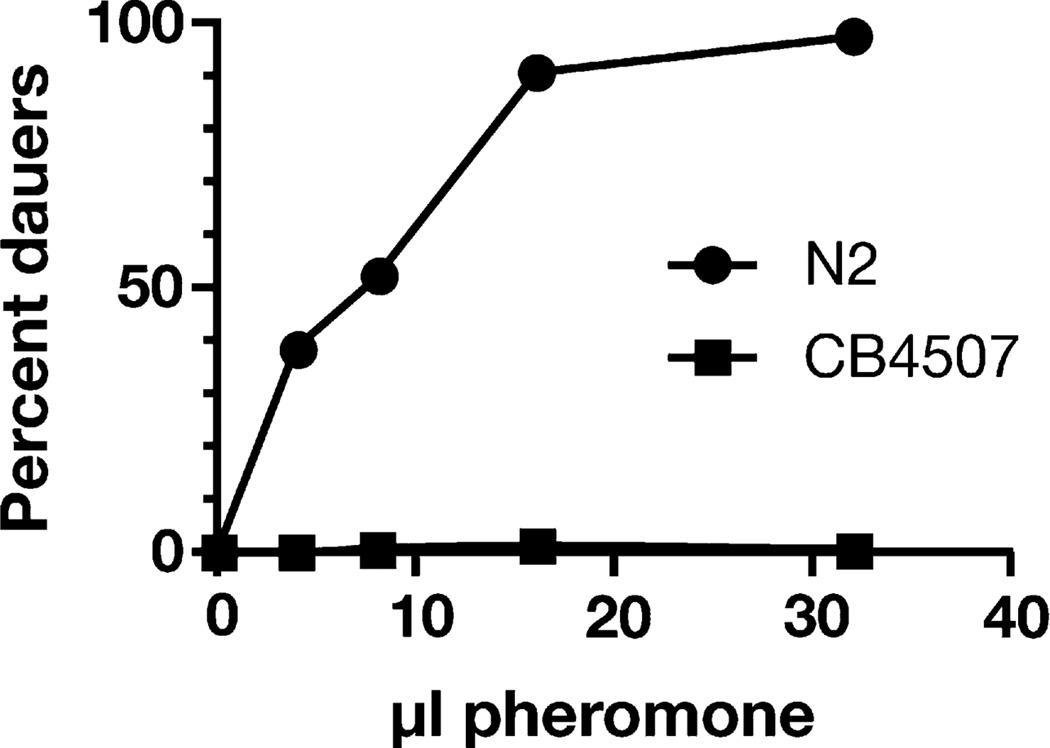
To test whether dauer induction shows any adaptation to different environments, we obtained 26 wild C. elegans strains from diverse locales and examined their sensitivity to dauer pheromone, compared to the wild-type laboratory strain (N2 Bristol). We found that CB4507, a C. elegans isolate from a palm oasis in the Anza Borrego desert in southern California [26], formed no dauers in response to exogenously added pheromone at 25°C (Figure 2). In comparison, N2 animals generally form many dauers. CB4507 also did not form dauers in plate starvation assays, indicating that the strain is dauer defective (data not shown). However, CB4507 formed dauers in response to pheromone at higher temperatures (27°C). These results suggest that CB4507 has adapted to desert conditions by decreasing dauer pheromone responsiveness at lower temperatures. Such adaptation of diapause is not unprecedented, given that a polymorphism of the Drosophila timeless circadian protein that promotes earlier diapause is found more frequently in northern than in southern Europe [27].
Figure 2. The Wild C. elegans Isolate CB4507 from the Anza Borrego Desert is Resistant to Exogenous Dauer Pheromone.
Increasing amounts of dauer pheromone were added to N2 and CB4507. The results of two parallel experiments were combined. Each point represents 100–200 assayed animals.
scd-2 Encodes an Anaplastic Lymphoma Kinase Ortholog
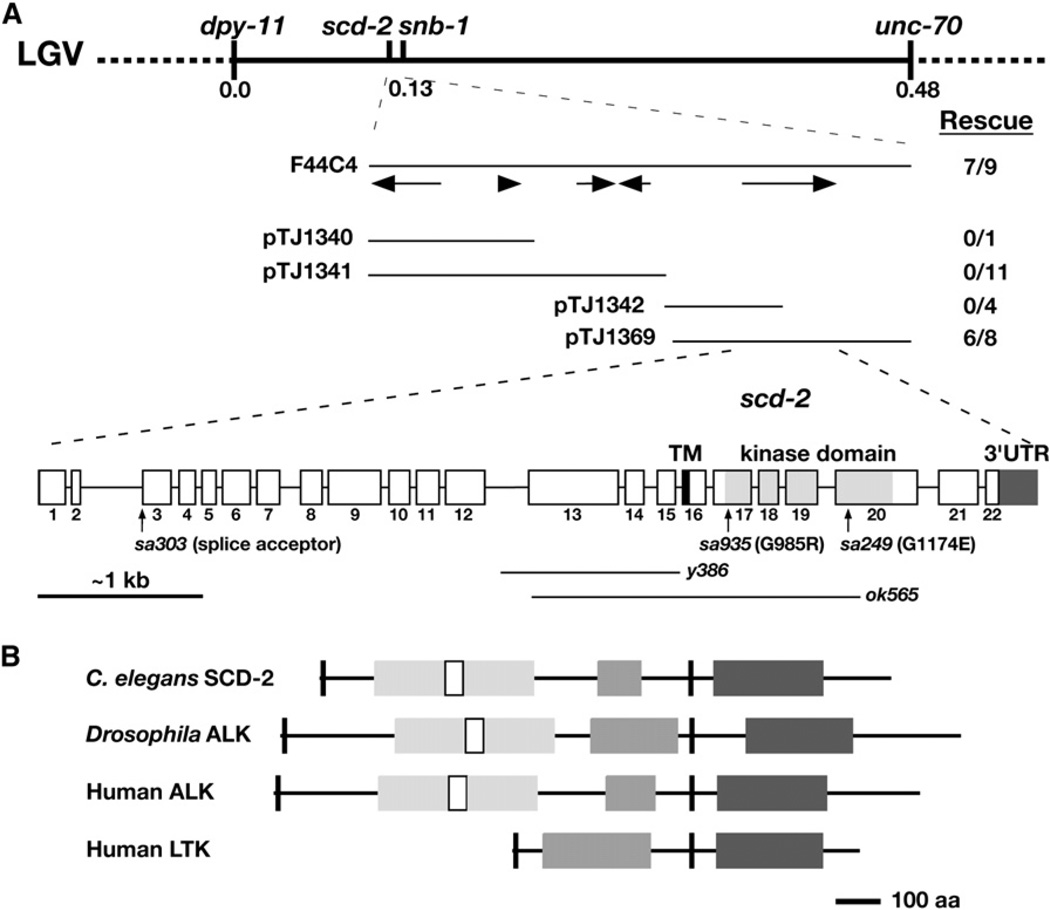
In genetic crosses, the CB4507 pheromone-resistance trait behaved as a single mutation, named sa935, which mapped to the center of chromosome V near the previously identified gene scd-2. A complementation test showed that sa935 is an allele of scd-2, which had been identified previously in screens for suppressors of Daf-c mutations in the dauer TGF-β pathway [25]. We cloned scd-2 by a series of mapping and transgenic experiments (Figure 3 and Supplemental Experimental Procedures, available online) and found that it corresponds to the open reading frame T10H9.2, which encodes the C. elegans ortholog of the human proto-oncogene ALK.
Figure 3. scd-2 Encodes the Nematode Anaplastic Lymphoma Kinase, a Proto-Oncogene Receptor Tyrosine Kinase.
(A) Genetic and physical maps of the scd-2 region. Arrows show open reading frames contained in the F44C4 cosmid. pTJ1340, pTJ1341, pTJ1342, and pTJ1369 are subclones of the cosmid F44C4. Transgenic rescue is shown as number of lines rescuing pheromone insensitivity per total lines generated for each subclone. scd-2 contains 22 exons. The two lines below the splice diagram represent the regions deleted in y386 and ok565. The locations of the sa303, sa935 and sa249 point mutations are also shown.
(B) Domain diagram of SCD-2, Drosophila ALK (dAlk), human ALK and human LTK, aligned at the transmembrane domain. Short black boxes represent the signal peptide and TM domain, white boxes represent the LDLRA motifs, light gray boxes represent the MAM motifs, dark gray boxes represent the glycine-rich region, and large C-terminal black boxes represent the kinase domains. We identified a GGFGGGGXXC motif toward the end of the glycine-rich region in every ALK/LTK ortholog examined, suggesting that this sequence motif is functionally significant.
ALK is a receptor tyrosine kinase (RTK), a member of a diverse group of receptors for intercellular signal transduction that share basic topological and catalytic features. The human ALK family of RTKs is part of the insulin-receptor superfamily of RTKs, and it consists of two members, ALK and LTK (leukocyte tyrosine kinase). The kinase domains of ALK, LTK, and other insulin-receptor superfamily members are very similar, but they diverge in the extracellular region.
The C. elegans ALK gene scd-2 contains 22 exons and produces a putative protein of 1421 amino acids that has all the distinctive sequence motifs of human ALK (Figure 3), including the conserved kinase domain. Distal to the transmembrane domain are a glycine-rich region and domains found in secreted proteins and receptors, arranged in a distinctive MAM-LDLRA-MAM (meprins/A5 protein/PTPmu and low density lipoprotein receptor type A [28, 29]) pattern. SCD-2 and human ALK share overall amino acid sequence identity of 23% and similarity of 35%. In the kinase domain, the identity is 47% and the similarity is 61% (Figure S1).
We sequenced the ALK gene from the three available scd-2 alleles and identified lesions for all three, proving that scd-2 encodes ALK. scd-2(sa935) and scd-2(sa249) contain G to A transitions resulting in G985R and G1174E substitutions, respectively. sa935 and sa249 replace highly conserved small glycine side chains in the kinase domain with bulky charged side chains; the glycine at position 985 is part of the ATP-binding motif and is conserved in all functional protein kinases, and the glycine at position 1174 is conserved in all ALK orthologs identified thus far and in most other tyrosine kinases. scd-2(sa303) contains a G to A transition in the splice-acceptor site for intron 2. Using a PCR-based methodology, we also isolated the deletion allele scd-2(y386) (see Supplemental Experimental Procedures). Another deletion allele, scd-2(ok565), was obtained from the C. elegans Gene Knockout Consortium. y386 deletes 1148 bp of T10H9.2, extending from the middle of intron 12 to the end of intron 15, and removes a glycine-rich region common to all ALK family members, but the subsequent reading frame is restored. ok565 deletes 2135 bp of T10H9.2, extending from exon 13 to exon 20, eliminating the glycinerich region, the transmembrane domain, and the kinase domain.
By phenotypic and/or molecular criteria, the scd-2 alleles sa249, sa935, y386, and ok565 are all candidate kinase null alleles. We used sa249 and y386 as canonical alleles for our genetic analyses because ok565 was obtained later, but all observed results were subsequently repeated with ok565 (Table 1, Figure S2 and Table S1). scd-2(sa303) causes a weaker phenotype than do the other alleles, and RT-PCR analysis showed that use of an in-frame cryptic splice acceptor in exon 3 restored some functional transcript and, presumably, some receptor function.
Table 1.
The scd-2 Pathway Regulates TGF-β Transcriptional Output
| Genotype | GFP Expression |
|---|---|
| cuIs5 | +++++ |
| daf-7(e1372); cuIs5 | + |
| daf-7(e1372); cuIs5; daf-3(e1376) | +++++ |
| daf-7(e1372); cuIs5; daf-12(m20) | + |
| daf-7(e1372); cuIs5; scd-2(sa249) | +++ |
| daf-7(e1372); cuIs5; scd-2(y386) | +++ |
| daf-7(e1372); cuIs5; scd-2(ok565) | +++ |
| daf-7(e1372); cuIs5; hen-1(tm501) | +++ |
| daf-7(e1372); cuIs5; hen-1(ut236) | +++ |
| daf-7(e1372); cuIs5; sma-5(n678) | +++ |
| daf-7(e1372); cuIs5; soc-1(n1789) | ++ |
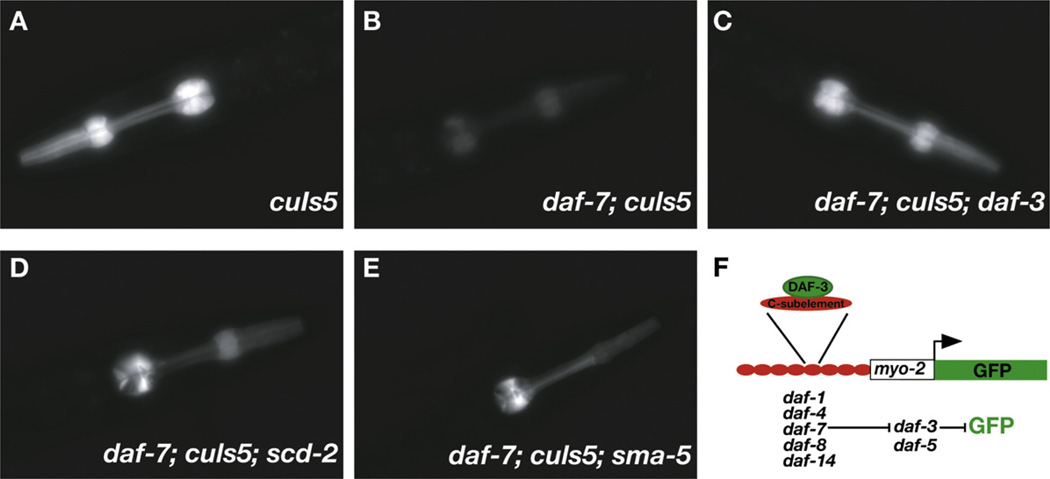
scd-2, hen-1, soc-1, and sma-5 regulate the activity of cuIs5, a reporter for the TGF-β branch of the dauer pathway. “+” represents barely visible pharyngeal GFP, whereas “+++++” represents wild-type cuIs5 levels (Figure 5). All experiments except those involving hen-1 and scd-2(ok565) were replicated with the independently isolated cuIs2 transgene. All gave the same results.
scd-2 Converges with or Acts in Parallel with the TGF-β Branch of the Dauer Pathway
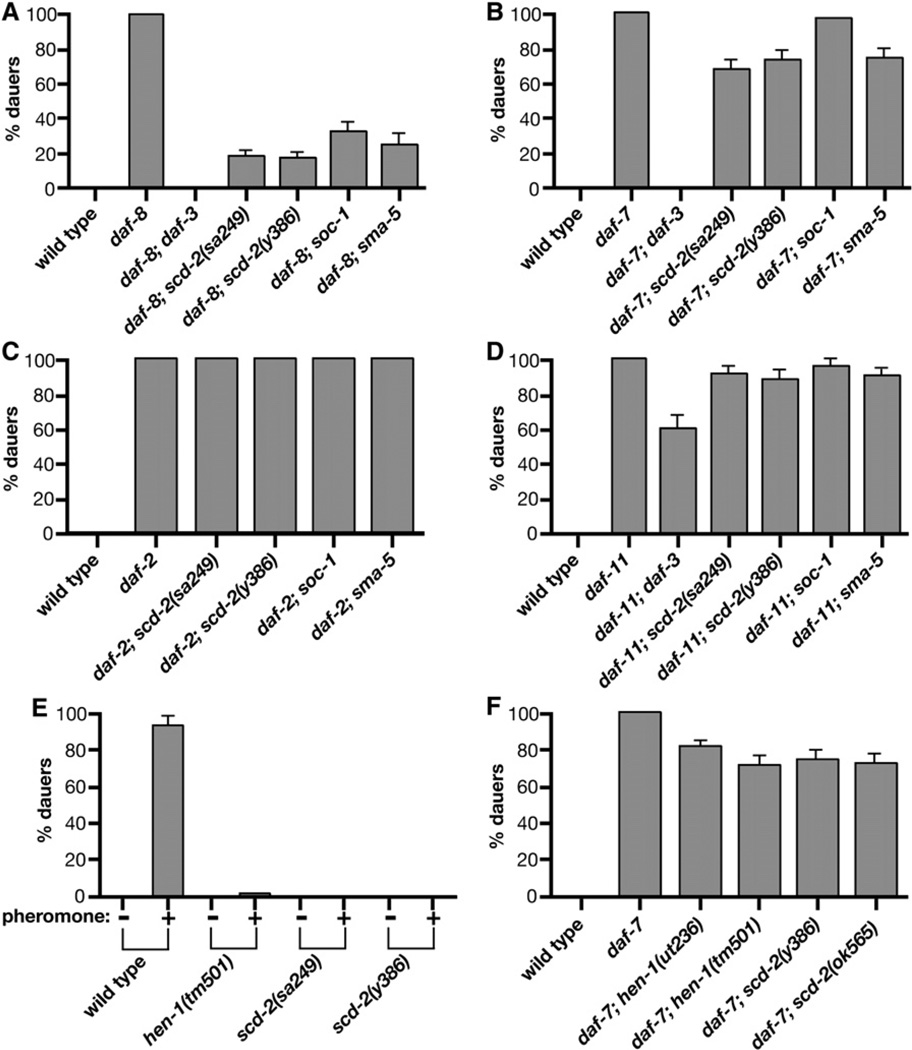
scd-2(sa249) was previously shown to suppress the Daf-c phenotypes caused by disruptions to the TGF-β branch of the dauer pathway [25]. These results suggested that scd-2 regulates dauer formation by interacting with the TGF-β pathway. To determine the placement of scd-2 more precisely in the dauer regulatory hierarchy, epistasis analyses were performed. We built double-mutant strains carrying scd-2(sa249), scd-2(y386), or scd-2(ok565) and one of the following selected Daf-c mutations: daf-7(e1372), daf-4(m63), or daf-8(e1393) in the ASI/TGF-β branch, daf-11(m47) in the ASJ dauer pheromone branch, and daf-2(e1370) in the insulin branch (Figure 4 and Figure S2). All of these Daf-c mutations are null or strong alleles, and all induce dauer formation robustly, except mutations in daf-8, which acts partially redundantly with the daf-14 Smad [30]. daf-8 mutants exhibit a moderate Daf-c phenotype, which provides a more sensitive background for analyzing pathway interactions.
Figure 4. Genes Encoding the SCD-2 Receptor Tyrosine Kinase and its Probable Ligand HEN-1, Adaptor SOC-1, and MAP Kinase SMA-5 Converge with the TGF-β Branch of the Dauer Pathway.
Assays measure dauer formation in double mutants defective in a Daf-c gene and a SCD-2 RTK pathway gene. All assays were at 25°C. Except for mutations in known Daf-c genes, none of the single mutants formed dauers at 25°C (data not shown). Error bars show the 95% confidence interval calculated based on sample size. (A–D) Dauer formation in double mutants carrying one of the following Daf-c mutations is shown: (A) daf-8(e1393) (B) daf-7(e1372) (C) daf-2(e1370), (D) daf-11(m47). scd-2(y386) and scd-2(sa249) also suppress daf-4(m63) (not shown). More than 200 animals were scored per genotype.
(E) Dauer pheromone response of wild-type, hen-1, and scd-2 animals. 32 µl of a dauer pheromone preparation was included in each plate. Typically, 100–200 animals were assayed for each pheromone concentration in each genotype.
(F) hen-1 has Daf-d phenotypes comparable to scd-2 and sma-5. scd-2(ok565) and mutations in hen-1 weakly suppress daf-11 and have no effect on daf-2 (data not shown). hen-1(ut236), hen-1(tm501), and scd-2(ok565) also suppress daf-8 at levels comparable to other scd mutations (data not shown).
scd-2(sa249), scd-2(y386), and scd-2(ok565) weakly suppress the Daf-c phenotype caused by daf-7 or daf-4 (data not shown) and strongly, but incompletely, suppress daf-8. scd-2 mutations do not suppress daf-2 mutations, and they significantly, but very weakly, suppress daf-11, mutations (Figure 4 and Figure S2). These results resemble the epistatic interactions between daf-3/Smad or daf-5/sno/ski and daf-7, daf-8, daf-11, and daf-2, only the suppression by scd-2 is weaker [13]. In contrast, mutations in the downstream daf-12 nuclear hormone receptor gene completely suppress daf-7, daf-8, daf-11, and daf-2 12]. scd-2 interactions are, therefore, clearly distinguishable from those of daf-12.
The very weak suppression of daf-11 by scd-2 is consistent with the moderate suppression of daf-11 by daf-3 12]. In addition to its requirement in ASJ-branch signaling, the DAF-11 transmembrane guanylyl cyclase also plays a minor role in ASI-branch signaling [15]. Consequently, mutations that perturb TGF-β branch signaling can inhibit signaling mediated by ASI, but not by ASJ, resulting in partial suppression of the daf-11 Daf-c phenotype.
We derive two conclusions from these epistasis analyses. First, scd-2 interactions with daf-11 and daf-2 are similar to those of daf-3/Smad (Figure 4 and Figure S2) and daf-5/sno/ ski (not shown), suggesting that scd-2 acts at a similar point in the dauer-regulatory network as daf-3 and daf-5. Second, the partial suppression of TGF-β Daf-c mutants by scd-2 suggests that scd-2 functions in parallel to, or converges with, the TGF-β signal. Whereas daf-3 and daf-5 are absolutely required for transmission of the DAF-7/ TGF-β signal, scd-2 null alleles only moderately suppress the daf-7 mutant phenotype, suggesting that scd-2 modulates the TGF-β signal or functions in parallel with it.
The dauer TGF-β pathway regulates multiple processes unrelated to dauer formation. Daf-c TGF-β branch mutations confer Cpy (clumpy distribution on food), Egl (egg-laying defective), and Din (dark intestine) phenotypes in addition to their dauer constitutive phenotype. The Cpy, Egl, and Din phenotypes are completely suppressed by strong alleles of daf-3 or daf-5, but Daf-d mutations in further-downstream genes like daf-12 suppress only the Daf-c phenotype [13]. We found that all five scd-2 mutations suppress the daf-7/TGF-β Daf-c phenotype but fail to suppress the Cpy, Egl, and Din phenotypes (data not shown). These observations indicate that scd-2 interacts with the TGF-β branch specifically in dauer formation and not in other processes.
scd-2 Modulates DAF-3 Activity
To test whether scd-2 converges with the TGF-β branch of the dauer pathway, we used two integrated GFP reporter transgenes, cuIs2 and cuIs5, which directly reflect the transcriptional output of the TGF-β pathway by virtue of their response to the DAF-3/Smad transcription factor [31]. In these reporters, the gfp gene is fused to the minimal myo-2 promoter, which drives transcription exclusively in pharyngeal muscle. Upstream of the minimal promoter are eight tandem copies of the C183 portion of the C subelement, a DNA sequence that is specifically recognized and bound by the DAF-3 protein in vitro. The C183 repeats confer DAF-3-mediated transcriptional repression on the reporters. In wild-type animals carrying either the cuIs2 or cuIs5 integrated reporter transgene, pharyngeal GFP expression is high because no nuclear DAF-3 activity is available to repress it. However, in TGF-β Daf-c mutants (e.g. daf-7 or daf-8), in which DAF-3 is de-repressed and active in the nucleus, GFP expression is greatly reduced. This reduction in GFP level is suppressed by mutations in daf-3 31]. Although it is unclear whether the single copy of the C subelement present in the endogenous myo-2 gene renders it a target of DAF-3 [32], the C subelement reporter transgenes function as accurate monitors of TGF-β-signaling activity in the dauer pathway.
We used both cuIs2 and cuIs5 reporters to assess DAF-3 transcriptional activity in different mutant backgrounds (Figure 5, Table 1, and Table S1). Although mutations in the TGF-β-pathway components strongly reduced GFP expression from both C subelement reporter transgenes, the specificity for the TGF-β branch had not been further validated by a test of the effects of the daf-2, daf-11, and daf-12 branches on the reporters’ expression. We found that the daf-2(e1370) mutation, which acts downstream of the TGF-β signal, did not repress cuIs2 and cuIs5 GFP expression. daf-11(m47) weakly repressed GFP expression in a daf-3-dependent manner, consistent with the weak role of daf-11 in the ASI TGF-β branch. A mutation in the downstream daf-12 steroid hormone receptor suppressed the Daf-c phenotype of daf-7 but did not alter daf-7 repression of GFP expression. These results suggest that the reporters reflect TGF-β-pathway output.
Figure 5. The TGF-β and scd-2 Branches Regulate Levels of GFP Expression from cuIs5, a Reporter of DAF-3 Transcriptional Activity.
The images show animals of different genotypes bearing the transgenic cuIs5 GFP reporter. (A) cuIs5, (B) daf-7; cuIs5, (C) daf-7; cuIs5; daf-3, (D) daf-7; cuIs5; scd-2, (E) daf-7; cuIs5; sma-5.
(F) A schematic of the reporter transgene.
We found that three different scd-2 alleles partially restore GFP expression in daf-7 or daf-8 mutant backgrounds, consistent with scd-2 modulation of the TGF-β pathway (Figure 5, Table 1, and Table S1). The incomplete restoration of GFP expression is consistent with the incomplete suppression by scd-2 of the daf-8 and daf-7 Daf-c phenotypes.
We conclude that scd-2 signaling directly regulates the activity of the TGF-β branch somewhere between the DAF-8/ DAF-14 Smads and the DAF-3/DAF-5 transcriptional complex. The fact that scd-2 encodes a receptor suggests that it is not simply a cytoplasmic or nuclear modifier of TGF-β signaling but, rather, it defines a novel signaling branch, with ligand and signal-transduction components, which converges and is integrated with the TGF-β branch to enhance DAF-3 activity.
Identification of the Ligand, Adaptor, and MAP Kinase for the SCD-2 Receptor Pathway
We reasoned that analysis of mutations in known C. elegans RTK-signaling components could identify additional members of the scd-2 RTK branch. Of thirteen genes tested (Figure S2), only three caused a Scd phenotype: moderate suppression of daf-7, strong suppression of daf-8, weak suppression of daf-11, no suppression of daf-2, and restoration of cuIs5 and/or cuIs2 pharyngeal GFP expression in a daf-7 or daf-8 background.
The hen-1 gene encodes a C. elegans ortholog of the ALK ligand [33–35], a putative secreted protein with an LDLRA motif that resembles the LDLRA motif in the SCD-2 extracellular region. We found that two mutant alleles of hen-1 confer dauer-defective phenotypes and show suppression patterns comparable to those of scd-2 mutations (Figure 4F; data not shown). Like scd-2 mutations, hen-1 mutations confer resistance to exogenously added dauer pheromone (Figure 4E) and restore cuIs5 GFP expression in daf-7 and daf-8 backgrounds (Table 1, Table S1).
Two mutations in soc-1, which encodes an RTK multi-adaptor protein related to DOS/Gab [36], caused a Scd phenotype, but one weaker than those caused by scd-2 mutations (Figures 4A–4D). soc-1 mutations also restored cuIs2 and cuIs5 GFP-expression levels in a daf-7 mutant background, but more weakly than did scd-2 mutations (Table 1). The weaker soc-1 Scd phenotype suggests that SOC-1 might not be the sole adaptor for SCD-2 RTK signaling.
A mutation in sma-5, which encodes the C. elegans protein most similar to human ERK5/MAP kinase 7 [37], also conferred dauer-defective phenotypes comparable in strength to scd-2 and hen-1 mutations (Figures 4A–4D). Like scd-2 and hen-1 mutations, the sma-5 mutation also restored cuIs2 and cuIs5 GFP expression (Figure 5E, Table 1, and Table S1).
Null mutations in two components of the same pathway would not be predicted to act synergistically. To test whether hen-1 and scd-2 function linearly or in parallel, we constructed the daf-7; scd-2; hen-1 triple mutant strain. As expected, we found that the degree of daf-7 suppression by scd-2(ok565); hen-1(tm501) together was comparable to the level of suppression by scd-2(ok565) and hen-1(tm501) separately (Figure S2F). These results suggest that hen-1, scd-2, soc-1, and sma-5 act in the same pathway and define a novel signaling branch that modulates the dauer TGF-β branch by activating the DAF-3 Smad transcription factor (Figure 6).
Figure 6. A Cellular and Molecular Model for Parallel Sensory Processing in Dauer Formation.
Environmental stimuli, cells, and proteins that promote dauer formation are shown in green, whereas those that inhibit dauer formation are in red. Arrows indicate a positive genetic interaction, and bars indicate an inhibitory genetic interaction. Dauer pheromone is known to promote the dauer developmental decision via signaling by the ASJ and ASI ciliated amphid sensory neurons (see Introduction). The activation of AIY by an additional sensory neuron is only one theoretical possibility. Alternatively, AIY could be activated by ASE or could be inhibited by another sensory neuron. Not shown in this model are the downstream DAF-2 insulin-receptor branch and the steroid hormone receptor DAF-12.
Constitutive scd-2 Activation Depresses Feeding Behavior
To further determine the function of the SCD-2 receptor, we constructed a constitutively active receptor that activates signaling independently of ligand activity. In wild-type RTKs, ligand binding stimulates dimerization of two RTK subunits, resulting in the transphosphorylation of individual receptor subunits and, hence, the activation of receptor signaling. Constitutive dimerization of an RTK can be achieved by replacing the normal receptor transmembrane (TM) domain with the mutant TM domain of the mammalian ErbB2/Her2/Neu RTK oncogene, referred to as neu*, which constitutively dimerizes, bypassing the need for ligand [38]. The SCD-2 TM domain was therefore replaced with the neu* TM domain, and the activated receptor was then expressed under the control of the daf-4 promoter.
The daf-4 promoter used for the scd-2(neu*) experiment is broadly expressed in most tissues, and the TGF-β-branch components have been shown to be broadly required for function in multiple tissues [30]. Given that the scd-2 branch modulates the TGF-β branch in these tissues and known scd-2::gfp expression patterns appear incomplete (see Discussion), we expressed the activated scd-2 in the same tissues as the those of the TGF-β receptor.
High expression of the constitutively activated SCD-2(neu*) receptor caused 100% of animals to arrest in the first larval stage (L1), with no overt anatomical defects. The arrested larvae behaved like wild-type L1 animals raised in the absence of food: they moved sluggishly, did not make feeding-related foraging movements, and failed to activate the pharyngeal pumping mechanism that ingests food. Lower expression of the constitutively activated SCD-2(neu*) receptor resulted in rare animals that grew beyond the L1 stage but developed slowly, were scrawny with pale intestines, and appeared malnourished like mutants defective in feeding [39]. The pharyngeal muscles and grinder of the pharynx appeared wild-type, as did the infrequent pharyngeal pumping, all suggesting that the feeding apparatus was capable of functioning normally. One interpretation of these results is that scd-2(neu*) negatively regulates feeding behavior, a topic amplified in the Discussion. However, it is unclear whether this effect reflects a wild-type function of SCD-2 or is the result of over-expression.
The early-arrest phenotype precluded assessment of the activated receptor’s effects on dauer formation. Activated SCD-2 might be expected to confer a dauer-constitutive phenotype; however, no dauers were among the rare animals that escaped L1 arrest. Thus, activation of the SCD-2 RTK branch might not be sufficient to induce dauer formation by itself but can only modulate TGF-β -branch activity. If so, scd-2(neu*) might act as an enhancer of TGF-β branch Daf-c mutations. Double-mutant combinations with scd-2(neu*) and TGF-β Daf-c mutations were subviable, preventing the testing of this idea. One interpretation is that the SCD-2 branch of the dauer pathway might transduce a food signal.
Discussion
We isolated a wild C. elegans strain from the desert and showed that it harbors a scd-2 mutation that prevents efficient entry into the dauer state. This finding suggests that loss of the SCD-2 sensory signal confers an adaptive advantage under harsh environmental conditions that would otherwise induce dauer formation. scd-2 encodes the C. elegans ortholog of the proto-oncogene anaplastic lymphoma kinase. We also identify hen-1, soc-1, and sma-5, which encode the ALK ligand, an adaptor protein, and a MAP kinase, respectively, as members of the scd-2 genetic pathway, a novel sensory input into the dauer-regulatory hierarchy. We used a GFP reporter specific for TGF-β pathway to show that scd-2 signaling is required for full transcriptional activity of DAF-3/Smad, a downstream TGF-β-pathway effector. These results show that the SCD-2 and TGF-β pathways converge and are integrated at the level of transcriptional regulation of DAF-3. Our study of scd-2 thus identifies a new pathway for chemosensory input into the dauer decision.
The SCD-2 RTK Defines a New Sensory Transduction Branch of the Dauer Pathway
An integrated view of scd-2 function emerges from disparate lines of evidence. The involvement of the HEN-1 ligand and the SCD-2 receptor in dauer formation implies the existence of a new branch in the dauer pathway. Three lines of evidence support this model. First, genetic analysis revealed the interaction between the SCD-2 RTK pathway and the previously known DAF-7/TGF-β branch. Second, analysis with the DAF-3-regulated GFP reporter indicates that the scd-2 branch controls DAF-3 transcriptional output. Third, the putative HEN-1 ligand is expressed in sensory neurons not previously implicated in dauer formation. We therefore propose that the scd-2 branch of the dauer pathway transduces a novel sensory input.
We propose that the HEN-1 LDLRA-motif-containing ligand is activated in response to a sensory stimulus, thus promoting SCD-2 RTK signaling. SCD-2 then signals via the SOC-1 adaptor protein to activate the SMA-5 ERK/MAP kinase. Ultimately, the information transduced by the SCD-2 branch is integrated with the information transduced by the DAF-7/TGF-β branch via promotion of the activity of the DAF-3 Smad transcription factor (Figure 1 and Figure 6).
The biological function of the C. elegans SCD-2 receptor is strikingly different from the function of the Drosophila DAlk receptor, which acts as a conventional growth-factor receptor to specify cell fate, but the molecular composition of the pathways in which these receptors function is similar. The ligand for DAlk is the LDLRA-domain-containing secreted protein Jeb, which is an ortholog of the putative SCD-2 ligand HEN-1. DAlk signals through aMAP kinase, which resembles the SMA-5 MAP kinase [33, 35, 40–42].
HEN-1 May Function as a Neuroendocrine Signal
HEN-1 expression was previously found in the ASE and AIY neurons in the head [34]. However, ectopic HEN-1 expression from amphid neurons AWB and AWC or relatively distant mechanosensory neurons is sufficient for rescue of the hen-1 mutant chemosensory defects [34], suggesting that HEN-1 functions nonautonomously, perhaps acting as an endocrine signal. To test the cellular basis for the HEN-1 signal, we genetically disrupted the function of the bilateral pair of ASE sensory neurons using mutations in che-1. Loss of the CHE-1 transcription factor is thought to specifically eliminate the function of the ASE sensory neurons [43, 44]. We showed that four different che-1 mutations suppress the Daf-c phenotype of daf-14(m77), confer resistance to exogenous dauer pheromone (Supplemental Results, Table S2), and weakly restore cuIs5 GFP levels in a daf-7 background. Though these results require additional validation, they suggest that the ASE gustatory neurons function as a partial source of HEN-1 ligand, perhaps in response to a food signal. The biological effects of the SCD-2 pathway are evident throughout the animal. For example, scd-2 regulates cuIs2 and cuIs5 reporter GFP expression in the pharynx and modulates the activity of downstream components of the dauer TGF-β signaling branch throughout the animal. However, HEN-1 expression is restricted to the ASE and AIY neurons in the head [34]. Therefore, HEN-1 might function analogously to DAF-7/TGF-β, whose expression is restricted to the ASI amphid sensory neurons that mediate pheromone response [9] but whose downstream effectors are broadly expressed and functionally required in many tissues of the animal [24, 30, 31].
This model predicts broad scd-2 expression, but thus far more limited expression of GFP driven by the scd-2 promoter has been observed with transcriptional and translational fusions (D.J.R. and B.J.M., unpublished data) [45, 46]. The limited expression pattern could reflect incomplete regulatory sequences in the reporter constructs or low levels of transgene expression. We note that scd-2 mutants affect reporter GFP expression in the pharynx, where scd-2 expression has not been reported. Furthermore, we have found a role for scd-2 in morphogenesis of embryonic epithelia and axons in the nervous system (D.J.R and B.J.M., unpublished results) that would also not have been predicted by the reporter expression pattern. The embryonic morphogenesis phenotypes occur at a time when no SCD-2::GFP expression has been observed. Therefore, it is likely that the scd-2 GFP fusions do not reproduce the full expression pattern of scd-2 and that scd-2 expression is broad.
Transcriptional Integration of Two Sensory Inputs into the Dauer Pathway
Our data, combined with previous work on TGF-β signaling and the dauer pathway, allow us to infer a mechanism by which two sensory inputs are integrated to form a single transcriptional output that regulates dauer formation. Using transcriptional GFP reporters as a read-out of the TGF-β-branch activity, we showed that SCD-2, acting through SMA-5/MAP kinase, promotes the activity of the DAF-3 transcription factor. Activation of DAF-3 by SMA-5 is unlikely to be direct, because DAF-3 lacks MAP kinase phosphorylation consensus sequences, and we found DAF-3 gel mobility to be unaltered when the SCD-2 branch was inactivated (data not shown). In contrast, DAF-5, a Sno/Ski homolog required for DAF-3 transcriptional activity [32], contains a MAP kinase consensus site, suggesting DAF-5 as a potential SMA-5 phosphorylation target for promotion of DAF-3 transcriptional activity. Alternatively, SMA-5 could regulate a different DAF-3 transcriptional coactivator or intermediate targets.
This model of SCD-2 RTK regulation of TGF-β signaling also explains why scd-2 mutants are pheromone resistant. Because daf-3 and daf-5 mutants are pheromone resistant [4], mutations that cause a failure to activate DAF-3 or DAF-5, like mutations in SCD-2-pathway components, would also be expected to cause pheromone resistance. Furthermore, this model suggests that scd-2 mutants could be pheromone resistant not because scd-2 itself transduces a pheromone signal but, rather, because mutations that disrupt scd-2 signaling prevent maximal activation of DAF-3 in response to pheromone.
SCD-2 and Different Sensory Modalities
Both pheromone and high temperature promote dauer formation, whereas the presence of food inhibits it. No other sensory input is known to modulate dauer formation [7]. In principle, the scd-2 branch could mediate any of these three inputs or an as-yet-undefined one.
scd-2 is unlikely to mediate a temperature signal. Although the AIY thermosensory interneuron expresses HEN-1 [34] and disruption of AIY function by a ttx-3 mutation perturbs the innate temperature sensitivity of recovery from the dauer state [11], mutations in the scd-2 branch do not perturb dauer pathway temperature sensitivity (Figure S2). These data argue against a primary role of hen-1/scd-2 signaling in transducing a thermal signal.
Either a pheromone or a food signal could account for our observed hen-1/scd-2-branch mutant phenotypes, but we favor a model in which SCD-2 mediates a food signal. In this model, a “high-food” state would inhibit scd-2 signaling, preventing activation of DAF-3 and antagonizing dauer formation. Four lines of evidence support this model. First, the constitutively active scd-2(neu*) causes L1 larval arrest, sluggish movement, little feeding and foraging behavior, and starved-appearing animals, consistent with a constitutive “no food” signal. Second, loss-of-function hen-1 and scd-2 mutants display no gross phenotypes in the presence of food, consistent with a “high food” signal. Third, scd-2 and hen-1 mutants, postulated to inappropriately signal a “high food” state, antagonize the dauer-constitutive phenotype of TGF-β-branch Daf-c mutations. Finally, previously described hen-1 chemosensory defects are also consistent with a “high food” signal.
Previous work examined chemosensory behavior of hen-1 mutants. In the absence of food, wild-type animals will cross an aversive Cu2+ barrier to reach a chemoattractive point of diacetyl, a perceived food source. Furthermore, wild-type animals will disperse in the absence of food, and well-fed animals respond robustly to associative-learning paradigms with starvation entrainment. In contrast, in the absence of food, hen-1 (hesitation) mutants fail to risk toxic Cu2+ to reach diacetyl, are defective in diacetyl chemotaxis at lower concentrations, and disperse poorly despite healthy locomotion. Furthermore, hen-1 mutant animals are partially defective in two types of chemosensory learning in which food starvation conditioning was applied [34]. Most of these are examples of integrative behaviors, in which the animals make decisions on the basis of multiple sensory inputs. Thus, the hen-1 mutant defects were interpreted as defects in integration of various sensory signals [34]. However, an alternative interpretation is that the hen-1 mutation disrupts a distinct sensory modality that also contributes to these behaviors, such as food. For example, an animal sensing a “high food” state would not risk an aversive barrier to chemotax toward a perceived food source.
Previous work on the function of ASE neurons is also consistent with the model that HEN-1 signals food availability. ASE neurons have been shown to respond to water-soluble attractants like Na+, Cl−, cAMP, and biotin but not to volatile odorants, suggesting that the ASEs are gustatory neurons, or “taste” neurons [47]. Our data argue that the two ASE sensory neurons compose a portion of the HEN-1-mediated signal (Supplemental Data). Therefore, the functions of the ASE neurons could shed light on the nature of the sensory cue mediated by the scd-2 branch. This branch might respond to water-soluble compounds, which could make up part of the food signal. Alternatively, the SCD-2 pathway could mediate pheromone response or another, as yet undiscovered, sensory input into dauer formation.
In conclusion, the HEN-1/SCD-2 pathway modulates diverse chemosensory processes, yet its loss can be tolerated under extreme environmental conditions. SCD-2 utilizes an RTK adaptor and MAP kinase in signaling and regulates the transcriptional output of the DAF-3 Smad. We implicate the ASE gustatory chemosensory neurons as one source of the HEN-1 signal, suggesting that the HEN-1/SCD-2 pathway mediates a novel chemosensory input into the dauer decision. This study provides significant insights into the sensation, transmission, and integration of sensory inputs, as well as their developmental and evolutionary impacts.
Experimental Procedures
Experimental procedures are included with the Supplemental Data.
Supplementary Material
Acknowledgments
We thank M. Koelle for advice on deletion libraries; N. Watanabe and Y. Ohshima for communicating results prior to publication; M. Hammarlund for information on the physical position of unc-70 prior to publication; J. Thatcher and P. Okkema for cuIs2 and cuIs5 strains; O. Hobert for che-1(ot27) and che-1(ot66);M. Stern for the NH#420 pNeu clone; Y. Kohara for cDNA clones; the C. elegans Gene Knockout Consortium at the Oklahoma Medical Research Foundation for isolating scd-2(ok565); B. Goldstein and C. J. Der for space and materials during the final stages of this project; C. Lutz, I. Carmi, G. Garriga, J. Kaplan, G. Patterson, and the Meyer and Garriga labs for helpful discussions; T. Harbaugh for endless mutagenesis; and the UC Berkeley worm community for assistance during deletion-library production. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. D.J.R. was a Damon Runyon Cancer Research Foundation Postdoctoral Fellow. M.A. was a Howard Hughes Medical Institute Predoctoral Fellow. This work was supported in part by NIH grant R01GM48700 to J.H.T. B.J.M. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental Data
Supplemental data include Supplemental Results and Experimental Procedures, two figures, and two tables and can be found with this article online at http://www.current-biology.com/cgi/content/full/18/15/1101/DC1/.
References
- 1.Bargmann CI, Kaplan JM. Signal transduction in the Caenorhabditis elegans nervous system. Annu. Rev. Neurosci. 1998;21:279–308. doi: 10.1146/annurev.neuro.21.1.279. [DOI] [PubMed] [Google Scholar]
- 2.Devaud JM. Experimental studies of adult Drosophila chemosensory behaviour. Behav. Processes. 2003;64:177–196. doi: 10.1016/s0376-6357(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 3.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 4.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 5.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 6.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 7.Riddle DL, Albert PS. Genetic and Environmental Regulation of Dauer Larva Development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Volume 1. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1997. pp. 739–768. [PubMed] [Google Scholar]
- 8.Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 9.Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JH, Inoue T. Methuselah meets diabetes. Bioessays. 1998;20:113–115. doi: 10.1002/(SICI)1521-1878(199802)20:2<113::AID-BIES3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 12.Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics. 137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKemy DD. Temperature sensing across species. Pflugers Arch. 2007;454:777–791. doi: 10.1007/s00424-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ailion M, Inoue T, Weaver CI, Holdcraft RW, Thomas JH. Neurosecretory control of aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1999;96:7394–7397. doi: 10.1073/pnas.96.13.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr. Biol. 2007;17:1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 23.Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue T, Thomas JH. Suppressors of transforming growth factor-beta pathway mutants in the Caenorhabditis elegans dauer formation pathway. Genetics. 2000;156:1035–1046. doi: 10.1093/genetics/156.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandrelli F, Tauber E, Pegoraro M, Mazzotta G, Cisotto P, Landskron J, Stanewsky R, Piccin A, Rosato E, Zordan M, et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science. 2007;316:1898–1900. doi: 10.1126/science.1138426. [DOI] [PubMed] [Google Scholar]
- 28.Beckmann G, Bork P. An adhesive domain detected in functionally diverse receptors. Trends Biochem. Sci. 1993;18:40–41. doi: 10.1016/0968-0004(93)90049-s. [DOI] [PubMed] [Google Scholar]
- 29.Fass D, Blacklow S, Kim PS, Berger JM. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Thomas JH. Targets of TGF-beta signaling in Caenorhabditis elegans dauer formation. Dev. Biol. 2000;217:192–204. doi: 10.1006/dbio.1999.9545. [DOI] [PubMed] [Google Scholar]
- 31.Thatcher JD, Haun C, Okkema PG. The DAF-3 Smad binds DNA and represses gene expression in the Caenorhabditis elegans pharynx. Development. 1999;126:97–107. doi: 10.1242/dev.126.1.97. [DOI] [PubMed] [Google Scholar]
- 32.da Graca LS, Zimmerman KK, Mitchell MC, Kozhan-Gorodetska M, Sekiewicz K, Morales Y, Patterson GI. DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF beta pathway to regulate C. elegans dauer development. Development. 2004;131:435–446. doi: 10.1242/dev.00922. [DOI] [PubMed] [Google Scholar]
- 33.Englund C, Loren CE, Grabbe C, Varshney GK, Deleuil F, Hallberg B, Palmer RH. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425:512–516. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell. 2002;109:639–649. doi: 10.1016/s0092-8674(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 35.Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425:507–512. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- 36.Schutzman JL, Borland CZ, Newman JC, Robinson MK, Kokel M, Stern MJ. The Caenorhabditis elegans EGL-15 signaling pathway implicates a DOS-like multisubstrate adaptor protein in fibroblast growth factor signal transduction. Mol. Cell. Biol. 2001;21:8104–8116. doi: 10.1128/MCB.21.23.8104-8116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe N, Nagamatsu Y, Gengyo-Ando K, Mitani S, Ohshima Y. Control of body size by SMA-5, a homolog of MAP kinase BMK1/ERK5, in C. elegans. Development. 2005;132:3175–3184. doi: 10.1242/dev.01895. [DOI] [PubMed] [Google Scholar]
- 38.Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- 39.Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loren CE, Scully A, Grabbe C, Edeen PT, Thomas J, McKeown M, Hunter T, Palmer RH. Identification and characterization of DAlk: a novel Drosophila melanogaster RTK which drives ERK activation in vivo. Genes Cells. 2001;6:531–544. doi: 10.1046/j.1365-2443.2001.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loren CE, Englund C, Grabbe C, Hallberg B, Hunter T, Palmer RH. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003;4:781–786. doi: 10.1038/sj.embor.embor897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss JB, Suyama KL, Lee HH, Scott MP. Jelly belly: a Drosophila LDL receptor repeat-containing signal required for mesoderm migration and differentiation. Cell. 2001;107:387–398. doi: 10.1016/s0092-8674(01)00540-2. [DOI] [PubMed] [Google Scholar]
- 43.Uchida O, Nakano H, Koga M, Ohshima Y. The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development. 2003;130:1215–1224. doi: 10.1242/dev.00341. [DOI] [PubMed] [Google Scholar]
- 44.Chang S, Johnston RJ, Jr, Hobert O. A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev. 2003;17:2123–2137. doi: 10.1101/gad.1117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao EH, Hung W, Abrams B, Zhen M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 2004;430:345–350. doi: 10.1038/nature02647. [DOI] [PubMed] [Google Scholar]
- 46.Hunt-Newbury R, Viveiros R, Johnsen R, Mah A, Anastas D, Fang L, Halfnight E, Lee D, Lin J, Lorch A, et al. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 2007;5:e237. doi: 10.1371/journal.pbio.0050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.