Abstract
Paraplegia was reported after occlusion of the segmental vessels during anterior spinal surgery. The aim of this study was to investigate the effect of occlusion of the segmental vessels on the somatosensory-evoked potential (SEP) monitoring and analyze its potential risk for cord ischemia. Thirty-one patients with thoracic scoliosis underwent anterior spinal surgery. T5–T11 segmental vessels on the convexity were occluded with microvascular clamps at the point 2 cm from the intravertebra foramen. The SEPs were recorded 5 min before occlusion and 2, 7, 12 and 17 min after occlusion. The SEPs were analyzed with two indices i.e. P40 latency and P40 amplitude. All SEP waveforms recorded during the test were regular and recognizable. Compared to 5 min before occlusion, the P40 latencies at 2 min and 7 min after occlusion significantly increased 3.39% and 2.76% on an average, the P40 amplitudes at 2 min after occlusion significantly declined 26% (peak to peak) or 22% (peak to baseline) on an average (P<0.05). But the changes of SEPs were temporary. The SEPs began to restore at 12 min after occlusion and returned to the pre-occlusion level at 17 min after occlusion. No neurologic complications occurred in all patients after surgery. These results suggest that SEP is a possible indicator for ischemia of the spinal cord which is a dynamic course and cannot be considered an “all-or–none” phenomenon. Without the factors such as developmental deformities of the spinal cord, vascular variation and potential cord ischemia, occlusion of the segmental vessels would be safe during the anterior spinal surgery.
Keywords: Ischemia, Occlusion, Segmental vessel, Somatosensory-evoked potential, Spinal cord
Introduction
A survey conducted in 1994 by the Scoliosis Research Society (SRS) showed a 0.72% acute neurologic complication rate in scoliosis surgery [16]. With the recent development of instrumented spinal surgery and the growing enthusiasm for its use, more and more neurologic complications may occur [4, 18, 19]. Since ischemia of the spinal cord is universally acknowledged as the etiology of these neurologic complications, intraoperative techniques are directed toward reducing the severity and duration of cord ischemia, increasing the tolerance of the cord to reduced oxygen tensions and early detecting of the spinal cord ischemia.
Segmental vessel system is the important blood supply network for the spinal cord. As for severe and rigid scoliosis, some procedures such as anterior release, osteotomy epiphysiodesis or strut fusion should be done before posterior correction surgery [14, 21]. In these procedures the segmental vessels should be occluded for exposure of the anterior spine. Ever since anterior spine surgery was done in large numbers in the early 1960s, there has been anxiety on the part of surgeons that ligating the segmental vessels might result in neurologic complication or bring about potential neurologic risk. Apel et al. [1] reported that spinal cord ischemia secondary to occlusion of the segmental vessels led to three paraplegia during anterior spinal surgery. For the early detection of spinal cord ischemia, some forms of spinal cord monitoring present. The somatosensory-evoked potential (SEP) is the electrical response of the brain to an applied somatosensory stimulus. This type of monitoring is one of the most popular clinical tools to identify impairment of the spinal cord during spinal surgery. Nuwer et al. [16, 17] reported that spinal cord disfunction caused by stretching or interruption of blood flow could be detected with SEP monitoring during scoliosis correction surgery with low false-positive and false-negative rates. Gharagozloo et al. [8] reported that arterial spinal cord ischemia after cross-clamping of the aorta during thoacoabdominal aortic aneurysm repair also could be detected with SEP monitoring. A technique of temporary occlusion of segmental vessels with concomitant SEP monitoring has been described in an attempt to intraoperatively identify vessels critical to the blood supply of the spinal cord [1].
In this study, we used SEPs to monitor the spinal cord conductive function. Our aim was to investigate the effect of occlusion of the segmental vessels on the SEP monitoring and analyse its potential risk for the cord ischemia.
Materials and methods
Thirty-one patients with thoracic scoliosis underwent anterior spinal surgery, including 25 anterior release, 3 epiphysiodesis and 3 strut fusion. 12 patients were male and 19 female. The average age was 15.7 years (range 13–23 years). The preoperative Cobb angle averaged 86.8°(range 80°–114°). There were 21 patients with idiopathic scoliosis, 9 patients with congenital scoliosis and one patient with Scheuermann’s disease associated with scoliosis. Preoperative clinical examinations of the nerve system and SEPs of every patient were normal. No spinal cord malformation or intra-canal vessels malformation was found in preoperative MRI in any patient. All patients were put under general anesthesia. The patients were placed in a lateral decubitus position with the convex portion of the spine facing up. The thoracic curves of all patients were convex to the right. So the patients were placed with the right side of the spine facing up. After the routine thoracotomy, the parietal pleura overlying the spine was divided longitudinally and then bluntly stripped from the spine. The segmental vessels were divided. T5–T11 segmental vessels on the convexity were occluded temporarily with microvascular clamps at the point 2 cm from the intra-vertebra foramen. The SEPs were recorded 5 min before occlusion and 2, 7, 12 and 17 min after occlusion. The blood pressure, temperature, SaO2 and the depth of anesthesia were kept stable during the course of monitoring.
SEP Monitoring: The posterior tibial nerve was stimulated by the subdermal needle electrodes inserted 2 cm apart at the right ankle. The stimulation current ranged from 10 to 30 mA and was kept constant once selected for a particular patient. The norm current density was adjusted to produce a small movement of the toes. Single-pulse stimulation with a frequency of 3.1 Hz and a duration of 200 μs was applied. The SEP signals were collected over Cz′ versus Fpz of the 10–20 international system. Standard adhesive gel Ag/Ag electrodes were used for Fpz recordings, and Ag/Ag chloride cups were used for Cz′ (2 cm behind Cz) recordings. The recorded signals were amplified and filtered between 5 Hz and 500 Hz. The responses to 200 stimuli were averaged.
In this study, the SEPs were analysed with two indices: (1) P40 latency. The latency was the delay from stimulation to the onset of the first peak. (2) P40 amplitude. The amplitudes were measured from peak to peak (P40-N50) and peak to baseline (P40-baseline).
The values of SEP latencies and amplitudes at 2, 7, 12 and 17 min after clamping were compared with that of 5 min before clamping by paired student t-test. The SEP latencies and amplitudes were expressed in terms of the mean value and standard deviation (SD). A significance level of P<0.05 was selected. A decrease in SEP amplitude of more than 50% or an increase in latency of more than 10% was considered to be the warning criteria for giving up clamping of the segmental vessels.
Results
The SEP waveforms of 31 patients recorded during the test were regular and recognizable.
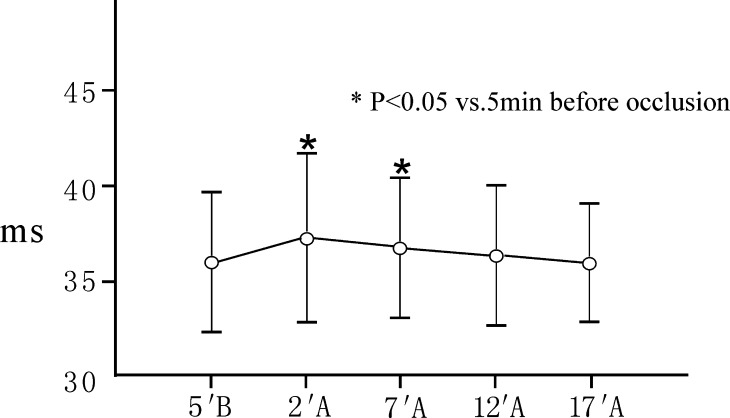
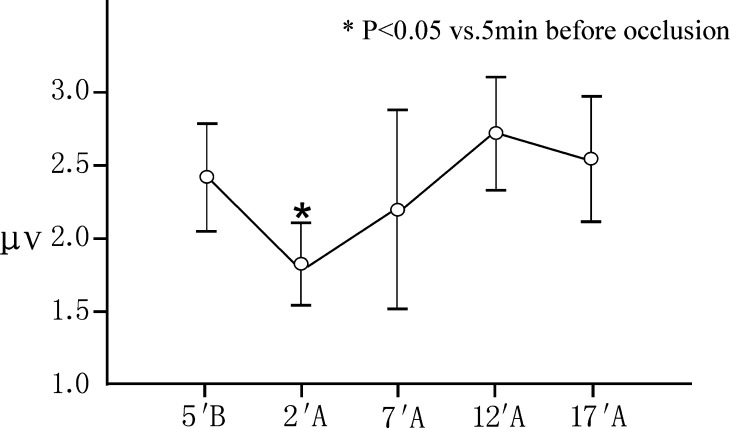
The values of the latencies and amplitudes of P40 at 5 min before occlusion and 2, 7, 12 and 17 min after occlusion are shown in Table 1. Compared to 5 min before occlusion, the SEP changed significantly within 7 min after occlusion of the segmental vessels, especially within 2 min after occlusion. The P40 latencies at 2 min and 7 min after occlusion significantly increased 1.22 ms (3.39%) and 0.82 ms (2.76%) on an average, the P40 amplitudes at 2 min after occlusion significantly declined 0.64 μV (26%) (peak to peak) or 0.28 μV (22%) (peak to baseline) on average (P<0.05). But the changes of SEPs were temporary. The SEP began to restore at 12 min after occlusion and returned to the pre-occlusion level at 17 min after occlusion. The P40 latencies and amplitudes at 5 min before occlusion and 12, 17 min after occlusion showed no significant difference (P>0.05). All changes in SEP latencies and amplitudes were within the applied 10/50% alarm levels (Figs. 1, 2, 3).
Table 1.
Values of P40 latencies and P40 amplitudes (peak to peak and peak to baseline) before and after occlusion of the segmental vessels(mean±standard deviation, n=31)
| P40 latency (ms) | P40 amplitude (μv) (peak to peak) | P40 amplitude (μv) (peak to baseline) | |
|---|---|---|---|
| 5 min before occlusion | 35.89±3.90 | 2.45±0.31 | 1.29±0.52 |
| 2 min after occlusion | 37.10±4.71 | 1.81±0.24 | 1.01±0.65 |
| 7 min after occlusion | 36.71±3.80 | 2.17±0.67 | 1.32±0.56 |
| 12 min after occlusion | 36.01±3.84 | 2.71±0.33 | 1.41±0.62 |
| 17 min after occlusion | 35.93±3.33 | 2.54±0.40 | 1.35±0.58 |
Fig. 1.
Changes in P40 latencies after occlusion of the segmental vessels(mean±standard deviation, n=31). A, after occlusion; B, before occlusion. Contrasted to 5 min before occlusion, the P40 latencies at 2 min and 7 min after occlusion significantly prolonged 1.22 ms (3.39%) and 0.82 ms (2.76%) on average (P<0.05). The P40 latencies at 5 min before occlusion and 12, 17 min after occlusion showed no significant difference (P>0.05)
Fig. 2.
Changes in P40 amplitudes (peak to peak) after occlusion of the segmental vessels(mean±standard deviation, n=31). A, after occlusion; B, before occlusion. Contrasted to 5 min before occlusion, the P40 amplitudes at 2 min after occlusion significantly declined 0.64 μV (26%) on average (P<0.05). But the P40 amplitudes at 5 min before occlusion and 7, 12 and 17 min after occlusion showed no significant difference (P>0.05)
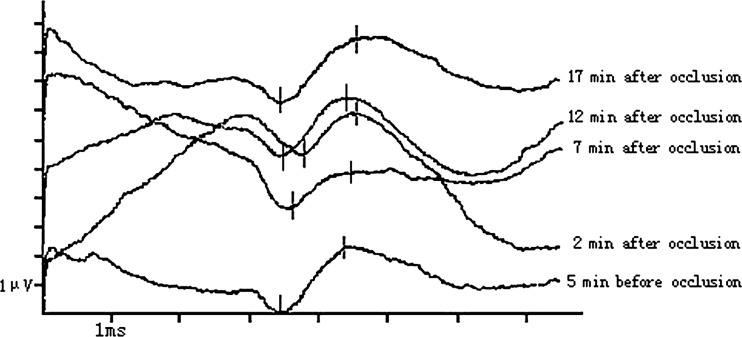
Fig. 3.
Case 1. Male, 15 years old, adolescent idiopathic scoliosis. Anterior spinal release was performed with SEP monitoring. All SEP waveforms were regular and recognizable. Contrasted to the P40 amplitude (peak to peak) at 5 min before occlusion (2.21 μV), the P40 amplitude at 2 min and 7 min after occlusion declined 0.76 μV (34%) and 0.78 μV (35%), respectively. But the changes of SEPs were temporary. The P40 amplitude began to restore at 12 min after occlusion (2.08 μV) and returned to the pre-occlusion level at 17 min after occlusion (2.28 μV). No neurologic complications occurred after surgery
All segmental vessels occluded temporarily with microvascular clamps were ligated and cut off at the end of the study. All patients were operated successfully with the anterior spinal surgery and no neurologic complications occurred.
Discussion
There are three arterial areas in the spinal cord distinguished by the number of radicular spinal branches and predominance of collateral circulation [12]. They are superior (cervicothoracic) area, intermediate (mid-thoracic) area and lower (thoracolumbar) area. The mid-thoracic area of the spinal cord has the least abundant blood supply [5]. The anterior spinal artery in the mid-thoracic region is supplied by single radicular vessel. Perimedullary anastomoses between the anterior spinal artery and the two posterior spinal arteries are numerous in the superior and lower area, but sparse in the intermediate region of the spinal cord. Central arteries penetrating into the gray and white matter of the spinal cord are typically smaller and fewer in number in the mid-thoracic region compared with the cervical and thoracolumbar regions [12]. The spinal cord varies in width and shape throughout its length. It is consistently narrowest in mid-thoracic region which extends from T4 down to T8. The narrow zone of the spinal canal corresponds almost exactly with that part of the cord to which the blood supply is least profuse. So Dommisse [5] termed the mid-thoracic region “the critical zone of the spinal cord”.
Theoretically, occluding segmental vessels may have the potential risk of reducing the blood supply to the spinal cord and causing the cord ischemia, especially in the mid-thoracic region. Winter et al. [20] reviewed 1197 consecutive anterior procedures. Approximately 6000 segmental vessels were ligated. No patient developed a paraplegia as a result of the segmental vessel ligation. Dommisse [5] reported that the segmental arteries divided into numerous branches at the intervertebral foramen and formed an anastomotic network in the loose connective tissue of the extra-dural space. The rich anastomotic channels offered “alternative pathways” for the arterial flow and served to maintain the flow under conditions of stress. It was owing to their presence that the spinal cord circulation was preserved after occlusion of the segmental vessels. Bassett et al. [2] reported that 84 segmental arteries were temporarily occluded in 15 patients and no SEP changes occurred. He thought that sufficient collateral circulation could be the reason for the lack of SEP changes. The collateral supply way included anastomoses at the same foraminal level distal to the ligated segmental artery or by radicular arteries originating from adjacent segmental vessels. But three paraplegias were noted during anterior approaches for congenital kyphoscoliosis by Apel et al[1]. They set up a prospective study using SEP monitoring with temporary occlusion of the segmental vessels and noted seven additional cases out of the 41 so monitored. In this study we found that the SEPs changed significantly within 7 min after occlusion of the segmental vessels, especially within 2 min after occlusion. But the effect of occlusion of the segmental vessels on the cord conductive function was temporary. The SEPs began to restore at 12 min after occlusion and returned to the pre-occlusion level at 17 min after occlusion. So we think that the spinal cord ischemia is a dynamic course and cannot be considered an “all-or-none” phenomenon. In this study, occlusion of the segmental vessels might cause transient ischemia of the spinal cord. Temporary changes in SEP amplitude and latency did occur. But with the compensation of the collateral blood circulation the cord blood supply could quickly restore before occurrence of irreversible damage of the spinal cord. So the SEPs could quickly return to normal and no paraplegia occurred after surgery.
SEP monitoring has been used for 30 years [15]. It offers the promise of being able to avert neurological compromise by the early detection of abnormal signal transmission from distal extremities to the cerebral cortex [7]. But SEP is considered to only reflect conduction of sensory information in the posterior column, which is not as sensitive to the ischemia processes as the anterior pathway [11]. A survey by the Scoliosis Research Society showed the overall false-negative rate to be 0.127% and the overall false-positive rate to be 10 times higher at 1.51% for SEP monitoring [16]. A large prospective study showed that in aortic aneurysm surgery SEP monitoring had high false-negative (13%) and false-positive (67%) rates, and could not significantly improve neurologic outcome [3]. Dong et al. [6] reported that compared with SEP, motor evoked potential (MEP), especially myogenic MEP, was more sensitive and specific in the detection of spinal cord ischemia in aortic aneurysm surgery. Lips et al. [13] reported that intraoperative absence of tcMEP signals for more than 1 h consistently resulted in paraplegia and extensive spinal cord infarction 72 h later, while spinal cord ischemia followed by prompt restoration of tcMEP signals corresponded with normal histopathology and normal motor function. So they concluded that intraoperative tcMEPs had a good prognostic value for neurologic outcome during procedures in which the spinal cord was at risk for ischemia. Though SEP is still the most convenient and economical method for intraoperative spinal cord monitoring [9, 10], we have to admit that MEP would have been a better choice. In this study, recovery of SEPs after transient changes corresponded with normal postoperative neurologic function. Because we had no patient with postoperative neurologic dysfunction we could not study the SEP change pattern of this kind of patients. So we only could infer that SEP is a possible indicator for the cord ischemia. As a general rule in SEP monitoring, a 50% reduction in amplitude or a 10% increase in latency is considered as significant for monitoring [1, 6, 10]. According to this rule, all SEP changes in this study are not significant. It is supportive of our result that no spinal cord injury occurred after surgery. Because we wanted to study the subtle change pattern of SEPs after clamping of the segmental vessels, we did not use this general rule when we did statistical analysis. In this study, a 50% reduction in SEP amplitude or a 10% increase in latency was considered to be the warning criteria for giving up occlusion. We think this criteria means that cord ischemia is so severe that some measures should be taken. Amplitude changes less than 50% or latency changes less than 10% does not mean that there is no cord ischemia. It means that perhaps cord ischemia already occurred, but was too slight to cause irreversible damage and should not be interfered.
Winter et al. [20] believed there would appear to be virtually no risk to segmental vessel ligation provided: (1) vessel ligation is unilateral, (2) done on the convexity of a scoliosis, (3) ligated at mid-vertebral body level, and (4) hypotensive anesthesia is avoided. Kostuik [20] thought the segmental vessels should not be divided without appropriate assessment during surgery, particularly in patients whose cords were at risk. He thought the cord at risk was in patients who had had multiple surgeries, kyphotic patients, patients with evidence of neurologic problems before surgery and patients whose cords were compressed with effacement of cerebral spinal fluid. We think that without the factors such as developmental deformities of the spinal cord, vascular variation and potential cord ischemia, occlusion of the segmental vessels would be safe during the anterior spinal surgery. But the morbidity of the cord ischemic injury could increase greatly in several cases: (1) Congenital scoliosis associated with vascular deformity or developmental deformities of the spinal cord; (2) Segmental vessels need to be occluded in many segments; (3) Severe kyphotic deformity. Many segmental vessels concentrate into the region of the top vertebra of angular kyphotic deformity. When stretched much, the spinal cord is in the condition of potential ischemia; (4) Patients with neurologic injury such as rotational dislocation in congenital scoliosis and kyphosis associated with incomplete paraplegia; (5) Hypotension, low temperature and low blood volume during surgery. For these patients whose cords are at risk we can temporarily clamp the segmental vessels and monitor the SEPs for 17 min. Clamping of the segmental vessels should be given up immediately if a decrease in SEP amplitude of more than 50% or an increase in latency of more than 10% occurred during monitoring.
Conclusions
The results of this study suggest that SEP is a possible indicator for ischemia of the spinal cord which is a dynamic course and can not be considered an “all-or-none” phenomenon. Occlusion of the segmental vessels may cause transient ischemia of the spinal cord. But the cord’s blood supply can quickly restore with the compensation of the collateral blood circulation. Without factors such as developmental deformities of the spinal cord, vascular variation and potential cord ischemia, occlusion of the segmental vessels would be safe during the anterior spinal surgery.
References
- 1.Apel D, Marrero G, King J, Tolo VT, Bassett GS. Avoiding paraplegia during anterior spinal surgery: the roel of SSEP monitoring with temporary occlusion of segmental spinal arteries. Spine. 1991;16:S365–370. [PubMed] [Google Scholar]
- 2.Bassett G, Johnson C, Stanley P. Comparison of preoperative selective spinal angiography and somatosensory-evoked potential monitoring with temporary occlusion of segmental vessels during anterior spinal surgrey. Spine. 1996;21:1996–2000. doi: 10.1097/00007632-199609010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Crawford ES, Mizrahi EM, Hess KR, Cosell JS, Safi HJ, Patel VM. The impact of distal aortic perfusion and somatosensory evoked potential monitoring on prevention of paraplegia after aortic aneurysm operation. J Thorac Cardiovasc Surg. 1988;94:275–285. [PubMed] [Google Scholar]
- 4.Dawson EG, Sherman JE, Kanim LE, Nuwer MR. Spinal cord monitoring: results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine. 1991;16:S361–S364. [PubMed] [Google Scholar]
- 5.Dommisse GF. The blood supply of the spinal cord. J Bone Joint Surg Br. 1974;56:225–235. [PubMed] [Google Scholar]
- 6.Dong CC, Macdonald DB, Janusz MT. Intraoperative spinal cord monitoring during descending thoracic and thoracoabdominal aneurysm surgery. Ann Thorac Surg. 2002;74:S1873–S1876. doi: 10.1016/S0003-4975(02)04137-1. [DOI] [PubMed] [Google Scholar]
- 7.Galla JD, Ergin MA, Lansman SL, McCullough JN, Nguyen KH, Spielvogel D, Klein JJ, Griepp RB. Use of somatosensory evoked potentials for thoracic and thoracoabdominal aortic resections. Ann Thorac Surg. 1999;67:1947–1952. doi: 10.1016/S0003-4975(99)00444-0. [DOI] [PubMed] [Google Scholar]
- 8.Gharagozloo F, Neville RF, Cox JC. Spinal cord protection during surgical procedure on the descending thoracic and thoracoabdominal aorta: a critical overview. Sem Thoracic Cardiovasc Surg. 1998;10:73–86. doi: 10.1016/s1043-0679(98)70022-x. [DOI] [PubMed] [Google Scholar]
- 9.Guerit JM, Dion RA. State-of-the-act of neuromonitoring for prevention of immediate and delayed paraplegia in thoracic and thoracoabdominal aorta surgery. Ann Thorac Surg. 2002;74:S1867–S1869. doi: 10.1016/S0003-4975(02)04130-9. [DOI] [PubMed] [Google Scholar]
- 10.Guerit JM, Witdoeckt C, Rubay J, Matta A, Dion R. The usefulness of the spinal and subcortical components of the posterior tibial nerve SEPs for spinal cord monitoring during aortic coarctation repair. Electroencephalogr Clin Neurophysiol. 1997;104:115–121. doi: 10.1016/S0168-5597(97)96661-2. [DOI] [PubMed] [Google Scholar]
- 11.Janusz MT, Qayumi AK, Jamieson WR, Fairholm DJ. Experimental use of somatosensory evoked potential for intraoperative identification of spinal cord blood supply. J Invest Surg. 1997;10:195–203. doi: 10.3109/08941939709032158. [DOI] [PubMed] [Google Scholar]
- 12.Lazorthes G, Gouaze A, Zadeh JO, Santini JJ, Lazorthes Y, Burdin P. Arterial vascularization of the spinal cord. J Neurosurg. 1971;35:253–262. doi: 10.3171/jns.1971.35.3.0253. [DOI] [PubMed] [Google Scholar]
- 13.Lips J, Haan PD, Jager SW, Vanicky I, Jacobs MJ, Kalkman CJ. The role of transcranial motor evoked potentials in predicting neurologic and histopathologic outcome after experimental spinal cord ischemia. Anesthesiology. 2002;97:183–191. doi: 10.1097/00000542-200207000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Master MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis: a study of one hundred and twelve patients. J Bone Joint Surg Am. 1999;81:1367–1383. doi: 10.2106/00004623-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Nash CL, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop. 1977;126:100–105. [PubMed] [Google Scholar]
- 16.Nuwer MR. Spinal cord monitoring with somatosensory techniques. J Clin Neurophysiol. 1998;15:183–193. doi: 10.1097/00004691-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. SEP spinal cord monitoring reduces neurologic deficits after scoliosis surgery:Results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-D. [DOI] [PubMed] [Google Scholar]
- 18.Rittmeister M, Leyendecker K, Kurth A, Schmitt E. Cauda equina compression due to a laminar hook: a late complication of posterior instrumentation in scoliosis surgery. Eur Spine J. 1999;8:417–420. doi: 10.1007/s005860050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilber RG, Thompson GH, Shaffer JW, Brown RH, Nash CL. Postoperative neurological deficits in segmental spinal instrumentation: a study using spinal cord monitoring. J Bone Joint Surg Am. 1984;66:1178–1187. [PubMed] [Google Scholar]
- 20.Winter RB, Lonstein JE, Denis F, Leonard AS, Garamella JJ. Paraplegia resulting from vessel ligation. Spine. 1996;21:1232–1234. doi: 10.1097/00007632-199605150-00017. [DOI] [PubMed] [Google Scholar]
- 21.Zeller RD, Dubousset J. Progressive rotational dislocation in kyphoscoliotic deformities: presentation and treatment. Spine. 2000;25:1092–1097. doi: 10.1097/00007632-200005010-00009. [DOI] [PubMed] [Google Scholar]