Abstract
In an effort to identify the enzymatic mechanism responsible for the synthesis of reactive oxygen species produced during the hypersensitive response, preparations of rose (Rosa damascena) cell plasma membranes, partially solubilized plasma membrane protein, and cytosol were assayed for the NADH- and NADPH-dependent synthesis of superoxide using assays for the reduction of cytochrome c (Cyt c), assays for the reduction of nitroblue tetrazolium, and assays for the chemiluminescence of N,N′-dimethyl-9,9′-biacridium dinitrate (lucigenin). Each assay ascribed the highest activity to a different preparation: the Cyt c assay to cytosol, the nitroblue tetrazolium assay to plasma membrane, and the lucigenin assay to the partially solubilized plasma membrane protein (with NADH). This suggests that no two assays measure the same set of enzymes and that none of the assays is suitable for comparisons of superoxide synthesis among different cell fractions. With the plasma membrane preparation, the presence of large amounts of superoxide-dismutase-insensitive Cyt c reductase confounded attempts to use Cyt c to measure superoxide synthesis. With the partially solubilized membrane protein, direct reduction of lucigenin probably contributed to the chemiluminescence. Superoxide synthesis detected with lucigenin should be confirmed by superoxide-dismutase-sensitive Cyt c reduction.
In plant systems the synthesis of ROS, including superoxide, hydrogen peroxide, and the hydroxyl radical, occurs as a by-product of normal metabolism. However, excesses of ROS are produced during particular periods of development and in response to various stresses. There has been a recent focus on ROS produced during the hypersensitive response to pathogen infection and to the presence of noninfective elicitors from pathogenic and nonpathogenic microbes.
One mechanism for the production of ROS is the single-electron reduction of O2 to form superoxide. Dismutation of superoxide forms hydrogen peroxide and in the presence of transition metals, principally ferrous iron, the Fenton reaction produces hydroxyl radicals from the substrate hydrogen peroxide. Thus, the formation of superoxide leads to the other species of ROS. The detection of superoxide and the quantification of superoxide synthesis is a challenge, because traditional spectrophotometric methods are not especially sensitive. A sensitive method that depends on the chemiluminescence of lucigenin has been used extensively; it is considered to be a specific indicator of superoxide because it shows little signal from hydrogen peroxide (Corbisier et al., 1987) and has been used in a large number of studies of ROS production (for refs., see Faulkner and Fridovich, 1993).
Using lucigenin, our laboratory (Auh and Murphy, 1995) reported the accumulation of superoxide in suspension cultures of rose (Rosa damascena) cells treated with a cell wall elicitor from Phytophthora cinnamomea. Subsequently, Murphy and Auh (1996) used lucigenin to measure the rate of synthesis of superoxide by enzymes in rose cell plasma membranes.
Recent reports (Faulkner and Fridovich, 1993; Liochev and Fridovich, 1997; Vásquez-Vivar et al., 1997) have cast doubt on the utility of lucigenin as a specific indicator of the presence or synthesis of superoxide. Liochev and Fridovich (1997) pointed out that the chemiluminescence of lucigenin requires an initial reduction followed by the reaction with superoxide. The ability of potassium superoxide alone to induce a transient chemiluminescence from lucigenin (Liochev and Fridovich, 1997) suggests that the superoxide radical can itself accomplish the initial reduction, the reduced form then reacting with an additional superoxide molecule. However, the ability of endothelial nitric oxide synthase (Vásquez-Vivar et al., 1997) and xanthine oxidase (Liochev and Fridovich, 1997) to elicit chemiluminescence from lucigenin under conditions in which these enzymes are not known to produce superoxide shows that reduction of lucigenin is sufficient to give a positive signal: reduced lucigenin can reduce oxygen and the resulting superoxide reacts with additional reduced lucigenin to give chemiluminescence. Because the reaction sequence still involves superoxide, chemiluminescence is inhibited by SOD, even though the enzyme activity that is measured is not superoxide synthesis.
Because of the importance of ROS production to many aspects of plant cell physiology, we considered it necessary to use other assays to confirm our earlier report of superoxide synthesis from rose plasma membranes. The following data demonstrate the difference in the characteristics and rates of NADH- and NADPH-dependent superoxide-synthetic enzymes in three fractions from cultured rose cells, as assayed by lucigenin luminescence, Cyt c reduction, and NBT reduction.
MATERIALS AND METHODS
Chemicals
SOD, Cyt c, and lucigenin were obtained from Sigma. DPI was obtained from Cookson Chemicals Ltd. (Southampton, UK). NBT was obtained from Kodak.
Cell Preparation
Cells of rose (Rosa damascena Mill. cv Gloire de Guilan) were grown in suspension in liquid medium as described by Murphy et al. (1979).
Extraction and Preparation of the S100, Plasma Membrane, and Supernatant II
Four-day suspension-cultured rose cells were washed once with water and filtered on Whatman 3MM paper. Polyvinylpolypyrrolidone (approximately 1 g g−1 fresh weight of cells) and grinding buffer (25 mm Tris-Mes, pH 6.5, 3 mm Na2EDTA, 4.94 mm DTT, 250 mm Suc, and 2 mg/mL BSA) were added to the cells. The cells were then sonicated four times for 15 s each, and the resulting solution was filtered through four layers of cheesecloth. PMSF was added to the filtered solution at 0.25 mm. The filtrate was centrifuged at 10,000g for 15 min. The supernatant was collected and centrifuged for 40 min at 100,000g. The supernatant from this centrifugation, labeled S100, was dialyzed against grinding buffer minus DTT and PMSF before it was used in assays. Pellets were resuspended in phase buffer (5 mm potassium phosphate, pH 7.6, 4 mm potassium chloride, and 250 mm Suc) and subjected to three cycles of phase partitioning using the PEG-dextran method of Larsson and co-workers as described previously (Murphy and Auh, 1996).
The final hydrophobic (PEG) phase was removed, diluted in resuspension buffer (1 mm Tris-Mes, pH 6.5, 1 mm magnesium sulfate, and 20% Suc), and centrifuged for 40 min at 100,000g. The resulting supernatant was labeled supernatant II, and the resulting pellets were resuspended in resuspension buffer to give the final plasma membrane preparation. The active enzyme (lucigenin assay) in supernatant II was concentrated and further purified by adsorbing it to a Mono-Q column (Pharmacia 5/5) in 20 mm bis-Tris-propane-chloride buffer, pH 7.5, and eluting it with a potassium-chloride gradient in the same buffer. A single peak of activity eluted at approximately 0.3 m potassium chloride.
Lucigenin Assay for Superoxide Synthesis
A standard assay mixture contained approximately 3 μg of plasma membrane protein, 6 μg of S100 protein, or 0.3 μg of supernatant II protein, 100 μm NADH or 30 μm NADPH, 0.02% (w/v) Triton X-100, 0.4 mm lucigenin, and buffer (0.1 m Gly-sodium hydroxide, 1 mm EDTA, pH 9.0) to make a total volume of 1 mL. Additionally, 40 units of SOD (as defined by the manufacturer) or 15 μm DPI was added to appropriate reaction mixtures. Chemiluminescence was measured in a scintillation counter (model 9800, Beckman) in the single-photon counting mode for 1 min at 15-min intervals (six cycles total). Calibration of this assay using xanthine and xanthine oxidase was described previously by Murphy and Auh (1996).
Cyt c Assay for Superoxide Synthesis
A standard assay mixture contained plasma membrane, S100, or supernatant II protein (amounts as given above), 100 μm NADH or 40 μm NADPH, 0.02% (w/v) Triton X-100, 100 μm Cyt c, and buffer (20 mm Tris-chloride, pH 7.5, 3 mm magnesium chloride) to make a total volume of 0.5 mL in a quartz cuvette. Additionally, 40 units of SOD or 30 μm DPI was added to appropriate reaction mixtures. Change in A550 was measured over the 1st min in a spectrophotometer (model DU-640, Beckman). Calculation of specific activity assumed an absorption coefficient of 21 mm−1 cm−1.
NBT Assay for Superoxide Synthesis
A standard assay mixture contained plasma membrane, S100, or supernatant II protein (amounts as given above), 100 μm NADH or 40 μm NADPH, 0.02% (w/v) Triton X-100, 100 μm NBT, and buffer (20 mm Tris-chloride, pH 7.5, 3 mm magnesium chloride) to make a total volume of 0.5 mL in a quartz cuvette. Additionally, 40 units of SOD or 30 μm DPI was added to the reaction mixture. Change in A530 was measured over the 1st min in the spectrophotometer. Calculation of specific activity assumed an absorption coefficient of 12.8 mm−1 cm−1.
Protein Assay
Protein concentration was measured for each sample with the Coomassie brilliant blue protein-staining reagent (Bradford, 1976). BSA was used as a protein standard.
Calculations
To calculate specific activity, background values were obtained with all reaction components except enzyme. These values were then subtracted from the results with corresponding reaction mixtures including enzyme, and the difference was divided by the amount of protein added to the reaction mixture.
SOD Assay
The SOD activities in representative preparations of plasma membrane, S100, and supernatant II were measured on microtiter plates using illuminated riboflavin as a source of superoxide and NBT as an indicator. Each well contained 100 μL of a defined dilution of sample (3-fold dilution series in 20 mm bis-Tris-propane-chloride buffer, pH 7.5, 0.075% dodecylmaltoside, and 250 mm Suc) plus 200 μL of reaction mixture (150 mm Tris-chloride, 300 mm EDTA, pH 7.5, 0.1 mm NBT, and 3 μm riboflavin). The plate was illuminated with intense white light until wells without SOD achieved a moderate blue color. The optical absorption of the wells was measured with an automatic plate reader (green filter). One unit was defined as the amount of activity that reduced the amount of colored reaction product by one-half. The sensitivity of the assay was compared with the nominal activity of commercial preparations of SOD and found to give values 3-fold higher than expected.
RESULTS
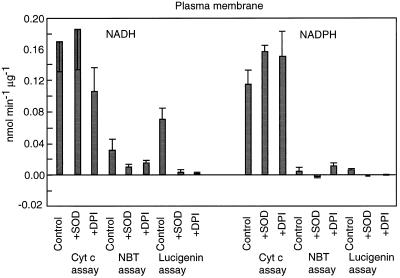
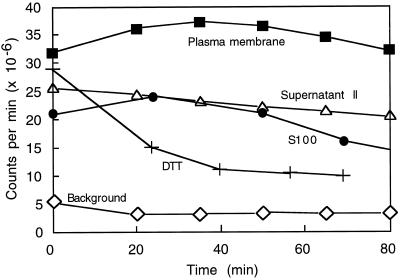
Plasma membrane-enriched preparations stimulated lucigenin chemiluminescence, Cyt c reduction, and reduction of NBT with both NADH and NADPH as electron donors (Fig. 1). The values for lucigenin chemiluminescence agreed with those reported previously (Murphy and Auh, 1996). As reported, chemiluminescence tended to increase for 15 to 30 min, then slowly decreased (Fig. 2). Also as found previously, luminescence was strongly reduced by the addition of SOD or DPI. The results for NBT reduction were similar in that the reactions were substantially reduced by SOD; however, NBT reduction was only partly sensitive to 30 μm DPI when NADH was used as the electron source, and was not sensitive to DPI when NADPH was used. The activity of plasma membrane in the Cyt c assay was higher than in either of the other assays. However, neither NADH- nor NADPH-dependent reduction of Cyt c was very sensitive to SOD or DPI. It is likely that the plasma membrane preparation contained substantial amounts of a Cyt c reductase that transferred electrons to Cyt c without forming superoxide. It is not known whether this enzyme was a component of the plasma membrane or a contaminant, perhaps the antimycin-A-insensitive Cyt c reductase of the ER.
Figure 1.
Specific activity of superoxide synthases from plasma membrane. Reaction mixtures were supplemented with either 100 μm NADH or 30 μm NADPH. Where indicated, reactions were conducted in the presence of 40 units of SOD or 30 μm DPI. Values are means ± se of at least five trials (Cyt c), four trials (NBT), or three trials (lucigenin).
Figure 2.
Time course of luminescence produced by plasma membrane (3.3 μg of protein), supernatant II (0.12 μg of protein), and S100 (6.0 μg of protein). Reaction components (protein, 100 μm NADH, 0.02% Triton X-100, 0.4 mm lucigenin, plus buffer to make 1.0 mL) were combined at time 0 and placed in a scintillation counter, and the luminescence, integrated over 1 min, was measured every 15 min. Background values were from mixtures of all reaction components except protein. Also shown is the luminescence produced by NADH, Triton X-100, and lucigenin in the presence of 50 μm DTT (the concentration in reactions receiving S100).
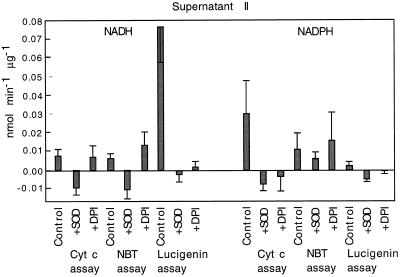
During studies of the plasma membrane we discovered that a substantial amount of NADH-dependent lucigenin-luminescence-stimulating activity was present in the supernatant of the last ultracentrifugation step in the plasma membrane-purification procedure. The total amount of enzyme was approximately equal to that in the plasma membrane pellet. Because this enzyme sedimented in the first ultracentrifugation, it was originally associated with large membrane fragments. We assume that it was partially solubilized during the phase partitions. The fact that it partitioned with the PEG phase suggests that the enzyme (or small membrane pieces to which it was attached) had chemical characteristics like those of plasma membrane. Although we have no conclusive evidence that this enzyme came from plasma membrane, its characteristics, including Km(NADH) and sensitivity to DPI, quinacrine, and azide, match those of the corresponding plasma membrane activity. This enzyme differs from plasma membrane enzyme in that it is more easily solubilized with detergents and chromatographs as a single peak (data not shown). We refer to this enzyme preparation as supernatant II.
With NADH as the reductant, supernatant II showed very high activity in the lucigenin-chemiluminescence assay. In fact, activity in this assay was higher than in the other two assays. As with plasma membrane, lucigenin luminescence was strongly inhibited by SOD and DPI (Fig. 3). In the NBT- and Cyt c-reduction assays this enzyme was also sensitive to SOD but not to DPI. With NADPH as the reductant, supernatant II showed little activity in the lucigenin assay and the highest activity in the Cyt c assay. In the other assays the enzyme showed sensitivity to SOD; however, it was sensitive to DPI only in the Cyt c assay and not in the NBT assay.
Figure 3.
Specific activity of superoxide synthases from supernatant II. Reaction mixtures were supplemented with either 100 μm NADH or 30 μm NADPH. Where indicated, reactions were conducted in the presence of 40 units of SOD or 30 μm DPI. Values are means ± se of at least five trials (Cyt c) or seven trials (NBT and lucigenin).
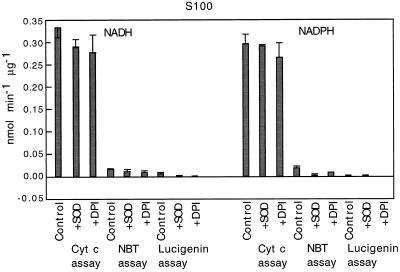
S100 was dialyzed to remove DTT, which interfered (gave positive indications) in all three assays. Figure 2 shows the effect of DTT on lucigenin. Its effect on Cyt c and NBT was even greater relative to enzymatic activity, probably because those assays measured reduction over a shorter time interval. S100 showed relatively low specific activity in the lucigenin assay (Fig. 4), but this activity was sensitive to SOD and DPI (Fig. 4). The highest activity of S100 appeared with the Cyt c assay; this activity was not sensitive to SOD or to DPI. In the NBT assay S100 activity was sensitive to SOD and DPI only when NADPH was used as the reductant.
Figure 4.
Specific activity of superoxide synthases from S100. Reaction mixtures were supplemented with either 100 μm NADH or 30 μm NADPH. Where indicated, reactions were conducted in the presence of 40 units of SOD or 30 μm DPI. Values are means ± se of two preparations, each assayed in triplicate.
A riboflavin-NBT assay was used to judge the SOD activity in two representative plasma membrane, supernatant II, and S100 preparations. Plasma membrane had ≤0.02 unit μL−1, supernatant II had no detectable activity, and the two samples of S100 had 0.02 and 0.06 units μL−1. All of the assays of superoxide synthesis described above used 10 μL of preparation.
DISCUSSION
It is clear from the results presented in Figures 1, 3, and 4 that success in detecting and quantifying a superoxide-producing enzyme depends on the assay used. Each assay ascribed the highest activity to a different preparation: the Cyt c assay to S100, the NBT assay to plasma membrane, and the lucigenin assay to supernatant II (with NADH). This suggests that no two assays measure the same set of enzymes. The differences may not be only in superoxide-generating enzymes. The three assay reagents will respond differently to the presence of SOD in the preparations. Liochev and Fridovich (1997) noted that superoxide reacts faster with Cyt c than with reduced lucigenin, and thus the Cyt c assay is less sensitive to SOD. Therefore, none of the assays is suitable for comparing superoxide synthesis in different cell fractions.
The results of the Cyt c assay probably also indicate the presence of a Cyt c reductase in plasma membrane. This means that the use of this assay with plant plasma membrane is problematic, because the high and variable activity can mask low levels of a superoxide-generating enzyme, even if—as is customary—the difference in rates with and without SOD are measured. An antimycin-A-resistant Cyt c reductase activity is generally considered a marker for the ER (Quail, 1979). It is not known whether the activity in our plasma membrane preparation represents contamination from residual ER or from a plasma membrane-specific enzyme.
The much higher specific activity of supernatant II in the lucigenin assay compared with the other assays suggests that this preparation contains an NADH-specific diaphorase that preferentially reduces lucigenin. According to Liochev and Fridovich (1997) and Vásquez-Vivar et al. (1997), the reduced lucigenin would react with oxygen to produce superoxide. As noted by these authors, the sensitivity of the luminescence to SOD is no indication that the enzymes are producing superoxide, because superoxide is essential to the luminescence whether it is formed by the enzyme or by the nonenzymatic oxidation of reduced lucigenin. The gradual increase in activity often observed over the first 15 min with plasma membrane (Fig. 2; figure 1 of Murphy and Auh, 1996) and occasionally with supernatant II (data not shown) is consistent with a two-step reaction mechanism, the increase reflecting the accumulation of the reduced form of either lucigenin, superoxide, or both. This problem can also apply to NBT (Picker and Fridovich, 1984).
The existence of a lucigenin diaphorase does not mean that a superoxide-synthase activity is not also present; in fact, the existence of SOD-inhibited NADPH-Cyt c oxidation-reduction activity indicates that supernatant II does produce superoxide. Further fractionation of this preparation will be needed to determine whether the activities measured by the three assays reflect the presence of a single enzyme or more than one enzyme.
DPI, an inhibitor of flavoenzymes, has been used to study the enzymatic synthesis of ROS (Cross and Jones, 1986; Deme et al., 1994). The inhibition of activity by DPI depended on the assay used. The NADH- and NADPH-stimulated activities of all three preparations were sensitive to 30 μm DPI when assayed with lucigenin but less so or not at all when assayed with NBT or Cyt c (with the exception of supernatant II and NADPH). In some cases, e.g. with Cyt c reductase of plasma membrane, an enzyme activity clearly different from activities that are sensitive to SOD, this is easy to understand. It is more difficult to understand why plasma membrane activities assayed with NBT and lucigenin, which are both sensitive to SOD, should be more sensitive to DPI with lucigenin than with NBT. Because DPI must be converted to a free radical before it is effective (O'Donnell et al., 1993, 1994), the possibility of interaction between DPI and the assay reagents should be investigated. In the mean time, the results of the present study confirm the utility of DPI in investigations of superoxide synthesis to this extent: because the generation of ROS by rose cells is strongly inhibited by less than 1 μm DPI (Bolwell et al., 1998), this process is unlikely to depend on an enzyme that is insensitive to this compound at 30 μm.
Abbreviations:
- DPI
diphenyleneiodonium
- lucigenin
N,N′-dimethyl-9,9′-biacridium dinitrate
- NBT
nitroblue tetrazolium
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
This work was supported in part by U.S. Department of Agriculture National Research Initiative-Cooperative State Research Service grant no. 94-37100-0788 to T.M.M.
LITERATURE CITED
- Auh C-K, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh C-K, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corbisier P, Houbion A, Remacle J. A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Anal Biochem. 1987;164:240–247. doi: 10.1016/0003-2697(87)90392-7. [DOI] [PubMed] [Google Scholar]
- Cross AR, Jones OTG. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils: specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme D, Doussiere J, De Sandro V, Dupuy C, Pommier J, Virion A. The Ca2+/NADPH-dependent H2O2 generation in thyroid plasma membrane: inhibition by diphenyleneiodonium. Biochem J. 1994;301:75–81. doi: 10.1042/bj3010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2·−. Free Radical Biol Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. Lucigenin (bis-N-methylacridinium) as a mediator of superoxide anion production. Arch Biochem Biophys. 1997;337:115–120. doi: 10.1006/abbi.1997.9766. [DOI] [PubMed] [Google Scholar]
- Murphy TM, Auh C-K. The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 1996;110:621–629. doi: 10.1104/pp.110.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TM, Hamilton CM, Street HE. A strain of Rosa damascena cultured cells resistant to ultraviolet light. Plant Physiol. 1979;64:936–941. doi: 10.1104/pp.64.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell VB, Smith GCM, Jones OTG. Involvement of phenyl radicals in iodonium compound inhibition of flavoenzymes. Mol Pharmacol. 1994;46:778–785. [PubMed] [Google Scholar]
- O'Donnell VB, Tew DG, Jones OTG, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker SD, Fridovich I. On the mechanism of production of superoxide radical by reaction mixtures containing NAD, phenazine methosulfate, and nitroblue tetrazolium. Arch Biochem Biophys. 1984;228:155–158. doi: 10.1016/0003-9861(84)90056-0. [DOI] [PubMed] [Google Scholar]
- Quail PH. Plant cell fractionation. Annu Rev Plant Physiol. 1979;30:425–484. [Google Scholar]
- Vásquez-Vivar J, Hogg N, Pritchard KA, Jr, Martasek P, Kalyanaraman B. Superoxide anion formation from lucigenin: an electron spin resonance spin-trapping study. FEBS Lett. 1997;403:127–130. doi: 10.1016/s0014-5793(97)00036-7. [DOI] [PubMed] [Google Scholar]