Abstract
Kyphoplasty (KP) is a minimally invasive technique for the percutaneous stabilisation of vertebral fractures. As such, this technique is highly dependent upon intraoperative fluoroscopic visualisation. In order to assess the range of radiation doses that patients are typically subjected to, 60 consecutive procedures using simultaneous bilateral fluoroscopy were analysed with respect to exposure time (ET). In a subset of 16 of these patients, a theoretical entrance skin dose (ESD) and effective dose was additionally calculated from intraoperatively measured dose area product. Average fluoroscopy time for single level cases reached 2.2 min (range 0.6–4.3) in the lateral plane and 1.6 min (range 0.5–3.0) in the anterior–posterior plane. For multiple level cases the corresponding ET per level was 1.7 min (range 0.6–2.9) per level in the lateral and 1.1 min (range 0.5–2.0) in the anterior-posterior plane. ESD was estimated as an average 0.32 Gy (range 0.05–0.86) in the anterior–posterior and 0.68 Gy (range 0.10–1.43) in the lateral plane. Effective dose (cumulative from both planes) averaged 4.28 mSv (range 0.47–10.14). Safety margins for the development of early transient erythema are respected within the presented fluoroscopy times. Longer ET in the lateral plane may however breach the 2 Gy threshold. Use of large c-arms and judiciously operating the exposure is recommended. With regard to effective dose, a single fluoroscopy guided KP performed for osteoporotic or traumatic vertebral fractures is a safe procedure.
Keywords: Kyphoplasty, Patient radiation exposure, Biplanar fluoroscopy, Spine
Introduction
During the last years spine surgery has witnessed a trend towards increasing numbers of minimally invasive procedures. Some of these, such as vertebroplasty (VP) and kyphoplasty (KP), rely heavily on X-ray guidance through fluoroscopy or computed tomography (CT). While the minimised soft tissue trauma and associated co-morbidity is an unquestionable asset of these techniques, the risk to the patient and the surgeon arising from the considerable X-ray exposure during the procedure is not incompletely understood. Recent investigations of the radiation exposure by surgeons and operating room personnel during fluoroscopy guided vertebral augmentation have revealed relevant doses that warrant specific safety measures such as whole body aprons and lead collars besides technical refinements of the equipment and fluoroscopes [6, 8, 9, 11, 14]. The relevance of the radiation exposure to the patient, who necessarily receives the brunt of the dose in VP and KP, has however only received limited attention [12].
The purpose of this investigation was therefore to analyse the biplanar fluoroscopy exposure time (ET) of KP patients and to estimate entrance skin dose (ESD) and effective dose (E) that the patient is typically subjected to in order to provide a basis for radiation associated risk assessment.
Materials and methods
Patient collective
Fifty-three successive patients (36 women, 17 men; average age 73 years, range 49–90) treated for osteoporotic or traumatic vertebral fractures through percutaneous KP were included in the investigation (Table 1). Within a year of the initial KP procedure five patients developed new fractures which were again treated with KP in seven separate operative sessions. These procedures were considered as separate cases for the purpose of determining intraoperative radiation exposure. Including these re-operations, 60 operative sessions were evaluated in which a total of 104 vertebrae from T4 to L5 were treated. Single fractures were treated in 33 operations and multiple fractures in 27 (average 2.6 vertebrae per multi-level operation; range 2–7). All operations were performed under general anaesthesia by surgeons with an experience of >200 KP procedures, eliminating any relevant learning curve effect.
Table 1.
Patient and exposure data
| Case | AS | VL | NL | ETap(min) | ETlat(min) | TVap(kV) | TVlat(kV) | DAPap(Gy×cm2) | DAPlat(Gy×cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 1 BI | 74 F | T7,8 | 2 | 1.5 | 2.9 | 73 | 64 | ||
| 2 BI | 74 F | T10 | 1 | 0.9 | 1.6 | 79 | 77 | ||
| 3 BI | 74 F | T11 | 1 | 1.0 | 2.2 | 71 | 68 | ||
| 4 BF | 90 M | T8,11 | 2 | 1.5 | 2.4 | 80 | 65 | ||
| 5 BF | 90 M | T6,7,12 | 3 | 2.0 | 4.3 | 69 | 64 | ||
| 6 BE | 72 F | T12,L3 | 2 | 1.7 | 3.2 | 80 | 92 | ||
| 7 CG | 83 F | T12,L4 | 2 | 1.4 | 1.9 | 87 | 102 | ||
| 8 DL | 79 F | T7,11 | 2 | 2.4 | 4.8 | 73 | 64 | ||
| 9 DE | 62 F | T12,L1,L3 | 3 | 3.4 | 5.0 | 66 | 99 | ||
| 10 EV | 75 F | L5 | 1 | 1.4 | 3.3 | 76 | 80 | ||
| 11 EM | 79 F | L4 | 1 | 1.2 | 2.1 | 69 | 68 | ||
| 12 EK | 65 M | T6,12,L3 | 3 | 3.0 | 5.3 | 67 | 64 | ||
| 13 FC | 59 F | T9-12 | 4 | 3.0 | 4.6 | 88 | 88 | ||
| 14 FA | 78 F | L1,2 | 2 | 2.8 | 3.8 | 69 | 98 | ||
| 15 GK | 55 M | T12 | 1 | 3.0 | 2.4 | 85 | 82 | ||
| 16 GJ | 64 M | T5-11 | 7 | 5.9 | 8.6 | 79 | 69 | ||
| 17 HG | 62 F | L1 | 1 | 2.0 | 2.3 | 68 | 65 | ||
| 18 HL | 74 M | L1,3 | 2 | 2.5 | 5.8 | 74 | 73 | ||
| 19 HC | 66 F | L3 | 1 | 2.5 | 2.8 | 83 | 110 | ||
| 20 HF | 76 F | L3 | 1 | 2.3 | 4.3 | 70 | 40 | ||
| 21 IE | 69 F | T8 | 1 | 1.8 | 2.5 | 70 | 63 | ||
| 22 IE | 69 F | T6,7,11 | 3 | 5.2 | 6.7 | 63 | 70 | ||
| 23 KT | 54 M | T4 | 1 | 1.9 | 3.3 | 63 | 98 | ||
| 24 KG | 50 M | L1 | 1 | 0.9 | 0.9 | 110 | 99 | ||
| 25 KM | 81 F | T6,7 | 2 | 0.9 | 3.7 | 69 | 67 | ||
| 26 LA | 75 F | T5,7 | 2 | 2.8 | 3.4 | 70 | 65 | ||
| 27 MA | 73 F | T12,L1,2 | 3 | 3.7 | 6.2 | 70 | 73 | ||
| 28 MJ | 74 F | T11 | 1 | 1.5 | 2.6 | 69 | 68 | ||
| 29 MF | 76 M | L2 | 1 | 0.9 | 1.3 | 74 | 82 | ||
| 30 MM | 82 F | L1 | 1 | 1.7 | 3.2 | 74 | 98 | ||
| 31 NE | 78 F | L3 | 1 | 0.5 | 1.6 | 78 | 77 | ||
| 32 PJ | 77 M | L3 | 1 | 1.3 | 2.3 | 68 | 78 | ||
| 33 PT | 89 F | L2 | 1 | 1.4 | 1.9 | 69 | 80 | ||
| 34 SH | 81 F | L2 | 1 | 2.8 | 0.7 | 78 | 75 | ||
| 35 SH | 69 F | T12,L2 | 2 | 2.4 | 4.2 | 70 | 79 | ||
| 36 SM | 71 F | L1 | 1 | 0.9 | 1.3 | 70 | 59 | ||
| 37 SM | 56 F | L1 | 1 | 2.8 | 3.1 | 79 | 80 | ||
| 38 SM | 79 F | T9,11 | 2 | 3.9 | 3.8 | 74 | 104 | ||
| 39 TA | 79 M | L3 | 1 | 1.6 | 3.3 | 98 | 90 | ||
| 40 TE | 73 M | T12,L3 | 2 | 3.1 | 3.3 | 70 | 92 | ||
| 41 UF | 80 F | L4 | 1 | 1.2 | 2.8 | 70 | 89 | ||
| 42 WA | 75 F | L4 | 1 | 1.1 | 1.7 | 66 | 75 | ||
| 43 WR | 71 F | T11,12,L3 | 3 | 5.7 | 7.7 | 81 | 95 | ||
| 44 ZG | 64 F | L3 | 1 | 1.3 | 1.8 | 64 | 64 | ||
| 45 CD | 74 F | L4 | 1 | 1.6 | 2.8 | 76 | 97 | 13.75 | 24.89 |
| 46 DH | 85 F | L1,2 | 2 | 3.0 | 3.6 | 104 | 110 | 13.32 | 36.81 |
| 47 ET | 83 F | L1 | 1 | 2.3 | 3.2 | 79 | 75 | 9.54 | 20.71 |
| 48 EV | 76 F | T12 | 1 | 1.0 | 0.8 | 66 | 73 | 3.17 | 5.77 |
| 49 FH | 86 M | L2 | 1 | 2.0 | 1.9 | 70 | 89 | 7.59 | 10.89 |
| 50 FC | 59 F | L1-4 | 4 | 2.2 | 5.8 | 88 | 110 | 26.85 | 25.53 |
| 51 FK | 86 F | L2 | 1 | 0.5 | 0.6 | 64 | 69 | 1.49 | 2.61 |
| 52 HH | 81 F | L2 | 1 | 0.9 | 1.5 | 69 | 88 | 4.24 | 6.70 |
| 53 IE | 69 F | T9,12,L1 | 3 | 3.8 | 3.5 | 80 | 62 | 12.28 | 13.68 |
| 54 JM | 49 F | T6,7 | 2 | 1.8 | 4.2 | 68 | 69 | 6.73 | 22.21 |
| 55 KE | 78 F | L1 | 1 | 1.2 | 2.1 | 71 | 77 | 4.60 | 15.93 |
| 56 PA | 82 M | T12,L1 | 2 | 1.9 | 1.8 | 78 | 81 | 10.26 | 15.68 |
| 57 SB | 66 M | L1,2 | 2 | 1.7 | 4.3 | 85 | 110 | 17.12 | 28.63 |
| 58 SE | 70 M | L1 | 1 | 2.4 | 2.2 | 77 | 74 | 9.56 | 13.05 |
| 59 WH | 82 M | L3 | 1 | 2.8 | 3.0 | * | 89 | 7.37 | 18.68 |
| 60 WM | 68 M | T11,12,L5 | 3 | 2.4 | 1.9 | 93 | 110 | 10.18 | 17.96 |
| Mean | 73 | 1.7 | 2.1 | 3.2 | 75 | 81 | 9.88 | 17.48 | |
| Min | 49 | 1 | 0.5 | 0.6 | 63 | 40 | 1.49 | 2.61 | |
| Max | 90 | 7 | 5.9 | 8.6 | 110 | 110 | 26.85 | 36.81 |
Case groups 1–3; 4 & 5; 10 & 48; 19 & 50; 21 & 22 & 53 respectively are the same patient treated in separate sessions and at different levels
AS age and sex of the patient; VL vertebral levels treated; NL number of levels treated; ET fluoroscopy exposure times in ap- and lat-plane; TV tube voltage for ap- and lat-plane; DAP dose–area product of ap- and lat- plane; * tube voltage was not recorded. Mean, Min, Max mean values per case, minimum and maximum values are presented
Operative technique and fluoroscopic radiation exposure
A bilateral transpedicular approach was routinely used for lumbar and thoracolumbar vertebrae [4]. Thoracic vertebrae above T11 were routinely treated through an unilateral extrapedicular approach [1, 2]. Uni- or bilateral treatment of thoracolumbar vertebrae was at the discretion of the surgeon according to vertebral size and fracture morphology.
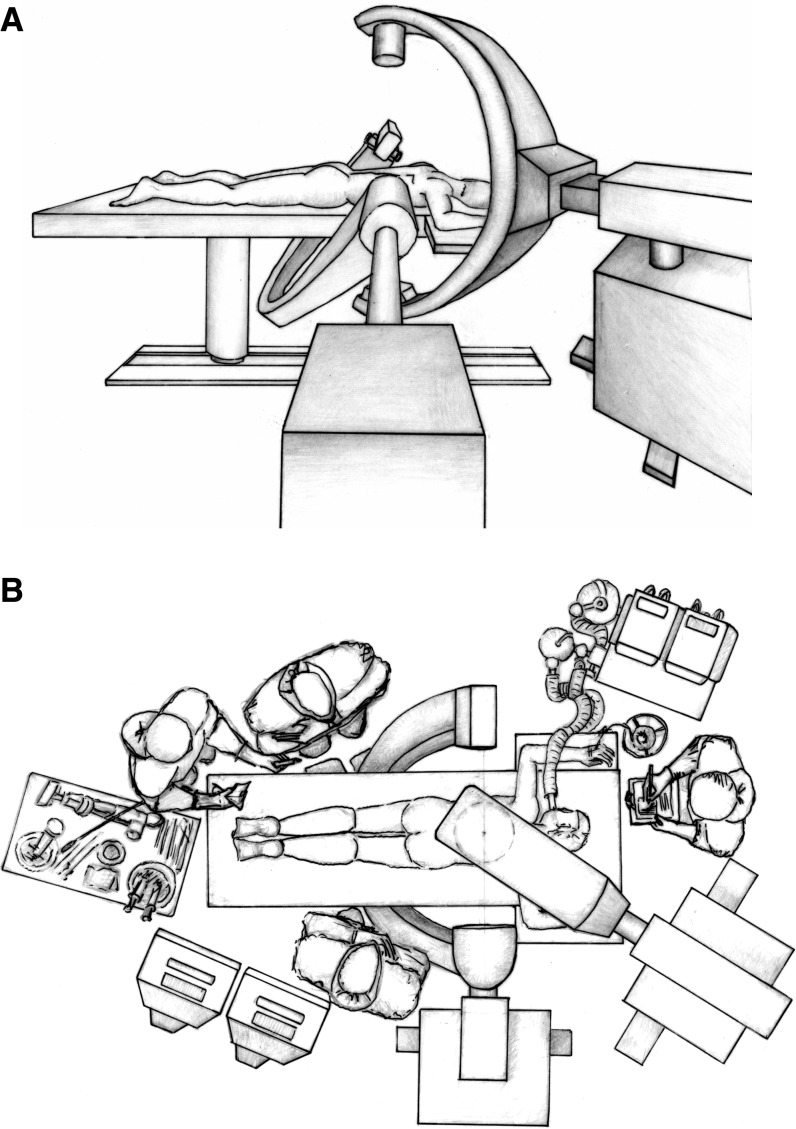
Simultaneous biplanar fluoroscopy using separate c-arms was used in all cases (Fig. 1a, b). All tool placement steps [1, 4] were simultaneously monitored in both fluoroscopic planes. Balloon inflation was monitored frequently at the discretion of the surgeon, as was polymethylmethacrylate (PMMA) injection. Whenever feasible with regard to the fluoroscopic visualisation, the PMMA injection of up to three adjacent vertebrae was done simultaneously. Exposure time was recorded in minutes separately for both c-arms via the built-in timers, beginning with patient positioning until the final documentation exposure. This ensured documentation of the entire ET of a given patient from entering to leaving the operating theatre. The surgeons controlled the exposure via foot switches. The c-arms were operated in automatic brightness control mode, with fixed diaphragms and in non-pulsed mode. No extraordinary effort was made to reduce ET that would have jeopardised the safety of the procedure.
Fig. 1.
Drawing of the typical operative set-up for percutaneous KP under biplanar fluoroscopic guidance in a lateral view (A) and birds eye view (B). The surgeon and assistant are positioned at the sides of the patient, while the scrub nurse is at the foot end and the anaesthesiologist is at the head of the table
Various c-arm fluoroscopes (Siemens Siremobil Compact, Siemens Siremobil 2000, Siemens Siremobil Iso-C) were used during the first 44 cases (1–44 in Table 1) in which ET in both planes (ETap and ETlat) as well as tube voltage (TVap and TVlat) were recorded at the end of each operative session (Table 1). Average ETs for single and multiple levels were calculated from these values (Table 2). Since the projections and patient positions did not vary significantly during operative sessions with treatment of up to three adjacent vertebrae, it was assumed, that the tube voltages TVap and TVlat established by the automatic brightness control unit, remained constant throughout the procedure. Variations of TV between non-adjacent levels and due to the application of contrast medium laden PMMA or the presence of tools in the X-ray beam could not be considered.
Table 2.
Fluoroscopic ETs per treated level of the case groups
| ETapper single level (min) | ETlatper single level (min) | ETapper multi-level (min) | ETlatper multi-level (min) | |
|---|---|---|---|---|
| All cases | 1.6 (0.5–3.0) | 2.2 (0.6–4.3) | 1.1 (0.5–2.0) | 1.7 (0.6–2.9) |
| Cases 1–44 | 1.6 (0.5–3.0) | 2.3 (0.7–4.3) | 1.1 (0.5–2.0) | 1.8 (1.0–2.9) |
| Cases 45–60 | 1.6 (0.8–2.5) | 2.0 (0.6–3.2) | 1.0 (0.6–1.5) | 1.5 (0.6–2.7) |
Mean exposure time in the respective plane (ETap or ETlat) in minutes of the entire collective (all), case group 1–44 and case group 45–60. Exposure times are broken down into minutes/level for single level cases and minutes per level for multi-level cases. Minimum and maximum values are respectively listed in brackets
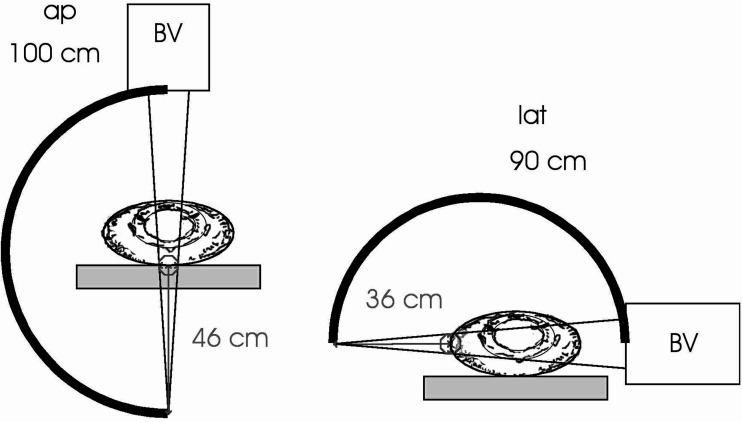
Two c-arm fluoroscopes (Siemens Siremobil Compact) equipped with dose-area-product (DAP) meters were available for the subsequent 16 cases (45–60 in Table 1), in which DAP was recorded in both planes in addition to ET and TV. The ap-unit was larger than the lat-unit with a span of 79 cm (100 cm focus to image intensifier distance) versus a span of 70 cm (90 cm focus to image intensifier distance). The larger unit was used in the ap-plane to allow greater freedom of movement during tool manipulation. Focus to skin distances for normal adult patients typically amounted to 46 cm in the ap-plane and to 36 cm in the lat-plane. Entrance field sizes determined by means of X-ray films were 37.4 cm2 for the ap-plane and 30.8 cm2 for the lat-plane (Fig. 2—online supplemental material). However, as individual patients may show a deviation from these field sizes due to body size and positioning, the subsequent dose estimations can only be regarded as a closer approximation.
Fig. 2.
Schematic representation of the fluoroscopy setting used to experimentally determine the entrance field sizes in both planes of view. A typical operative set-up was simulated and X-ray films were exposed at the usual distance of the patient from the focus (46 cm focus to skin distance ap and 36 cm focus to skin distance lat). Field sizes of 37.4 cm2 in the ap plane and 30.8 cm2 in the lat plane were determined. ap anterior–posterior fluoroscopy unit with 100 cm focus to image intensifier distance, lat lateral fluoroscopy unit with 90 cm focus to image intensifier distance, BV image intensifier
Patient dose
The estimation of patient dose was based on the intraoperatively gathered radiation exposure data of cases 45 to 60 recorded in Table 1, in which the values of DAP were known from the direct measurement of the DAP meters. The proper function of the DAP meters was controlled by the Technical Inspection Agency (TÜV Süddeutschland) by means of a calibrated dosemeter (PTW Diados E, calibration date 09.2003). For all fluoroscopes involved, the total filtration amounted to 3 mm aluminium. It was decided to determine ESD and E as descriptors of patient dose.
ESD relates to the dose dependant threshold for development of early transient and main erythema [15] at the entrance side of the patient. E is the only available dose descriptor related to the risk of developing radiation induced cancers, which enables comparison with patient doses acquired from different exposure situations, such as CT. Entrance skin dose and E were calculated via Eqs. 1, 2, 3, 5 (see supplemental online material) for cases 45–60 and are presented in Table 3.
Table 3.
Patient doses of cases 45 to 60
| Case | ESDap(Gy) | ESDlat(Gy) | Eap(mSv) | Elat(mSv) | Etot(mSv) |
|---|---|---|---|---|---|
| 45 CD | 0.44 | 0.97 | 2.92 | 3.08 | 6.00 |
| 46 DH | 0.43 | 1.43 | 3.75 | 5.14 | 8.90 |
| 47 ET | 0.31 | 0.81 | 2.11 | 1.89 | 4.00 |
| 48 EV | 0.11 | 0.22 | 0.57 | 0.51 | 1.08 |
| 49 FH | 0.24 | 0.42 | 1.47 | 1.23 | 2.70 |
| 50 FC | 0.86 | 0.99 | 6.57 | 3.57 | 10.14 |
| 51 FK | 0.05 | 0.10 | 0.26 | 0.21 | 0.47 |
| 52 HH | 0.14 | 0.26 | 0.81 | 0.75 | 1.56 |
| 53 IE | 0.39 | 0.53 | 2.75 | 0.92 | 3.66 |
| 54 JM | 0.22 | 0.87 | 1.26 | 1.79 | 3.05 |
| 55 KE | 0.15 | 0.62 | 0.91 | 1.51 | 2.41 |
| 56 PA | 0.33 | 0.61 | 2.24 | 1.58 | 3.82 |
| 57 SB | 0.55 | 1.12 | 4.06 | 4.00 | 8.06 |
| 58 SE | 0.31 | 0.51 | 2.06 | 1.17 | 3.23 |
| 59 WH | 0.24 | 0.73 | * | 2.11 | * |
| 60 WM | 0.33 | 0.70 | 2.62 | 2.51 | 5.13 |
| Mean | 0.32 | 0.68 | 2.29 | 2.00 | 4.28 |
| Minimum | 0.05 | 0.10 | 0.26 | 0.21 | 0.47 |
| Maximum | 0.86 | 1.43 | 6.57 | 5.14 | 10.14 |
ESD entrance skin dose in ap- and lat-plane, E effective dose in ap- and lat- plane and total (ap + lat), * Eap and Etot could not be determined in this case as the tube voltage was not recorded
ICRP 60 [7] provides “nominal probability coefficients” for developing cancers in a lifetime after a single, acute irradiation. The coefficient is 0.05 (=5%) per Sievert (Sv−1) of E for fatal cancers; 0.01 (=1%) for non-fatal cancers and 0.06 (=6%) for any cancer. While these coefficients principally relate to a homogenous irradiation of the whole body, an application in the fluoroscopy setting is not ruled out. The coefficients will however be less accurate in this application. Equations (4) and (5) allow a theoretical estimation of the increase in lifetime risk for developing cancers after a single KP procedure for the obtained mean and maximum E values of cases 45–60 (Table 3).
Dosimetrical methodology
Entrance skin dose was calculated via Eq. (1). Entrance skin dose values for both planes (ESDap, ESDlat) of cases 45–60 are presented in Table 3.
While the use of E to describe patient exposures after small field size examinations, like in this study, is not unproblematic and may even cause criticism, it has to be seen as a makeshift as there is no other way to achieve a patient dose risk relation in a fluoroscopy application such as KP. Ideally, E is determined from a given set of selected organ doses through Eq. (2), incorporating so-called tissue weighting factors, which account for the probability of non-deterministic effects (malignancies) of specific organs after irradiation [7]. In the context of this study, however, it was unthinkable to provide organ dose conversion coefficients to calculate organ doses from entrance dose for all the tube voltages, projections and vertebrae involved, to achieve E with Eq. (2). All the more because the radiation fields were very small and the decision, which organs were affected to which degree, was impossible to reach with the necessary precision. Fortunately, there is an earlier empirical finding, namely that E can be roughly estimated from DAP even without precise knowledge of field size and field position with regard to the patient with Eq. (3) [10]. This work provides conversion coefficients to calculate E from DAP. The only information required to select the conversion coefficients is the tube voltage, beam filtration, the body region examined (e.g. lumbar spine) and the projection (either ap or pa/lat). Following this procedure, the E values for each plane (Eap and Elat for cases 45 to 60 were estimated. As E, is defined as an additive quantity [7], the sum of the values of both planes (Etot = Eap + Elat) was included in Table 3.
Although DAP was not measured amongst the first 44 cases, disallowing the direct calculation of ESD and E, an endeavour was made to determine the range of patient dose in these cases in order to exclude significantly higher values than ascertained for cases 45–60. To achieve this, the relation between the measured DAP and corresponding ET of cases 45–60 was determined and regression lines were established (Fig. 3). Via these, fictitious DAP-values for cases 1–44 could be determined from their ET-values (DAPap= 4.77×ETap and DAPlat= 6.19×ETlat). The application of Eqs. (1) and (3) subsequently allowed the determination of the range of theoretical patient doses.
Fig. 3.
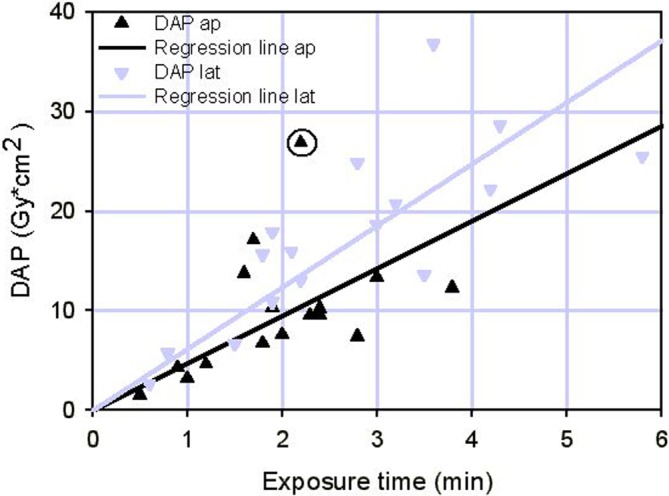
Dependence of DAP on fluoroscopic ET for cases 45 to 60
Formulas
Entrance skin dose (mGy) can easily be determined from the measured DAP (mGy×cm2) values with:
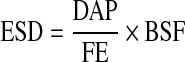 |
1 |
with FE (cm2) is the field size in focus to skin distance and BSF the so-called backscatter factor, which considers the contribution of radiation, backscattered by the patient, to ESD. An average value of BSF (BSF=1.2) for all occurring tube voltages was derived from the literature [5, 13].
E (mSv) is principally calculated from a given set of selected organ doses HT (mSv) along:
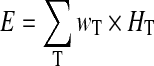 |
2 |
with wT so-called tissue weighting factors, which account for the probability of non-deterministic effects (malignancies) in organ T after irradiation [7].
E is alternatively estimated along conversion coefficients fE (mSv×mGy−1 ×cm−2) taken from [10] from DAP along:
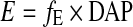 |
3 |
where fE is the dose conversion coefficient. The fE-values by Le Heron [10] are given in 10 kV steps of tube voltage, the fE-values for the actual tube voltages were attained by interpolation.
The risk Rfatal for radiation induced fatal cancers is estimated with:
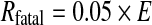 |
4 |
from the effective energy E (Sv) with 0.05 (Sv−1) the nominal probability coefficient taken from [7].
In an analogous way the risk for non-fatal cancers Rnon-fatal is estimated with:
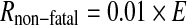 |
5 |
with 0.01 the nominal probability coefficient from [7]. The risk of developing any cancer, fatal or non-fatal is derived by adding the obtained Rfatal and Rnon-fatal values or by substituting 0.06 as the nominal probability coefficient in the formula.
Results
No complications occurred during the procedures and no procedure had to be aborted because of inadequate fluoroscopic visualisation.
Exposure time
Variations in ET were only due to technical operative factors such as visualisation and fracture complexity. With very few exceptions, ETlat was found to be longer than ETap with an average of 3.2 min (range 0.6–8.6) versus 2.1 min (range 0.5–5.9) per case (Table 1). When broken down into ET per level, average ETlat was 2.2 min (range 0.6–4.3) per level for single fracture cases and 1.7 min (range 0.6–2.9) per level for multiple fracture cases (Table 2). ETap and consistently remained below ETlat in this per level breakdown. Mean ET was not found to differ relevantly between the case groups 1–44 (no direct DAP measurement) and 45–60 (direct DAP measurement).
Patient dose
Entrance skin dose of case group 45–60 derived from Eq. (1) was found to range from a minimum of 0.05 Gy in the ap plane to 1.43 Gy in the lat plane (Table 3). The average value of the lat plane was markedly higher than for the ap plane with 0.68 versus 0.32 Gy respectively (Table 3).
Cumulative E values of case group 45–60 (Table 3), deduced from Eq. (3), range from 0.47 mSv to 10.14 mSv with an average of 4.28 mSv.
Using these values in Eqs. (4) and (5), the lifetime risk of developing a cancer after a single KP procedure is theoretically increased by 0.025% (0.02% for fatal cancers) for the mean E value of 4.28 mSv and by 0.06% (0.05% for fatal cancers) for the maximum E value of 10.14 mSv.
The correlation of ET and DAP of case group 45–60 in Fig. 3 reveals, that the DAP-values scatter considerably along the fitted regression line (as could have been expected due to varying patient thickness) and that they are higher for the lat-projection (larger patient diameter), than for the ap-projection. Figure 3 also shows that it is mainly the ET, which governs the DAP. Nevertheless, in some cases the measured DAP-value was higher than predicted by the regression line by a factor of up to 2.7 (case 50 FC ap in Table 1 and encircled in Fig. 3). Using the regression lines to determine DAP from ET values in case group 1–44 may therefore lead to an underestimation by a factor of 2.7 in the worst case. Assuming that patients on the whole were comparable between the case groups, this factor could affect the dimension of subsequently calculated ESD and E in an occasional individual of the latter case group. This will however not relevantly affect the mean patient doses in the whole case group, all the more so since the mean ETs of the groups are comparable (Table 2). Mean patient doses in the whole collective would therefore have been rather the same as in the case group 45–60 (Table 3). Nevertheless, it cannot be excluded, that there are deviators hidden in the case group 1–44, which received 2–3 times higher patient doses than those in Table 3. Since the maximum ET in a single patient (case 16 GJ in Table 1) treated for seven levels is higher than the average by a factor of 1.5, a four fold higher maximum patient dose than that established in Table 3, cannot strictly be excluded for this patient on the basis of the data collected in this study. Therefore, assuming a worst case E value of ~40 mSv for this patient would lead to a theoretical increase in lifetime risk for developing a cancer by 0.24% (0.2% for fatal cancers) through application of Eqs. (4) and (5).
Discussion
Exposure time
Simultaneous biplanar fluoroscopy was used in order to decrease radiation exposure (Fig. 1a, b). The rationale behind this set-up is that once an optimal setting has been found, it is maintained throughout the procedure and no radiation is “wasted” during readjusting to the second plane of view.
The finding that longer ET was almost always required in the lat view (Table 1) is due to the monitoring of the PMMA injection which relies heavily on the lat view for recognising even minimal epidural or paravertebral venous leakage. On an average, ETlat of 2.2 min was required in cases treated for single levels (Table 2). When treating multiple levels, the ETlat per level was reduced to 1.7 min per level (Table 2). This is because up to three adjacent vertebrae can be visualised with the same c-arm setting, occasionally allowing simultaneous PMMA injection.
The addition of ETap and ETlat to a total ET per treated level or case is not rational from a dosimetrical standpoint as they relate to different projections. Nevertheless, total ET per level provides the clinician with a rough guideline as to the amount of radiation that is “used” and may allow an-acknowledgeably unscientific-comparison between different operative set-ups and techniques. With this restriction in mind, average total ET per level in single fracture cases calculated from Table 1 was 3.8 min (range 1.1–6.6 min). In multiple level cases, the average total ET per level was 2.8 min (range 1.1–4.5 min). Corresponding total ET per level in multiple level VP cases (average 4.25 vertebrae per session) by Harstall et al. [6] was slightly lower with 2.2 min. This may reflect the greater technical demand of KP over VP during tool introduction and balloon inflation. In the only other dosimetrical investigation of patient exposure in KP by Perisinakis et al. [12], the average total ET was 10.1 min (4.7 min ETap and 5.4 min ETlat) in 11 patients. Although not clearly stated by these authors, their procedures appear to have been conducted with a single fluoroscopy unit. In this case, the significantly shorter ET of our collective would serve to underline the radiation saving rationale behind simultaneous biplanar fluoroscopy.
Patient radiation dose
As the patient exposure data presented in Table 1 was obtained in a genuine operative setting by experienced surgeons without unrealistic efforts to reduce exposure, the derived patient doses in Table 3 may be considered as a genuine baseline for discussing the hazards of radiation exposure to patients during KP.
However, while ET can be measured accurately, the patient doses derived from these values are subject to several sources of error: (1) The experimentally determined field sizes (Fig. 2) do not allow for individual variations due to patient size and positioning, so that the calculations of ESD (Table 3) from Eq. (1) have to be regarded as the closest possible approximation assuming average patient dimensions and exactly centred positioning. (2) The determination of E (Table 3) from a fluoroscopy application with small and varying fields of exposure (such as in KP) as per Eq. (3) has not been scientifically verified. The same applies for the subsequent calculations of the lifetime risk for developing cancers as per Eqs. (4) and (5). These values therefore have to be regarded as theoretical until confirmed by further investigations.
The calculated ESD values of the patients in Table 3 show that the 2 Gy threshold to early transient erythema [15] was not reached in either plane. Correspondingly, no signs of erythema developed in any of the patients. ESDap was well within the safety limit with an average of 0.23 Gy and even the maximum value only reached 0.86 Gy. ESDlat is of greater concern, as the average value reached 0.68 Gy and the maximum value rose to 1.43 Gy. DAP is however not only related to ET, but also reflects the voltage and current adjustment of the automatic brightness control unit according to patient thickness. Using Eq. (1) and the regression line calculation, a theoretical ETlat of ~8 and ~25 min respectively would be necessary for the induction of a transient early erythema (2 Gy threshold) or main erythema (6 Gy threshold [15]) in an average patient treated with our operative set-up and a permanent field of exposure. These findings reveal our chosen operative setting to be disadvantageous with regard to the smaller lateral c-arm, as it almost always had the longer ET, but is closest to the patients skin (Table 1, Fig. 2). In cases that are difficult to visualise (e.g. pre-existing scoliosis) or that require continuous live fluoroscopy during PMMA injection due to complex pathology (e.g. posterior wall disruption or osteolysis) it is conceivable that ETlat could reach levels of concern. The use of a larger c-arm, would hereby markedly reduce ESD, as skin exposure drops according to an inverse second order function with increased skin to focus distance.
Effective dose values were found to reach an average of 4.28 mSv with a maximum of 10.14 mSv in the case of group 45–60. While higher deviations of a factor 2–4 theoretically cannot be excluded for all individuals of case group 1–44 according to the regression line determination (Fig. 3), the comparable ET values between the case groups (Table 2) allow a reasonable assumption of a comparable range of patient doses between the groups. Our mean E values are found to compare favourably with those of Perisinakis et al. [12], which averaged 8.5–12.7 mSv, in dependence of the treated region, in 11 patients with an average total ET of 10.1 min.
The lifetime risk of developing a cancer after a single KP procedure is theoretically increased by 0.02% – 0.06% in case group 45–60. These values are to be seen in relation to a baseline of 20% – 25% cause of death from cancer in the Western European population. This 20%–25% lifetime risk of developing a fatal cancer is therefore theoretically increased by 0.02% for the mean E value of 4.28 mSv and by 0.05% for the maximum E value of 10.14 mSv after a single KP procedure. In the estimated worst case of E ~40 mSv the risk would increase by 0.2%. Although low, the risk of developing an early transient erythema or a cancer cannot be totally disregarded.
While data for comparison is lacking, it is unlikely that comparable fractures could be treated with lesser E-values through CT guided KP as repeated scanning and additional fluoroscopy during PMMA injection is usually necessary. The mean value for Etot (4.28 mSv), as reported in Table 3, is lower than E-values resulting from common, not specially dose reduced CT-examinations of the whole abdomen, with only the maximum value (10.14 mSv) equalling the typical mean value for abdominal CT [3]. According to the review by Perisinakis et al. [12], our mean KP patient dose of 4.28 mSv is well within the range of other common interventional fluoroscopic procedures such as radiofrequency catheter cardiac ablation (5.7 mSv), endoscopic retrograde cholangiopancreatography (12.4 mSv) and enteroclysis (14.0 mSv).
Reducing radiation exposure in KP
Exposure was directed by the surgeon via foot pedals. As the entire finesse of the procedure is in the hands of the surgeon, this is the only way of optimising fluoroscopy exposure without endangering the patient and hampering the flow of the operation. It is highly doubtful, whether exposure guided by a radiology technician would have been able to achieve comparable values or in any way have contributed positively to the success of the procedure.
A reduction of ET in this could have been achieved through pulsed imaging. The concern of missing PMMA leakage however prompted the surgeons to use non-pulsed imaging in short bursts. Nevertheless, pulsed imaging should be used where justifiable (e.g. for tool introduction). Studies will need to be directed towards comparing the risk of PMMA leakage in pulsed and non-pulsed imaging before formulating recommendations in this regard.
Acknowledgements
Figure 1 was provided through courtesy of Spinegraphics.
Footnotes
Sources of support: No financial support was received for this investigation.
References
- 1.Boszczyk BM, Bierschneider M, Hauck S, Vastmans J, Potulski M, Beisse R, Robert B, Jaksche H. Kyphoplastik im konventionellen und halboffenen Verfahren. Orthopäde. 2004;33:13–21. doi: 10.1007/s00132-003-0575-2. [DOI] [PubMed] [Google Scholar]
- 2.Brugieres P, Gaston A, Heran F, Voisin MC, Marsault C. Percutaneous biopsies of the thoracic spine under CT guidance: transcostovertebral approach. J Comput Assist Tomogr. 1990;14:446–448. doi: 10.1097/00004728-199005000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Galansky M, Nagel HD, Stamm G. CT-Expositionspraxis in der Bundesrepublik Deutschland, Fortschritte auf dem Gebiet der Röntgenstrahlen und bildgebenden Verfahren. RöFo. 2001;173:R1–R66. doi: 10.1055/s-2001-19474. [DOI] [PubMed] [Google Scholar]
- 4.Garfin SR, Hansen AY, Reiley MA. Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Harrison RM. Backscatter factors for diagnostic radiology. Phys Med Biol. 1982;27:1465–1474. doi: 10.1088/0031-9155/27/12/005. [DOI] [PubMed] [Google Scholar]
- 6.Harstall R, Heini PF, Mini RL, Orler R (2005) Radiation exposure to the surgeon during fluoroscopically assisted percutaneous vertebroplasty—a prospective study. Spine (in press) [DOI] [PubMed]
- 7.International Commission on Radiological Protection (1991) 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60, Pergamon Press, Oxford
- 8.Kallmes DF, O E, Roy SS, Piccolo RG, Marx WF, Lee JK, Jensen ME. Radiation dose to the operator during vertebroplasty: prospective comparison of the use of 1-cc syringes versus an injection device. Am J Neuroradiol. 2003;24:1257–1269. [PMC free article] [PubMed] [Google Scholar]
- 9.Kruger R, Faciszewski T. Radiation dose reduction to medical staff during vertebroplasty: a review of techniques and methods to mitigate occupational dose. Spine. 2003;28:1608–1613. doi: 10.1097/00007632-200307150-00024. [DOI] [PubMed] [Google Scholar]
- 10.Le Heron JC. Estimation of effective dose to the patient during medical X-ray examinations from measurement of the dose-area product. Phys Med Biol. 1992;37:2117–2126. doi: 10.1088/0031-9155/37/11/008. [DOI] [PubMed] [Google Scholar]
- 11.Mehdizade A, Lovblad KO, Wilhelm KE, Somon T, Wetzel SG, Kelekis AD, Yilmaz H, Abdo G, Martin JB, Viera JM, Rüfenacht DA. Radiation dose in vertebroplasty. Neuroradiology. 2004;46:243–245. doi: 10.1007/s00234-003-1156-0. [DOI] [PubMed] [Google Scholar]
- 12.Perisinakis K, Damilakis J, Theocharopoulos N, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Patient exposure and associated radiation risks from fluoroscopically guided vertebroplasty or kyphoplasty. Radiology. 2004;232:701–707. doi: 10.1148/radiol.2323031412. [DOI] [PubMed] [Google Scholar]
- 13.Petoussi-Henss N, Zankl M, Drexler G, Panzer W, Regulla D. Calculation of backscatter factors for diagnostic radiology using Monte Carlo methods. Phys Med Biol. 1998;43:2237–2250. doi: 10.1088/0031-9155/43/8/017. [DOI] [PubMed] [Google Scholar]
- 14.Theocharopoulos N, Perisinakis K, Damilakis J, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J Bone Joint Surg. 2003;85(A):1698–1703. doi: 10.2106/00004623-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71–84. doi: 10.1016/s1051-0443(94)71456-1. [DOI] [PubMed] [Google Scholar]