Abstract
This study was conducted to refine a small animal model of scoliosis, and to quantify the deformities throughout its growth period. Subcutaneous scapula-to-contralateral pelvis tethering surgery was selected due to its minimally invasive nature and potential applicability for a large animal model. The procedure was performed in 7-week-old New Zealand white rabbits. Group A animals (n=9) underwent the tethering procedure with a suture that spontaneously released. Group B animals (n=17) had the identical procedure with a robust tether and pelvic fixation, which was maintained for 2 months during growth. All animals developed immediate post-operative scoliosis with a Cobb angle of 23° (range, 6–39°) in group A and 59° (range, 24–90°) in group B animals. During the 2 month post-tethering, group A animals lost their tether and scoliosis resolved, whereas all animals in group B maintained their tether until scheduled release at which time the mean scoliosis was 62°. Immediately after tether release, group B scoliosis decreased to a mean 53°. Over the following 4 months of adolescent growth, the scoliosis decreased to a mean of 43° at skeletal maturity; the decrease usually occurred in animals with less than 45° curves at tether release. Radiographs revealed apical vertebral wedging (mean 19°) in all group B animals. Sagittal spinal alignment was also assessed, and for group B animals, the scoliotic segment developed mild to moderate kyphosis (mean 28°) and torsional deformity, but the kyphosis resolved by 4 months after tether-release. Complications specific to this technique included a high rate of transient scapulothoracic dissociation and cases of cor pulmonale. In conclusion, this tethering technique in immature rabbits consistently produced scoliosis with vertebral wedging when the tether was intact through the first 2 months of the protocol. The transient exaggeration of kyphosis suggests that the production of scoliosis is not necessarily dependent on lordosis in this model. Because this technique does not violate thoracic or spinal tissues, it may be useful in the investigation of secondary physiologic effects of mechanically-induced scoliosis, and may be scalable to larger animal species.
Keywords: Animal model, Scoliosis, Lordosis, Kyphosis, Tether
Introduction
Prospective randomized clinical trails provide the most accurate and clinically relevant information on the treatment of a disease. However, the application of experimental techniques in randomized human studies of scoliosis treatment is limited by ethical considerations. For this reason, a practical animal model of scoliosis would have great potential for gaining insight into the progression of and treatments for scoliosis. Specifically, our institution is interested in potential non-fusion treatments of scoliosis.
Scoliosis models have been created in animals by many techniques. Teratogenic scoliosis was induced by using endocrine or pharmacological treatments [3, 11, 13–15, 17, 18, and 31]. Other techniques had produced neuromuscular types of scoliosis [1, 2, 5, and 22]. Immobilization techniques were previously used [4, 8, 9, 12, 20, and 27], and some scoliosis induction techniques included rib resection and/or muscle release [7, 23, 28, and 29]. Other surgical models have used a variety of tethering procedures, typically in small animals [16, 24, 25, and 30].
The purpose of this pilot study was to refine a minimally invasive scoliosis model in a small mammalian species for use in directly investigating physiologic changes resulting from a mechanically produced curve, which could later be applied to a larger animal model in which human-sized technologies and devices would be applicable. The ideal technique would result in an unfused spine with a reproducible Cobb angle between 30° and 60° and would not violate the tissues surrounding the vertebra so that future corrective treatments could be applied. After a careful review, the technique of scapula-to-contralateral pelvis tether was identified as the most promising method meeting the above requirements [30]. The specific aim of this study was to quantify the coronal and sagittal deformities resulting from spinal growth in the presence of a static scapula-to-contralateral pelvis tether, and to assess the reliability of such a model of scoliosis.
Materials and methods
New Zealand white rabbits were used in this experimental study of the scapula-to-contralateral pelvis tethering surgical technique. Institutional Animal Care and Use Committee approval was obtained. Based upon the pilot data, two groups were analyzed: group A rabbits had a tether that spontaneously released (usually within 4 weeks), and group B rabbits had a robust tether that required surgical release. All animals were approximately 7 weeks old at the time of the tethering procedure. Animals of this age were chosen because the tethering period and release could still be performed prior to maturity of the animal [19]. This allowed observation of the post-tethered rabbit spinal deformity during remaining adolescent growth.
Preoperatively, all animals received antibiotics [gentamicin 5 mg/kg subcutaneously (SQ)], and were anesthetized with ketamine (35 mg/kg), xylazine (5 mg/kg), and acepromazine (0.75 mg/kg) administered intramuscularly. Animals were divided into two treatment protocol groups based on the type of tether.
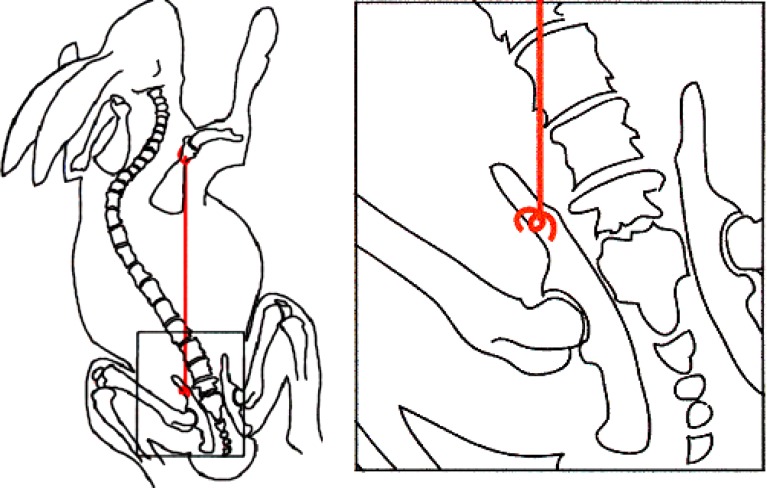
Operative tethering of the scapula to the contralateral pelvis in all animals consisted of an incision over the scapular neck and another incision over the contralateral iliac crest after which a subcutaneous tunnel was formed using blunt techniques. The tether was then passed through the subcutaneous tunnel with group A animals (n=9) using size O permanent braided sutures (Ethibond, Ethicon, Cincinnati, OH, USA). The suture was shortened so that the pelvis and scapula were drawn together to 90–95% of the original scapula-to-pelvis distance. The tether was easily palpable just below the skin of all animals. Group A animals reliably lost their tether typically within 4 weeks. Group B animals (n=17) had an identical procedure but with a robust non-resorbable No. 5 suture (Ethibond) shortened to 85–95% of the original scapula-to-pelvis distance, of which none failed. The more robust nature of the group B tether allowed for slightly greater shortening. Additionally, fixation to the pelvis entailed use of 0.9 mm stainless steel wire that had been formed into a figure-of-eight-shaped “toggle pin” (Fig. 1). This toggle pin was placed in such a manner that it distributed force against the outer table of the iliac wing when tension was applied to the suture. A percutaneous tether release surgery was performed on all animals with intact tethers 2 months after the index tethering procedure.
Fig. 1.
Schematic of scapula-to-contralateral pelvis tethering procedure in group B (2 months intact tether) animals. Inset shows figure-of-eight-shaped “toggle pin”
Post-operative care included analgesics (buprenorphine 0.05 mg/kg SQ every 8 h) and antibiotics (enrofloxacin 5 mg/kg per day, SQ) for 3 days. All animals had weekly clinical assessment for weight gain and mobility (subjective visual analysis of animal’s gait, as they crossed a 2 m track). Animals were followed until 6 months post-operatively at which time they were euthanized. Animals that spontaneously lost their tethers were euthanized when their scoliosis had normalized to approximately zero degrees. Euthanasia was achieved by intracardiac injection of 20 mEq potassium chloride. Necropsy was performed in all animals to confirm the presence or absence of deformity and to correlate the deformity to the radiographs. Additionally, manual spinal flexibility of each specimen was qualitatively evaluated at the time of necropsy.
Posteroanterior (PA) and lateral radiographs of the spine were taken with animals under general anesthesia at preoperative and immediate post-operative time points, and monthly thereafter, until 4 months after surgical tether release by which time animals had grown into mature adults. Radiographs were evaluated for spinal deformity and other bony abnormalities. The coronal and sagittal plane deformities were measured on PA and lateral radiographs by a single observer using the Cobb method [32]. Intra-observer variation was assessed by measuring the sagittal and coronal Cobb angles from five radiographs on three different days. Observer error was estimated using analysis of the components of variance. This analysis found an observer standard deviation of 6.3° for kyphosis and 4.5° for scoliosis.
Apical wedging and spinal length were measured from PA radiographs. Wedging was measured as previously described [26] immediately after tether release and at 4 months after tether release (6 months after initial tethering). Lines were drawn digitally on PA radiographs across superior and inferior endplates of the apical vertebrae. Wedging was then measured as the angular difference between these lines, and the opening end of the angle was noted to be oriented toward the concavity or convexity of the curve. Some degree of disc wedging was also noted in the apical region but was not quantified in this study. Spinal length was measured at the time of initial tethering and at 4 months after tether release from the T1-2 disc space to the L5-6 disc space.
Statistical analysis included linear regression to calculate the correlation coefficient between (i) the percent shortening of the suture and initial coronal curve (ii) the final coronal curve and percent shortening of the suture, and (iii) the magnitude of the main scoliotic curve and the degree of apical wedging. A paired t-test was used to compare the curve magnitudes between the monthly radiographs. p values less than 0.05 were considered statistically significant.
Results
Preoperative radiographs of all animals in group A (n=9) confirmed no pre-existing scoliosis, and all survived the index surgery. The mean post-operative scoliosis of group A animals was 23° (range, 6–39°). Seven of the animals lost their tethers before the 1-month X-ray, and the remaining two animals lost their tethers before the 2-month X-ray. Clinically, one could detect the loss of the tether in the subcutaneous tissue by palpation through the rabbit’s thin skin. In these animals with spontaneous release of their tethers, there was a corresponding resolution of their scoliosis. Necropsies performed in this group confirmed tether failure, as well as the mode of failure. Four of the nine animals lost fixation at the pelvis due to the tensioned suture pulling through the weak pelvic bone. Intrasubstance failure of the size O non-absorbable braided suture occurred in the other five animals.
Of the 17 animals in group B, 11 survived through 6 months follow-up to skeletal maturity. Two rabbits succumbed to anesthesia-related complications, one died of an unknown cause 1 week postoperatively, and two animals were euthanized due to wound infections that did not respond to a minimum of 10 days of antibiotics. One rabbit, with scoliosis of 110° at 6 weeks post-tethering, continued to have cyanosis and failure to thrive despite tether release 2 weeks early. This animal was euthanized early due to presumed cor pulmonale.
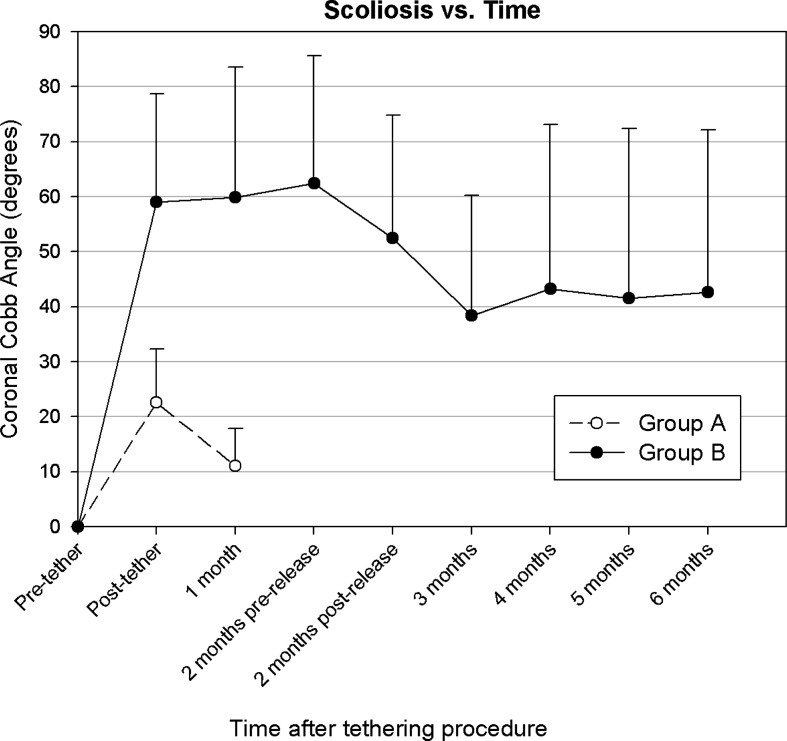
Preoperative radiographs revealed no scoliosis in any of the group B animals. After tethering surgery, an immediate scoliosis was obtained with a mean Cobb angle of 59° (range, 24–90°, n=14), as shown in Fig. 2. This was significantly different from pre-operative values (p<0.001), and all curves were concave toward the side of the tethered scapula. At 1 month, the scoliosis in this group was a mean of 60° (range, 15–110°, n=14). At 2 months, just prior to the time of the tether release, the mean Cobb angle was 62° (range, 32–120°, n=11). Immediately after tether release, scoliosis decreased to a mean 52° (range, 32–110°, n=11, p=0.008). By skeletal maturity at 6 months after tethering surgery (and 4 months after tether release), the mean scoliosis significantly decreased from its immediate post-release value to 43° (range, 11–123°, n=11; p=0.02). Four of six animals with scoliosis greater than 45° maintained or had progression of their scoliosis. Curves stabilized by 2 months after tether release, and changed by only a mean 0.6° (range, −9–9°) in the last 2 months of the study. Initial Cobb angles significantly correlated with the percent of suture shortening (R2=0.37, p=0.006). However, the percent of suture shortening and initial curve did not correlate with the final curve (p=0.57 and 0.37, respectively). Conversely, the curvature immediately after tether release did have a highly significant correlation with the final curvature (R2=0.59, p<0.0001).
Fig. 2.
Coronal plane curvature (mean Cobb angle, and error bars indicate one SD) for each experimental animal group as a function of time and tether integrity. Group A=self-releasing tether; group B=2 months intact tether
All apical vertebral bodies were wedged toward the curve concavities. Apical wedging angles averaged 11° (±7° standard deviation) immediately after tether release and 19° (±11°) at 4 months post-release. The final scoliotic curve was proportional to the degree of wedging after tether release (R2=0.67, p=0.002) and at 4 months post-release (R2=0.51, p=0.01). Animals with greater than 10° of vertebral body wedging maintained or had progression of their scoliosis. The initial spinal length was 18.4±1.3 cm, and by the 6 month duration (4 months post-tether), animals had grown by a mean 46±6% to 26.9±1.8 cm. The percent spinal growth did not correlate well with the degree of final scoliosis (R2=0.03, p=0.6).
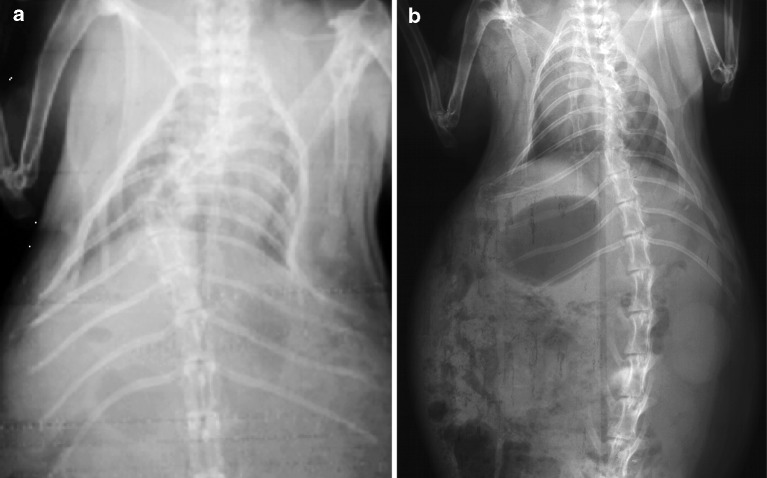
By 4 months post-tether release, there were eight animals in group B with single scoliotic curves; seven had the apex of their scoliosis between T5 and T10 (Fig. 3), and one with a lumbar curve had the apex at L3; three animals had double thoracic curves. As for animals with multiple curves, only the largest curve was used in the statistical analysis.
Fig. 3.
Example of group B coronal radiographs demonstrating cases of single (a) and double (b) scoliosis at skeletal maturity (6 months and 4 months after tether release)
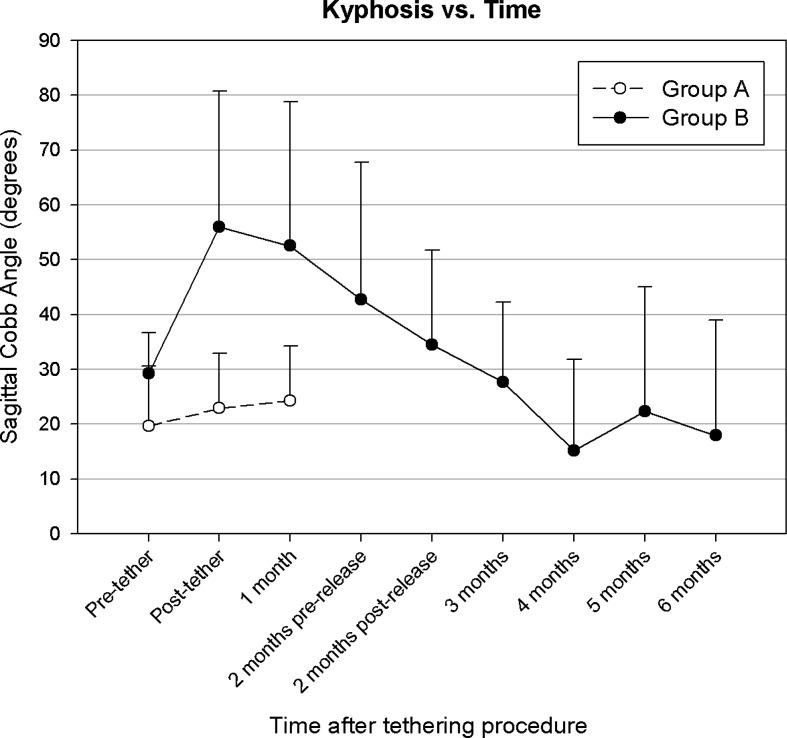
Sagittal radiographs found that the scoliotic segment became more kyphotic compared to preoperative measurements in 16 of 17 group B animals 1 month after tethering surgery, with a mean increase of 28° (p<0.001, Fig. 4). However, this increase in kyphosis was transient (Fig. 5). By 4 months after tether release, the kyphosis resolved in all but one animal (kyphosis of 65°). This animal had severe scoliosis and radiographs also revealed a high degree of torsional deformity. The group A animals, which had no lasting scoliosis, did not have any significant change in their sagittal alignment.
Fig. 4.
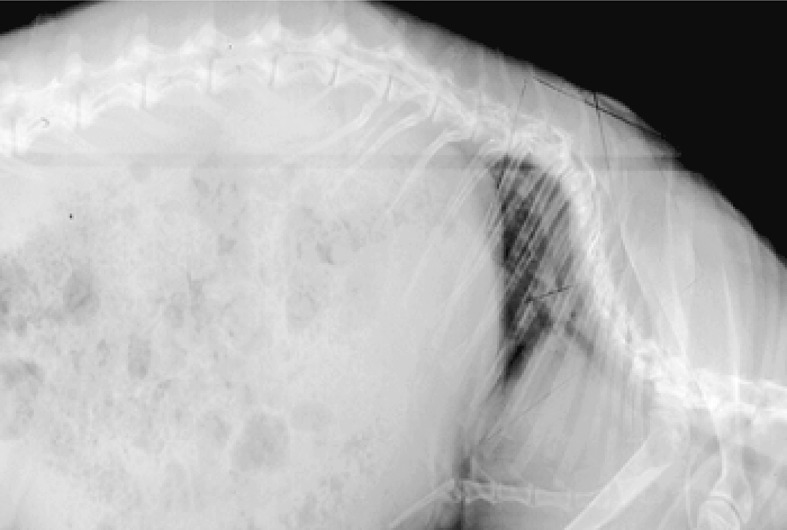
Example of group B sagittal radiograph demonstrating kyphosis and axial torsion
Fig. 5.
Sagittal plane curvature (kyphosis, mean and error bars indicate one SD) for each experimental group as a function of time and tether integrity. Group A=self-releasing tether; group B=2 months intact tether
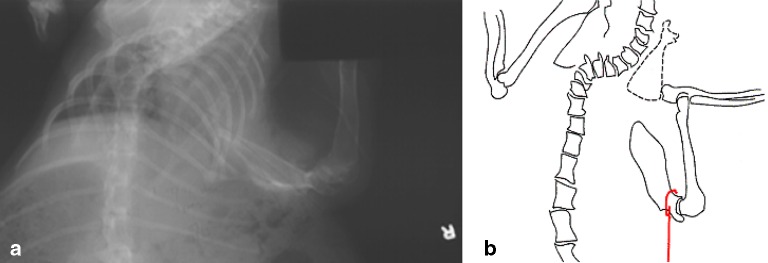
Front limb gait abnormalities were noted in the tethered extremity in all but two of group B animals 1 month after tethering surgery. Radiographs of these animals at 1 month identified varying degrees of scapulothoracic dissociation (Fig. 6). These gait disturbances and deformities were resolved by 4 months after tether release in all but one animal.
Fig. 6.
a Radiographic evidence of typical scapulothoracic dissociation, and b a schematic illustrating the magnitude of the dissociation
Post-mortem radiographs and necropsies revealed scoliosis and a rotation of the vertebrae in all animals of group B. Wedging of the apical vertebral bodies on the concave aspect of the curves confirmed radiographic findings. Radiographs also confirmed gowth-plate closure of all animals of 6-month duration. Gross examination revealed stiff but unfused segments at the apex of the curve.
Discussion
In this minimally invasive rabbit model of scoliosis, all animals maintaining an intact tether developed scoliosis with a mean final curve of 43°. Curves also exhibited sagittal and rotational deformity, and apical vertebral wedging toward the concavity of the scoliotic curve. The current study had more rigorous analysis than a prior study of this tethering scoliosis animal model [30]. The results of the present study found many similarities to this previous study, and differences as well. Comparison between group A group B demonstrated that reliable production of scoliosis required an intact tether for a full 2-month period. This time period corresponds to approximately one-third of the rabbit growth period. Group A, which may be considered a sham control group, also demonstrated that concave surgical scarring did not result in scoliosis. It should be noted that although the degree of surgical invasiveness was similar to groups A and B, the amount of tether shortening was slightly greater for group B. This is the most likely reason for the difference in the initial scoliosis curves between the two groups.
Group B tethers were surgically released after 2 months, by which time the rabbits had reached the age of 15 weeks. Published growth curves show that at 15 weeks, the New Zealand White rabbit reaches only 67% of its adult weight [19]. The model, therefore, produced an adolescent type of scoliosis at the 15-week time point, which was one goal of this study. In future applications of this model, it may be possible to remove the tether earlier in order to allow for a greater time period for growth-related treatments of scoliosis. However, early tether release may only be possible in animals with vertebral wedging greater than 10° and scoliosis over 45°; smaller values would otherwise lead to a subsequent decrease in the magnitude of scoliosis as suggested by group A results. Prolonged tethering of larger curves may result in severe scoliosis and the risk of developing cor pulmonale.
The magnitude of scoliosis in the present study had considerable variation, and ranged from 11° to 123°. Initial tether shortening correlated with the initial degree of curvature, but the final degree of curvature did not correlate with the initial tethered curve. Initial tension of the tether was not directly measured, but was assumed to have been proportional to the percent shortening. However, the tether tension likely changed over time due to pelvic tilt, scapular subluxation, tether or soft tissue creep, spinal growth, and animal activity. Changes in tether tension rather than length may explain the variability in final curvature and the poor correlation of the final curve and initial tether shortening. Future studies with an implantable load cell and telemetry, over the length of the study, would allow measurement of tension due to the initial tether shortening and over time related to soft tissue relaxation, and spinal growth. A tension threshold could possibly be established for progressive versus non-progressive scoliosis using the current animal model.
While the final curve magnitude could not be accurately predicted at the time of tethering, the degree of curvature and vertebral wedging just after tether release were good predictors of the final curves. That is, once a larger curve was established, it was unlikely to resolve. In a prior study by Tsaung et al. [30], curvatures over 45° were maintained or progressed by a mean 15° at final follow up. Similarly, 3 of 11 animals in the current study with scoliosis over 45° had maintenance of the curvature, whereas smaller curves stabilized at a lower value. In our study, the information on early curve magnitude could be used to reduce error when randomizing animals to treatment groups in future applications of this model. That is, variability can be reduced by performing experimental treatments only on the rabbits that have a scoliosis of 40° to 45° or more after their tether release. Animals with post-tether curves of less than 40° often had a decrease in curve magnitude over time and thus would create false positives for treatment effects. Somewhat analogous to human scoliosis, larger curves are more likely to progress, and prediction of scoliosis progression is a key factor in the decision whether or not to surgically intervene. Similar to greater curves at tether release predicting greater final curves, greater apical vertebral body wedging at the time of tether release predicted greater final curves and is similar to a previously described study [6]. Furthermore, while the amount of total spinal growth over the 6 month-study period did not seem to affect the degree of scoliosis, it is still possible that the growth rate during the tether period may be related to curve magnitude.
Despite success in consistently producing scoliosis, this animal model had several limitations. A modification of the originally described protocol [30] was necessary to ensure suture/pelvic bone integrity for the entire tethering period. In addition, as reported previously in other rabbit studies, survival rates were relatively low [10, 30], and 6 of the 26 animals in this study died prematurely. Scapulothoracic dissociation and associated gait abnormalities were also noted in most animals. However, this condition resolved after tether removal in all animals.
The animal model used in the present study had many similarities to human idiopathic scoliosis, which is characterized as a three-dimensional deformity with curvature in the coronal plane, axial rotation of the vertebrae, progression during growth (particularly for curves over 45°), and vertebral wedging at the apex of the curve [26]. Additionally, compared to other scoliosis models cited in the introduction, the technique of the present study also produced cases of double curves. In humans, scoliosis may also be accompanied by decrease in the normal thoracic kyphosis. Some authorities believe that loss of thoracic kyphosis is essential to the etiology of scoliosis as a three-dimensional deformity. However, others have found that hypokyphosis is variable in scoliosis patients [10, 21]. Unlike our results, a prior study of the tethered rabbit model of scoliosis reported lordoscoliosis, yet similar to the present study, found axial rotational deformity [30].
The present application of this model resulted in a transient kyphoscoliotic deformity during curve formation. Exaggeration of a rabbit’s normal kyphosis may be expected in this model as the caudal and medial tether forces draw the scapula toward the pelvis, thereby increasing the kyphotic profile already present in the rabbit. So, while the initiation of scoliosis in the animal model is different than in human scoliosis, the findings of this study support the concept that coronal curve development and progression do not require a lordotic sagittal spinal profile.
While the scoliotic spines in this model may differ in size from those in human cases, the unseperated adolescent scoliotic spines produced in this model were truly three-dimensional, and therefore provide a valuable research tool. In addition to the coronal and sagittal plane deformities, torsional and vertebral wedging components were identified and warrant high-resolution axial CT scans with reconstructions in future studies. This would quantify vertebral rotation, associated rib and chest deformities, as well as three-dimensional vertebral body asymmetry. Furthermore, the lack of surgical intervention directly to the spine lends this model to histologic analysis of the apical endplates, which became wedged in the asymmetric load environment. The many similarities of this animal model to the human case also allow analysis of secondary conditions related to scoliosis such as thoracic volume changes and pulmonary restrictive disease. Finally, application of this technique to a larger animal may allow more measurements on the human scale, and would enable surgical intervention using techniques intended for human use.
Conclusion
This study confirmed that tethering of sufficient duration at the time of adolescent growth of animals predictably produced scoliosis with a mean curve magnitude between 40° and 45°. This model exhibited several similarities to human scoliosis including variability of the curve magnitude, progression of large curves during adolescent growth and a three-dimensional deformity including wedging of the apical vertebrae. This small animal model may be applied to studies of secondary conditions related to a mechanically-derived curve, and may have applicability to larger animals for evaluation of treatments for scoliosis in humans.
Acknowledgement
The authors thank Inion, Ltd. for providing the financial support required to complete this study.
References
- 1.Barrios C, Arrotegui JI. Experimental kyphoscoliosis induced in rats by selective brain stem damage. Int Orthop. 1992;16:146–151. doi: 10.1007/BF00180206. [DOI] [PubMed] [Google Scholar]
- 2.Barrios C, Tunon M, Salis J, et al. Scoliosis induced by medullary damage: an experimental study in rabbits. Spine. 1987;12:433–439. doi: 10.1097/00007632-198706000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Beuerlein M, Wang X, Moreau M, et al. Development of scoliosis following pinealectomy in young chickens is not the result of an artifact of the surgical procedure. Microsc Res Tech. 2001;53:81–86. doi: 10.1002/jemt.1071. [DOI] [PubMed] [Google Scholar]
- 4.Binette MA, Stokes IAF, Aronsson DD, et al. Mechanical modulation of intervertebral disc growth: implications for scoliosis progression. Spine. 1996;21:1162–1167. doi: 10.1097/00007632-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Borodic GE (1991) Chemomodulation of curvature of the juvenile spine. US Patent 5,053,005, October 1
- 6.Braun JT, Akyuz E. Prediction of curve progression in a goat scoliosis model. J Spinal Disord Tech. 2005;18:272–276. [PubMed] [Google Scholar]
- 7.Braun JT, Ogilvie JW, Akyuz E, et al. Experimental scoliosis in an immature goat model: a method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine. 2003;28:2198–2203. doi: 10.1097/01.BRS.0000085095.37311.46. [DOI] [PubMed] [Google Scholar]
- 8.Coillard C, Rhalmi S, Rivard CH. Experimental scoliosis in the minipig: study of vertebral deformations. Ann Chir. 1999;53:773–780. [PubMed] [Google Scholar]
- 9.Court C, Colliou OK, Chin JR, et al. The effect of static in vivo bending on the murine intervertebral disc. Spine. 2001;26:239–245. doi: 10.1016/s1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 10.Dickson RA. Idiopathic scoliosis: foundation for physiological treatment. Ann R Coll Surg Engl. 1987;69:89–96. [PMC free article] [PubMed] [Google Scholar]
- 11.Geiger B, Steenbock H, Parsons H. Lathyrism in the rat. J Nutr. 1933;6:427. doi: 10.1111/j.1753-4887.1976.tb05778.x. [DOI] [PubMed] [Google Scholar]
- 12.Hakkarainen S. Experimental scoliosis: production of structural scoliosis by immobilization of young rabbits in a scoliotic position. Acta Orthop Scand. 1981;52:1–57. doi: 10.3109/ort.1981.52.suppl-192.01. [DOI] [PubMed] [Google Scholar]
- 13.Inoh H, Kawakami N, Matsuyama Y, et al. Correlation between the age of pinealectomy and the development of scoliosis in chickens. Spine. 2001;26:1014–1021. doi: 10.1097/00007632-200105010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura S, Ohara S, Suwa T, Nakagawa K. Studies of vitamin requirements of rainbow trout, Salmo Gairdneri-I. on the ascorbic acid. J Jpn Soc Fish. 1965;31:818. [Google Scholar]
- 15.Lalic JJ, Angevine DM. Dysostosis in adult rats after prolonged B-aminopropyonitrile feeding. Arch Path. 1970;90:22. [PubMed] [Google Scholar]
- 16.Lawton JO, Dickson RA. The experimental basis of idiopathic scoliosis. Clin Orthop. 1986;11:9–17. [PubMed] [Google Scholar]
- 17.Lim D, Lovel TR. Pathology of the vitamin C deficiency syndrome in channel catfish (Ictalurus punetatus) J Nutr. 1978;108:1137. doi: 10.1093/jn/108.7.1137. [DOI] [PubMed] [Google Scholar]
- 18.Machida M, Murai I, Miyashita Y, et al. Pathogenesis of idiopathic scoliosis. Experimental study in rats. Spine. 1999;24:1985–1989. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Masoud I, Shapiro F, Kent R, et al. A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J Orthop Res. 1986;4:221–231. doi: 10.1002/jor.1100040211. [DOI] [PubMed] [Google Scholar]
- 20.Mente PL, Stokes IAF, Spence H, et al. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine. 1997;22:1292–1296. doi: 10.1097/00007632-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Newton PO, Gollogly S, Faro F, Marks M (2004) Sagittal hypokyphosis varies with the location of the apex of the coronal curve: is there an explanation that suggests an etiology in idiopathic scoliosis. In: 39th annual meeting of the scoliosis research society, Buenos Aires, 6–9 September 2004
- 22.Pincott J, Davies JS, Taffs LF. Scoliosis caused by section of dorsal spinal nerve roots. J Bone Joint Surg. 1984;66B:27–29. doi: 10.1302/0301-620X.66B1.6693473. [DOI] [PubMed] [Google Scholar]
- 23.Robin GC. Scoliosis induced by rib resection in chickens. J Spine Disord. 1995;8:179–185. doi: 10.1097/00002517-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sarwark JF, Dabney KW, Salzman SK, et al. Experimental scoliosis in the rat. I. Methodology, anatomic features, and neurologic characterization. Spine. 1988;13:466–471. doi: 10.1097/00007632-198805000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Smith R, Dickson RA. Experimental structural scoliosis. J Bone Joint Surg. 1987;69B:576–581. doi: 10.1302/0301-620X.69B4.3611161. [DOI] [PubMed] [Google Scholar]
- 26.Stokes IA, Aronsson DD. Disc and vertebral wedging in patients with progressive scoliosis. J Spinal Disord. 2001;14:317–322. doi: 10.1097/00002517-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Stokes IA, Mente PL, Iatridis JC, et al. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg. 2002;84A:1842–1848. doi: 10.2106/00004623-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Thomas S, Dave PK. Experimental scoliosis in monkeys. Acta Orthop Scand. 1985;56:43–46. doi: 10.3109/17453678508992978. [DOI] [PubMed] [Google Scholar]
- 29.Thometz JG, Liu XC, Lyon R. Three-dimensional rotations of the thoracic spine after distraction with and without rib resection: a kinematic evaluation of the apical vertebra in rabbits with induced scoliosis. J Spinal Disord. 2000;13:108–112. doi: 10.1097/00002517-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Tsuang YH, Yang RS, Chen PQ, et al. Experimental structural scoliosis in rabbits. J Formos Med Assoc. 1992;91:886–890. [PubMed] [Google Scholar]
- 31.Wang X, Moreau M, Raso J, et al. Changes in serum melatonin levels in response to pinealectomy in the chicken and its correlation with development of scoliosis. Spine. 1998;23:2377–2381. doi: 10.1097/00007632-199811150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Whittle MW, Evans ML. Instrument for measuring the Cobb angle in scoliosis. Lancet. 1979;1:414. doi: 10.1016/S0140-6736(79)90887-0. [DOI] [PubMed] [Google Scholar]