Abstract
A prospective clinical and radiographic evaluation of 33 consecutive patients with severe and rigid idiopathic scoliosis (average Cobb angle 93°, flexibility on bending films 23%) were treated with combined anterior and posterior instrumentation with a minimum follow-up of 2 years. All patients underwent anterior release and VDS-Zielke Instrumentation of the primary curve. In highly rigid scoliosis, this was preceded by a posterior release. Finally, posterior correction and fusion with a multiple hook and pedicle screw construct was performed. Thirty patients were operated in one stage, three patients in two stages. Preoperative curves ranged from 80 to 122° Cobb angle. Frontal plane correction of the primary curve averaged 67% with an average loss of correction of 2°. The apical vertebral rotation of the primary curve was corrected by 49%. In all but three patients, sagittal alignment was restored. There were no neurological complications, deep wound infections or pseudarthrosis. Combined anterior and posterior instrumentation is safe and enables an effective three-dimensional curve correction in severe and rigid idiopathic scoliosis.
Keywords: Idiopathic scoliosis, Anterior fusion, Posterior fusion, VDS, Posterior instrumentation
Introduction
Only very few studies have been conducted reporting the treatment strategies of severe and rigid idiopathic scoliosis. The most common treatment is anterior release and a posterior correction and instrumentation with some authors advocating a period of halo traction between both procedures [3, 4, 10, 16, 18, 21]. Furthermore, there is a controversial debate on whether combined anterior and posterior procedures should be performed in one or two stages [16, 18]. Other authors again report on satisfactory results in these curves with an exclusive posterior approach utilizing segmental pedicle screw instrumentation [1, 5, 12].
To optimize curve correction, to minimize the neurological risk and to eliminate both patient discomfort and a prolonged hospital stay resulting from halo traction, we combined the anterior Zielke Instrumentation (VDS) with a posterior multiple hook and pedicle screw construct in mostly one-stage surgery to treat severe and rigid idiopathic curves. The clinical and radiographic results of 33 prospectively evaluated patients are presented.
Methods
Patients and evaluation
Between 1997 and 2002, 33 consecutive patients with severe and rigid idiopathic scoliosis were surgically treated at our institution. Inclusion criteria were idiopathic curves with Cobb angles of at least 80° and a flexibility of less than 40% on bending films. All patients were prospectively evaluated with an average follow-up of 34 months (range 24–78 months). No patient was lost to follow-up. Twenty-eight patients had adolescent and five patients adult idiopathic scoliosis. Twenty-nine patients were female, four patients were male with an average age of 17.6 years (11–39 years) at the time of surgery. There were 13 thoracic and 20 double major curves. The curves were classified according to the Lenke classification (Table 1) [13].
Table 1.
Data on the patients
| Patient | Age/sex | Lenke Classification | Cobb angle pre-op (°) | Cobb angle bending (°) | Cobb anglepost-op (°) | Anterior fusion length | Posterior fusion length | Op. time (min) | Blood loss (ml) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 15/F | 4AN | 122 | 118 | 56 | T7–L1 | T2–L3 | 445 | 2,100 | |
| 2 | 13/F | 6CNTL | 116 | 72 | 32 | T11–L4 | T4–L5 | 510 | 2,000 | |
| 3 | 13/F | 2AN | 90 | 60 | 36 | T7–T11 | T5–L2 | 250 | 400 | |
| 4* | 11/F | 4A+TL | 112 | 110 | 28 | T7–L1 | T4–L2 | 480 | 1,300 | |
| 5 | 13/F | 4CN | 80 | 54 | 20 | T6–T10 | T4–L4 | 280 | 250 | |
| 6 | 12/F | 6C-TL | 104 | 80 | 39 | T12–L4 | T4–L5 | 420 | 2,000 | |
| 7 | 16/F | 3CN | 96 | 78 | 36 | T12–L3 | T5–L4 | 270 | 1,200 | |
| 8* | 14/F | 3AN | 91 | 68 | 20 | T6–T12 | T3–L1 | 480 | 1,800 | Ventilator support (24 h) |
| 9 | 17/F | 3A+ | 81 | 58 | 22 | T6–T12 | T3–L2 | 360 | 900 | Ventilator support (12 h) |
| 10 | 17/M | 2C+ | 84 | 52 | 35 | T6–T11 | T2–L1 | 520 | 2,200 | |
| 11* | 16/F | 2BN | 92 | 68 | 40 | T7–L1 | T2–L2 | 420 | 3,400 | Hyperuresis |
| 12 | 12/F | 4CN | 87 | 68 | 14 | T6–T12 | T5–L4 | 410 | 1,900 | |
| 13 | 15/F | 3C+ | 80 | 59 | 30 | T6–T11 | T5–L3 | 450 | 1,500 | |
| 14 | 38/F | 6CN | 80 | 48 | 20 | T11–L3 | T4–L3 | 420 | 1,200 | Sec. chest tube |
| 15* | 28/F | 4A+ | 95 | 90 | 55 | T7–T12 | T4–L3 | 420 | 4,500 | Ventilator support (24 h) VDS rod breakage |
| 16 | 39/F | 3C+TL | 99 | 80 | 50 | T6–T11 | T3–L4 | 360 | 2,000 | |
| 17 | 15/F | 3C+ | 82 | 74 | 30 | 6–T11 | T5–L4 | 300 | 400 | |
| 18* | 16/M | 2BN | 112 | 74 | 36 | T6–T12 | T4–L2 | 480 | 3,000 | |
| 19* | 29/F | 4CN | 82 | 78 | 44 | T6–T12 | T4–L1 | 390 | 1,200 | |
| 20 | 15/F | 2CN | 88 | 51 | 16 | T7–T12 | T2–T12 | 360 | 1,000 | |
| 21 | 15/M | 4CN | 96 | 60 | 28 | T6–T12 | T3–L1 | 360 | 750 | |
| 22* | 14/F | 2AN | 96 | 78 | 30 | T7–L2 | T4–L3 | 540 | 1,200 | Subileus |
| 23 | 14/F | 6CN | 85 | 85 | 22 | T12–L4 | T4–L4 | 400 | 800 | |
| 24 | 15/F | 6CN | 80 | 54 | 14 | T12–L3 | T4–L4 | 360 | 800 | |
| 25* | 28/F | 4A+ | 106 | 90 | 41 | T6–T12 | T3–L4 | 480 | 1,800 | Ventilator support (6 h) |
| 26* | 14/F | 4C+ | 102 | 80 | 22 | T6–L1 | T5–L4 | 510 | 1,100 | |
| 27 | 12/F | 3C+ | 84 | 60 | 21 | T7–T12 | T5–L4 | 440 | 700 | |
| 28 | 14/F | 4C- | 114 | 78 | 35 | L1–L4 | T3–L4 | 420 | 1,500 | Superficial revision |
| 29* | 15/F | 4A+ | 109 | 65 | 33 | T7–T12 | T2–L2 | 480 | 1,000 | |
| 30 | 14/F | 6CN | 80 | 50 | 22 | T12–L4 | T5–L4 | 410 | 900 | |
| 31 | 2/F | 4A+ | 96 | 88 | 18 | T6–T12 | T4–L1 | 480 | 1,200 | |
| 32 | 16/F | 3B+ | 95 | 72 | 47 | T5–T11 | T3–L2 | 325 | 500 | |
| 33* | 16/M | 2A- | 108 | 68 | 36 | T6–T12 | T4–L3 | 530 | 4,700 |
Patient marked with an asterisk (*) received posterior release prior anterior–posterior instrumentation
Preoperatively, all patients underwent a careful history and physical examination. Preoperative radiographs consisted of long cassette anterior-posterior and lateral standing radiographs (Fig. 1a, b), as well as supine right and left bending films (Fig. 1c, d). In cases of juvenile onset, rapid curve progression or absent superficial abdominal reflexes, magnetic resonance images of the spinal canal and its contents were obtained.
Fig. 1.
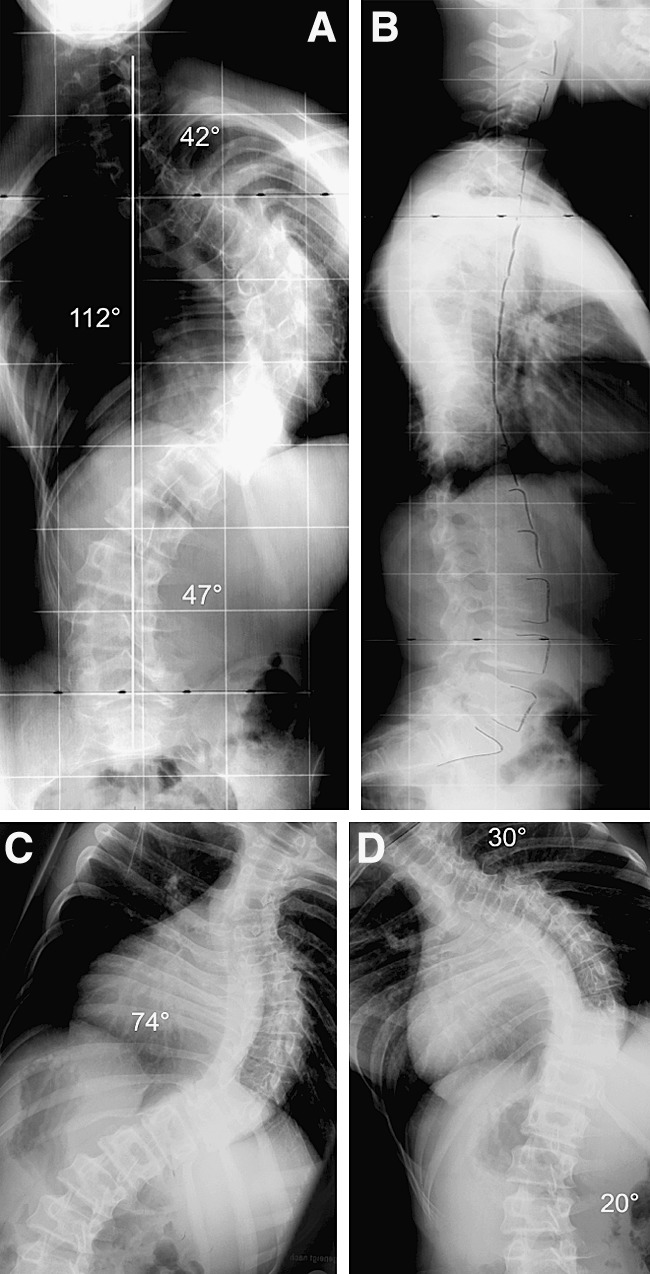
a–d A 15-year-old boy (patient 18) with a severe idiopathic thoracic scoliosis type Lenke 2BN and a 112° Cobb angle corrected in bending films to 74°
Patient’s evaluation consisted of clinical (Fig. 4a–c) and radiographic (Figs. 2a, b, 3a, b) analysis preoperatively, postoperatively and at final follow-up. Clinical measurements included rib hump, lumbar hump, trunk decompensation and shoulder levels. Preoperative parameters such as duration of surgery, intraoperative blood loss, use of cell saver, amount of blood transfusions and prolonged ventilator support as well as the day of the removal of the chest drain were documented. Complications were differentiated into intraoperative, early postoperative (during hospital stay) and late complications (after dismissal).
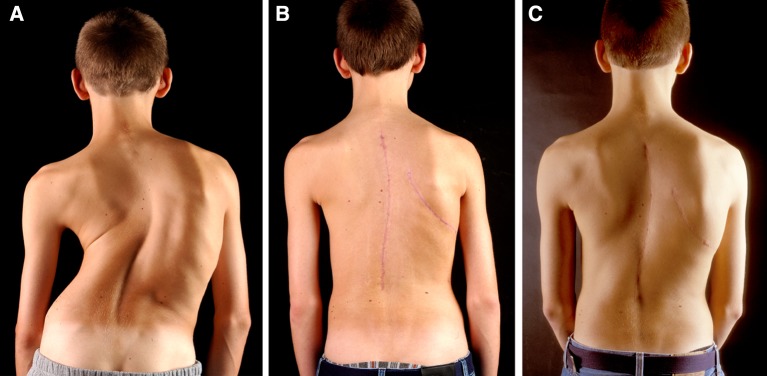
Fig. 4.
Clinical pictures of patient 18 preoperatively (a), 2 weeks postoperatively (b) and 2 years after operation (c)
Fig. 2.
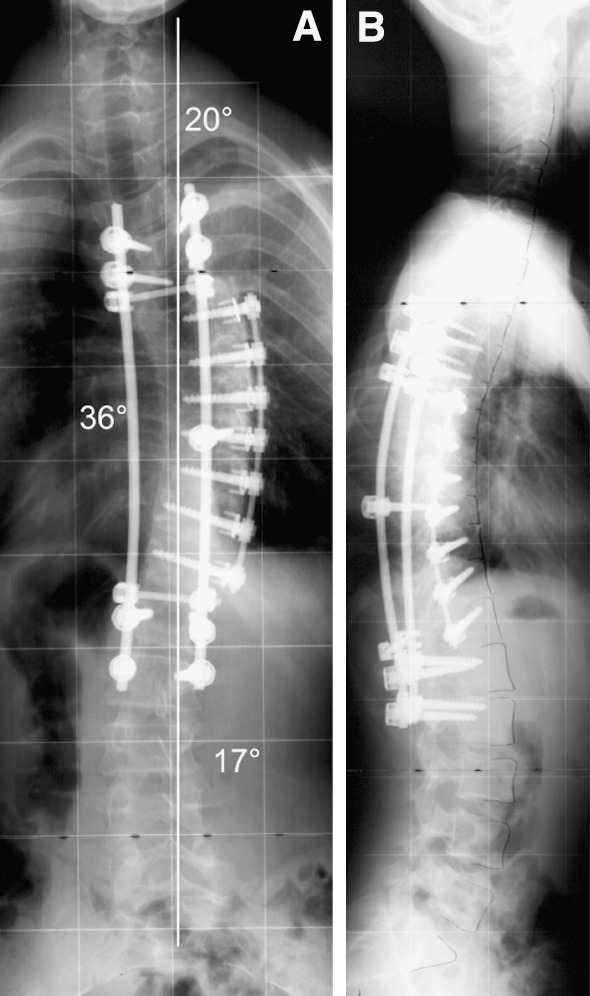
a, b The patient underwent a posterior release, anterior instrumentation from T6 to T12 and posterior instrumentation from T4 to L2 with thoracoplasty. Postoperatively the Cobb angle was corrected from 112 to 36° with a normal sagittal profile
Fig. 3.
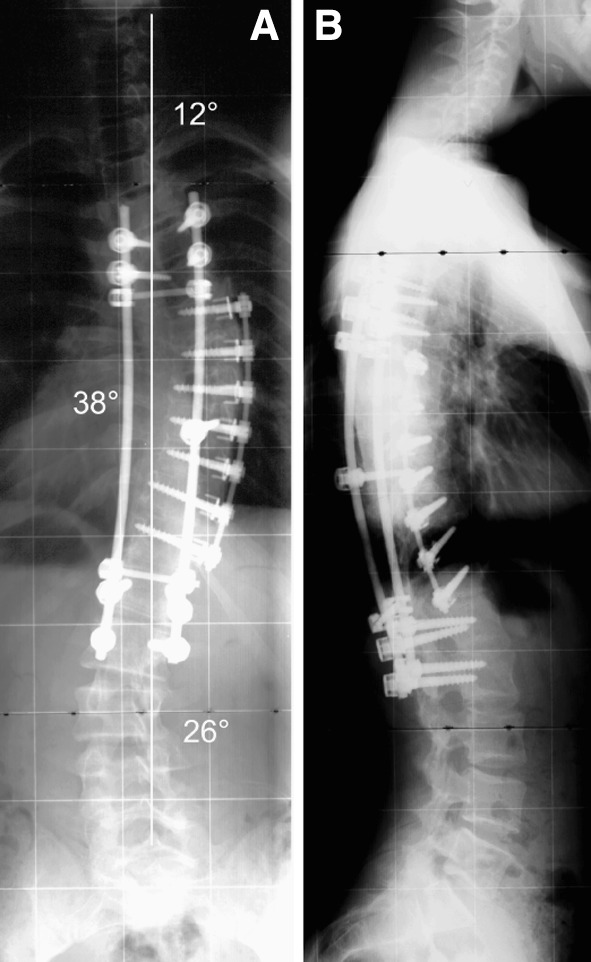
a, b At the 24 months follow-up no relevant loss of correction in the thoracic curve but slight lumbar curve progression to from 17 to 26° Cobb angle was observed
Radiographic analysis included Cobb angle measurements of the primary and secondary curves. The apical vertebral rotation was measured according to Perdriolle [15]. Furthermore, the tilt angle of the lower end-vertebra was documented. Translation of the apical vertebra of the primary and secondary curve was measured as the distance of the centre of the apical vertebra from the centre sacral vertical line (CSVL) in centimetres [13]. Shoulder balance was measured comparing the points of intersection of the clavicles with the first or second rib. Trunk decompensation was determined as the deviation of the plumb line from the spinous processes of C7 and S1 in centimetres. Sagittal plane analysis included measurement of thoracic kyphosis (T4–T12), thoracolumbar junction (T10–L2) and lumbar lordosis (L1–L5). Sagittal trunk decompensation was measured as the deviation of the plumb-line from C7 to the centre of the disc L5/S1 in centimetres [2].
Implants and surgical technique
In all patients, anterior release with complete disc excision and a Zielke-VDS Instrumentation of the main curve were performed [22]. In eight patients, the lumbar curve and in 25 patients, the thoracic curve was exposed anteriorly. All anterior thoracic instrumentations were done under single-lung ventilation, which was changed to a regular tube for the posterior procedure. In twelve patients, with an average Cobb angle of 102° and an averaged flexibility of less than 20%, the anterior instrumentation was preceded by a posterior release with osteotomies of the ankylosed facet joints including concave rib osteotomies. Finally, the patient was placed in a prone position and a posterior instrumentation, correction and fusion was performed using the posterior dual rod system (Depuy Spine, Leeds, England). In the upper thoracic spine, mostly hooks were used, the middle and lower thoracic as well as the lumbar spine were instrumented with pedicle screws. Posterior correction was achieved via the rod rotation manoeuvre in combination with the cantilever technique. The posterior instrumentation did not add any further correction to the anteriorly instrumented spine, but addressed the adjacent segments and the structural secondary curves. After posterior correction, a wake-up test was performed in all patients. Autologous bone graft either from the iliac crest or from the ribs was used for fusion. In two patients with a persistent prominent rib hump, a convex thoracoplasty was done during the posterior procedure. In three patients, the anaesthesiologist recommended to stage the posterior procedure due to an increased operating time with a high blood loss and an impaired cardiopulmonary condition. In these patients the posterior instrumentation was carried out 12–14 days after the anterior procedure.
Until the chest drain was removed all patients received intensive breathing exercises and i.v. antibiotics. All patients were mobilized without any external support and sport activities, apart from swimming were not allowed until one year postoperatively.
Statistical evaluation was performed with the Wilcoxon signed-rank test and a level of significance of 5%.
Results
Basic data
Surgery was performed in one stage in all but three patients. A posterior release was done in 12 highly severe and rigid curves with a mean Cobb angle of 102° and averaged flexibility of 20%. Total operating time averaged 412 min (standard deviation/SD 78 min, range 250–540 min). Mean intraoperative blood loss was 1,533 ml (SD 963 ml, 250–4,500 ml). In nearly all cases, the cell saver system was employed with an average retransfusion of 372 ml (0–2,100 ml). Furthermore, patients received 2.2 units (0–4 units) of predonated blood on average. Fifteen patients needed additionally an average of 2.5 units (1–4 units) of homologous blood. Twenty-nine patients were extubated immediately after surgery. Four patients required a postoperative ventilatory support on the intensive care unit for an average of 24 h. The chest drain was removed after a mean time of 4.3 days (3–6 days). Patients were discharged on average 15 days (13–25 days) postoperatively.
Clinical and radiographic data
The rib hump was reduced from 23° preoperatively to 11° postoperatively without any loss of correction at final follow-up (52% correction). The lumbar hump was corrected from 10 to 3° without relevant changes during follow-up (70% correction).
The average number of anteriorly fused segments was 5.3 (SD 1.1, 3–8 segments) with an average number of segments of the primary curve being 6.0 (SD 1.0, 4–8 segments). The number of posteriorly fused segments was 11.2 on average (SD 1.2, 9–13 segments). All thoracic curves were included into the fusion. Two patients were fused to L5, twelve patients to L4, six patients to L3, seven patients to L2, five patients to L1 and one patient to T12 (Table 1). In the thoracic spine, the proximal fusion level corresponded in most cases to the upper end-vertebra. The lumbar spine was included into the fusion in cases of a structural lumbar curve (Lenke Type 3C, 4C and 6C).
The preoperative Cobb angle of the primary curve averaged 93.4° (SD 12.2°, 80–122°) and corrected to 72.1° (SD 17.1°, 48–118°) on bending films (23% correction). Primary curve correction averaged 67% with a mean postoperative Cobb angle of 31.0° (SD 11.8°, 14–56°). Final correction at follow-up was 65% on average with a mean loss of correction of 1.9°. In 25 patients with a major and anteriorly instrumented thoracic curve, this was corrected from 93.4° (SD 11.9°, 80–122°) to 72.9° (SD 17.9°, 51–118°) on bending films and to 32.1° (SD 12.2°, 14–56°) postoperatively with 1.3° loss of correction at follow-up. In eight patients with a major and anteriorly instrumented lumbar curve, this was corrected from 93.5° (SD 14.6°, 80–116°) to 69.5° (SD 15.0°, 48–85°) on bending films and to 27.2° (SD 9.9°, 14–39°) postoperatively with 4.1° loss of correction at follow-up.
The mean apical vertebral rotation of the primary curve was 38.9° (SD 9.0°, 25–60°) preoperatively and 19.7° (SD 7.8°, 10–42°) postoperatively without any loss of correction during follow-up (49% correction). The tilt of the lowest instrumented vertebra was corrected from 31.1° (SD 11.3°, 10–60°) to 9.9° (SD 6.1°, 0–28°) and measured 8.9° (SD 4.5°, 0–20°) at final follow-up (71% correction). Translation of the thoracic apical vertebra from the CSVL was corrected from 7.0 cm (SD 2.9 cm, 1.5–12.5 cm) to 1.2 cm postoperatively (SD 1.9 cm, 2.5–7.7 cm) and to 1.9 cm (SD 1.5 cm, 0–5.3 cm) at follow-up. In the lumbar curves, the translation of the apical vertebra was reduced from 2.5 cm (SD 2.1 cm, 0–8.0 cm) preoperatively to 1.9 cm (SD 1.3 cm, 0–4.6 cm) postoperatively and to 1.6 cm (SD 1.1 cm, 0–4.6 cm) at final follow-up. Shoulder imbalance measured 0.9 cm (SD 1.1 cm, 0–5 cm) preoperatively, 0.9 cm (SD 0.7 cm, 0–2.5 cm) postoperatively and 0.5 cm (SD 0.5 cm, 0–1.5 cm) at follow-up. Frontal plane trunk decompensation averaged 1.2 cm preoperatively, 1.5 cm postoperatively and 1.0 cm at final follow-up.
Thoracic kyphosis measured 35.4° (SD 17.0°, −10–77°) preoperatively, 32.5° (SD 11.4°, 10–58°) postoperatively and 32.7° (SD 13.2°, 8–72°) at follow-up. Out of 12 patients with an either hyperkyphotic (n=10) or hypokyphotic (n=2) thoracic spine, a normal kyphosis could be restored in nine cases. Mean thoracolumbar junction measured 10.9° (SD 10.9°, 0–42°) preoperatively, 6.3° (SD 5.4°, 0–22°) postoperatively and 4.9° (SD 3.8°, 0–14°) at final follow-up. Six patients had a preoperative thoracolumbar hyperkyphosis of more than 20°. In all these cases, a correction to normal values was achieved. Two of these patients (patient 2, 6) have had a curve type 6C and were instrumented with the VDS in the lumbar spine. The kyphosis was corrected from 39° to 3° in patient 2 and from 42° to 10° in patient 6. Mean lumbar lordosis measured −50.3° (SD 15.1°, -23° to −82°) preoperatively, −43.3° (SD 11.7°, −22 to −69°) postoperatively and −45.7° (SD 9.1°, −27 to −63°) at final follow-up.
Complications
One patient required an additional chest tube on the contralateral side due to progressive pleural effusion on the third postoperative day. Another patient developed a subileus which was treated conservatively. One patient experienced polyuria which subsequently settled with conservative treatment and without any further adverse effects. In one patient, a superficial wound revision was required on the third postoperative day due to a torn wound drain. There were no neurological complications or any deep wound infections. In one patient, a fracture of the threaded VDS rod in the cephalad segment without breakage of the posterior rods was noted after 6 months without any loss of correction or signs of pseudarthrosis. In one patient with a persistent rib hump (patient 2), a secondary rib-hump resection was performed 4 years after the index procedure.
Discussion
To optimize curve correction, minimize the neurological risk and to eliminate both patient discomfort and a prolonged hospital stay resulting from halo traction we combined the anterior Zielke Instrumentation with a posterior multiple hook and pedicle screw construct in mostly one-stage surgery to treat severe and rigid idiopathic scoliosis. In 12 highly rigid and severe curves a posterior release was performed prior to the anterior instrumentation to achieve a 360° release. The presented results of 33 prospectively followed patients with a mean preoperative Cobb angle of 93° demonstrate an average primary curve correction of 67% despite a preoperative curve flexibility in the supine bending test of only 23% on average. Compared to the supine bending films traction or fulcrum bending films have shown to correlate better with the operatively achieved correction especially in thoracic curves and posterior fusion [6, 9, 19]. However, supine bending films are still needed to determine the fusion levels [19]. In the present study the bending tests were applied to determine fusion levels and to have comparable data as most studies even on severe curves use the bending test (Table 2).
Table 2.
Data on the literature
| Authors | Procedure | Study design | FU in month | n | Age in year | Pre-op Cobb angle | Flexibility | Post-op Cobb angle | Correction | Loss of correction | Complications | Op time (min) | Blood loss (ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arlet et al. [1] | PIF screws + hooks | r | 32 (18–64) | 15 th. AIS | 15 (11-18) | 79° (70–90°) | 32% | 35° (25–40°) | 54% | 1.1° | 1 exc. bleeding 2 deep infections |
314 | 1,875 |
| Chang et al. [5] | PIF screws | r | 34 (24–52) | 26 AIS | 31 (11-61) | 98° (75–135°) | 18% | 32° | 67% | 1 hemothorax 1revision |
|||
| Kuklo et al. [12] | PIF screws | r | 39 (24–62) | 20 th. AIS | 19 (11-44) | 100° (90–135°) | 29% | 32° (9–73°) | 68% | 2 medial screws 2 temp. decrease MEP |
|||
| Byrd et al. [4] | AIF+PIF (n=14) AR +PIF (n=12) two-staged | r | 49 (24–114) | 26 AIS | 38 (19-58) | 83° (45–160°) | 29% | 44° (10–97°) | 47% | 6° | 1 death 2 neurol. transient palsy 2 implant related compl. |
520 | 3,831 |
| Bradford et al. [3] | AR+PIF | r | 24 | 7 AIS | 24 (7-54) | 91° (80–118°) | ? | 37° (22-60°) | 59% | 3 dural tear 1 deep infection 1 quad. palsy |
730 | 5,500 | |
| Korovessis et al. [11] | AIF+PIF two-staged | r | 32 (12–72) | 34 AIS | 16 (13-29) | 73° th. (40–103°) 70° lu. (40–103°) |
26% th. 53% lu. |
35° (5–75°) th 21° (4–44°) lu. |
52% th. 70% lu.. |
1 exc. bleeding 1 Harrington rod fracture 1 meralgia paresthetica 6 sympathectomysyndrom |
2,662 | ||
| Klockner et al. [10] | AIF+PIF two-staged | r: n=9 p: n=14 | 176 | 23 AIS King II | 14,7 | 68° th. 61° lu. |
33% th. 39% lu.. |
13,2° th. 17,8° lu. |
71% th. 55% lu. |
2 prox. VDS screw loosening | 463 | 2,000 | |
| Shufflebarger et al. [16] | AR+PIF hooks one-staged n=16, two-staged n=10 | R | 35 (24–72) | 26 AIS/75 | (14-71) | 45% one-staged 37% two-staged |
1 respiratory insufficience 1 acute tubulus necrosis 1 hook displacement |
378 one-staged 510 two-staged | 1,580 one-staged 3,060 two-staged | ||||
| Tokunaga et al. [18] | AR + PIF | R | 96 (42–158) | 14 AIS/21 | 17 (10-25) | 107° | 26% Traction | 56° (34–100°) | 52% | 2.5° | 1 transient neurol. complication 3 prolongated ventilation |
293 +PIF | 1,600 +PIF |
| Winter et al. [21] | AIF+PIF two-staged | R | 5 adult | 38 (33-43) | 58° th. 79° lu. |
31% th. 29% lu. |
24% th. 46% lu. |
5° | None | 2,080 | |||
| Present study | AIF+PIF (hooks+screws) one-staged n=30, two-staged n=3 | P | 34 (24–78) | 33 AIS | 18 (11-39) | 93° (80–122°)° | 23% | 31° (14–56°) | 67% | 1.9° | 1 add. chest tube 1 subileus, 1 polyuria 1 superficial wound revision 4 prolongated ventilation |
412 | 1,533 |
AIS Adolescent idiopathic scoliosis, AR Anterior release, AIF Anterior correction and instrumented fusion, PIF Posterior correction and instrumented fusion, p Prospective study, R Retrospective study
A commonly performed procedure in the more severe and rigid idiopathic curves is posterior instrumentation combined with an anterior release. Byrd et al. published a series of 26 cases with a preoperative Cobb angle of 83° and a flexibility of 29% on preoperative bending films [4]. With anterior release and posterior Harrington Instrumentation, they achieved a final curve correction of 47%. Using a multiple posterior hook instrumentation preceded by an anterior release Shufflebarger reported curve correction rates between 37 and 45% in severe idiopathic curves [16]. Bradford performed an anterior vertebral column resection with posterior instrumentation in seven patients with high degree idiopathic curves (average Cobb angle 91°) and achieved an average correction of 59% [3]. Other authors applied Halo traction between the anterior release and posterior instrumentation and reported an average curve correction of 52% in 14 patients with severe idiopathic curves [18]. In 12 out of 33 patients with extremely severe curves beyond 100° or a flexibility of on average less than 20% a posterior release was done prior to the anterior procedure. Harms et al. recommend a posterior release prior to an anterior instrumented correction and fusion in thoracic curves larger than 75° Cobb angle [8]. Overall, we could not find an increased blood loss or operating time in comparison to other studies on the surgical treatment of severe idiopathic scoliosis (Table 2). There were no complications related to the posterior release.
Few authors report on exclusive posterior instrumentation without an additional anterior release in these type of curves. Chang et al. published a series of 41 patients with rigid and severe curves with an average preoperative Cobb angle of 98° and a preoperative flexibility of less than 30% (18% on average). The curves were either congenital, neuromuscular or idiopathic. The authors achieved a curve correction of 67% with an exclusive posterior approach applying the cantilever technique in combination with a multiple pedicle screw instrumentation [5]. Arlet et al. reported on 15 patients with idiopathic thoracic scoliosis with a Cobb angle between 70 and 90° and a flexibility index of 32% and a correction of 54% with third-generation posterior segmental instrumentation [1]. Kuklo et al. recently reported on 20 patients with idiopathic thoracic scoliosis and a Cobb angle of more than 90° and a mean flexibility of 29% on bending films. With posterior segmental pedicle screw instrumentation, a correction of 68% was achieved. Only in three cases, an anterior release was done prior to posterior instrumentation [12]. Data on the transverse plane correction including clinical data on rib-hump correction, however, were not provided. The authors concluded that due to the superior biomechanical properties of a segmental pedicle screw construct anterior release procedures are only indicated in very few cases.
In our study, the primary curve was released and instrumented anteriorly with additional posterior instrumentation to achieve a safe and satisfactory three-dimensional curve correction. The combination of anterior and posterior instrumentation in the management of severe idiopathic scoliosis has been published by several authors. Korovessis et al. combined the Zielke and the Harrington instrumentation for idiopathic scoliosis of up to 110° Cobb angle and achieved an average curve correction of 70% in lumbar and 51% in thoracic curves [11]. Klockner et al. published a series of moderate-sized curves of less than 70° Cobb angle and reported an average curve correction between 70 and 80% [10].
In our study, 30 of 33 patients were operated in one stage. In the remaining three patients the anaesthesiologist recommended a staged procedure due to an increased operating time with a high blood loss and an impaired cardiopulmonary condition of the patient. Shufflebarger et al. compared one-stage vs two-stage procedures and found a better curve correction as well as a reduced morbidity in the one-stage group [16]. This is supported by Bradford et al. who advocate a one-stage surgery unless the operating time for the anterior procedure exceeds 3 h or the intraoperative blood loss exceeds 1,000 ml [3]. Other authors argue in favour of a staged procedure in order to apply an interoperative period of halo traction [11].
In this series there were no cases of pseudarthrosis, which may be referred to the combined anterior and posterior fusion of the primary curve. The high safety of the presented treatment strategy in terms of no neurological complications might be explained with the anteriorly compressing and shortening effect of the Zielke-VDS and its prevention of any potential overdistraction by the posterior instrumentation. Bradford et al. reported on two neurological complications after anterior–posterior vertebral column resection and posterior instrumentation in a series of seven idiopathic scoliosis [3]. There was one patient with a transient unilateral foot muscle weakness which resolved after 1 week and one patient had an unilateral quadriceps weakness requiring revision surgery with decompression [3]. Shufflebarger et al. pointed out that there were more complications in the staged than in the continuous group. The authors report on one death and one transient cauda equina syndrome, both patients with neuromuscular curves and staged procedure [16]. Neurological complications after combined anterior and posterior instrumentation have not been published [10, 11].
Segmental pedicle screw instrumentation especially on the concave side might theoretically increase the neurological risk since the pedicles are smaller and the spinal cord is shifted to the concavity with a very limited epidural safe zone. Thus, the spinal cord may be jeopardized in cases of medial pedical screw perforation [14]. However, clinical studies have not been able to demonstrate an increased rate of pedicle screw malplacement in the more severe curves [12, 17]. In the cohort of Kuklo et al. one patient with two medial misplaced screws had been revised.
Delank et al. pointed out the risks of a too aggressive correction with pedicle screws baring the risk of overdistraction and ischaemia of the spinal cord. Considerable pressure loads on the spinal cord can occur through traction over an intraspinal hypomochlion [7]. Wilber et al. defined an intraoperative correction exceeding the preoperative bending correction as one of the factors related to an increased risk for spinal cord injury [20]. The advantages of anterior instrumentation in severe scoliosis are in our mind the reduced neurological risk due to convex compression and thus shortening of the spine, the true segmental derotation and the increased stability in combination with posterior instrumentation. However, it remains unclear which approach is the best in these severe and rigid curves.
Posterior instrumentation and correction only or in combination with an anterior release may be associated with an increased neurological risk and is certainly not able to provide a true segmental derotation as achieved with the anterior instrumentation. Furthermore, pedicle screw instrumentation in the concavity in severe curves is technically difficult, the pedicles are very thin and the spinal cord is shifted to the concavity with literally no epidural safe zone [14].
Conclusion
The clinical and radiographic results of 33 patients with severe and rigid idiopathic scoliosis treated by means of combined anterior and posterior instrumentation were evaluated. Correction of the primary curve averaged 67% without any relevant loss of correction at the latest follow-up. There were no cases of pseudarthrosis, deep wound infections or any neurological complications. One-stage anterior and posterior instrumentation is safe and enables an effective three-dimensional curve correction. Recent reports on segmental pedicle screw instrumentation indicate that with this technique an exclusive posterior procedure might be sufficient. We recommend anterior and posterior instrumentation in curves with a Cobb angle ≥90° and a flexibility of ≤30%, in extremely severe and rigid curves with Cobb angles beyond 100° a posterior release prior to the anterior–posterior procedure is beneficial.
References
- 1.Arlet V, Jiang L, Ouellet J. Is there a need for anterior release for 70–90° thoracic curves in adolescent scoliosis? Eur Spine J. 2004;13:740–745. doi: 10.1007/s00586-004-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines thoracolumbar junction. Spine 1989. 1989;14:717–721. doi: 10.1097/00007632-198907000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bradford DS, Tribus CB. Vertebral column resection for the treatment of rigid coronal decompensation. Spine. 1997;22:1590–1599. doi: 10.1097/00007632-199707150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JA, Scoles PV, Winter RB, Bradford DS, Lonstein JE, Moe JH. Adult idiopathic scoliosis treated by anterior and posterior spinal fusion. J Bone Joint Surg Am. 1987;69:843–850. [PubMed] [Google Scholar]
- 5.Chang K-W. Cantilever bending technique for treatment of large and rigid scoliosis. Spine. 2003;28:2452–2458. doi: 10.1097/01.BRS.0000092063.63315.D5. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KM, Luk KDK. Prediction of correction of scoliosis with use of the fulcrum bending radiograph. J Bone Joint Surg Am. 1997;79:1144–1150. doi: 10.2106/00004623-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Delank KS, Delank HW, Konig DP, Popken F, Furderer S, Eysel P. Iatrogenic paraplegia in spinal surgery. Arch Orthop Trauma Surg. 2005;125:33–41. doi: 10.1007/s00402-004-0763-5. [DOI] [PubMed] [Google Scholar]
- 8.Harms J, Jeszenszky D, Beele B. Ventral correction of thoracic scoliosis. In: Bridwell K, DeWald R, editors. The textbook of spinal surgery. 2. Philadelphia, PA: Lippincott-Raven; 1997. p. 40. [Google Scholar]
- 9.Klepps SJ, Lenke GL, Bridwell KH, Bassett GS, Whoton J. Prospective comparison of flexibility radiographs in adolescent idiopathic scoliosis. Spine. 2001;26:E74–E79. doi: 10.1097/00007632-200103010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Klockner C, Walter G, Matussek J, Weber U. Ventrodorsal correction and instrumentation in idiopathic scoliosis. Orthopade. 2000;29:571–577. doi: 10.1007/s001320050495. [DOI] [PubMed] [Google Scholar]
- 11.Korovessis P. Combined VDS and Harrington instrumentation for treatment of idiopathic double major curves. Spine. 1987;12:244–250. doi: 10.1097/00007632-198704000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Kuklo TR, Lenke LG, O’Brien MF, Lehman RA, Polly DW, Schroeder TS. Accuracy and efficacy of thoracic pedicle screws in curves over 90 degrees. Spine. 2005;30:222–226. doi: 10.1097/01.brs.0000150482.26918.d8. [DOI] [PubMed] [Google Scholar]
- 13.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169–1181. [PubMed] [Google Scholar]
- 14.Liljenqvist UR, Allkemper T, Hackenberg L, Link TM, Steinbeck J, Halm HFH. Analysis of vertebral morphology in idiopathic scoliosis with use of magnetic resonance imaging and multiplanar reconstruction. J Bone Joint Surg Am. 2002;84:359–368. doi: 10.2106/00004623-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Perdriolle R. The torsion meter: a critical review. J Pediatr Orthop. 1991;10:909–1015. [PubMed] [Google Scholar]
- 16.Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16:930–933. doi: 10.1097/00007632-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Suk S, Kim WJ, Lee SM, Kim JH, Chung ER. Thoracic pedicle screw fixation in spinal deformities. Spine. 2001;26:2049–2057. doi: 10.1097/00007632-200109150-00022. [DOI] [PubMed] [Google Scholar]
- 18.Tokunaga M, Minami S, Kitahara H, Isobe K, Nakata Y, Moriya H. Vertebral decancellation for severe scoliosis. Spine. 2000;25:469–474. doi: 10.1097/00007632-200002150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan JJ, Winter RB, Lonstein JE. Comparison of the use of supine bending and traction radiographs in the selection of fusion area in adolescent idiopathic scoliosis. Spine. 1996;21:2469–2473. doi: 10.1097/00007632-199611010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Wilber RG, Thompson GH, Shaffer JW, Brown RH, Nash CL., Jr Postoperative neurological deficits in segmental spinal instrumentation. A study using spinal cord monitoring. J Bone Joint Surg Am. 1984;66:1178–1187. [PubMed] [Google Scholar]
- 21.Winter RB. Combined Dwyer and Harrington instrumentation and fusion in the treatment of selected patients with painful adult idiopathic scoliosis. Spine. 1978;3:135–141. doi: 10.1097/00007632-197806000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Zielke K. Ventral derotation spondylodesis. Results of treatment of cases of idiopathic lumbar scoliosis. Z Orthop und Ihre Grenzgeb. 1982;120:320–329. doi: 10.1055/s-2008-1051620. [DOI] [PubMed] [Google Scholar]