Abstract
A novel single-stranded RNA (ssRNA) virus specifically infecting the bloom-forming diatom Rhizosolenia setigera (R. setigera RNA virus [RsRNAV]) was isolated from Ariake Sea, Japan. Viral replication occurred within the cytoplasm, and the virus particle was icosahedral, lacked a tail, and was 32 nm in diameter on average. The major nucleic acid extracted from the RsRNAV particles was an ssRNA molecule 11.2 kb in length, although smaller RNA molecules (0.6, 1.2, and 1.5 kb) were occasionally observed. The major structural proteins of RsRNAV were 41.5, 41.0, and 29.5 kDa. Inter- and intraspecies host specificity tests revealed that RsRNAV is not only species specific but also strain specific and that its intraspecies host specificity is diverse among virus clones. The latent period of RsRNAV was 2 days, and the burst sizes were 3,100 and 1,010 viruses per host cell when viruses were inoculated into the host culture at the exponential and stationary growth phases, respectively, at 15°C under a 12-h-12-h light-dark cycle of ca. 110 μmol of photons m−2 s−1 with cool white fluorescent illumination. To our knowledge, this is the first report describing the biological properties of a virus infecting a diatom. Further studies on RsRNAV will be helpful in understanding the ecological relationship between diatoms and viruses in nature.
Diatoms (Bacillariophyceae) are considered to be the most widespread group of plants on earth. They are abundant not only in all waters but also in soil and on moist surfaces of rocks and plants (7). The contribution of diatoms to the world net primary production was estimated to be as high as 20 to 25% (40). Diatoms contain a great number of species and include various harmful bloom-forming species such as Coscinodiscus wailesii (21), Eucampia zodiacus (22), Rhizosolenia imbricata (28), and Pseudonitzschia species (8). Thus, a large number of studies regarding diatoms have been conducted (40).
The bloom-forming diatom Rhizosolenia setigera belongs to the order Centrales and occurs widely throughout the world, i.e., in the North Atlantic Ocean, North Sea, Baltic Sea, English Channel, Mediterranean Sea, and Pacific Ocean. In Japan, R. setigera is also commonly observed on the Pacific coast in estuaries during all seasons (33). Blooms of R. setigera have been frequently recorded during low-temperature seasons (autumn, winter, and spring), when mainly larger cells of R. setigera appear (33). A notable point is that R. setigera is one of the main species forming diatom blooms from winter through early spring in the Ariake Sea in western Kyushu, Japan, where cultivation of the seaweed laver (Porphyra tenera) is of significant economic importance. Diatom blooms have often caused depletion of nutrients and damaged P. tenera cultures due to discoloration of the thalli (22, 26, 27). In particular, from the end of 2000 through the beginning of 2001, a large diatom bloom occurred in the Ariake Sea and led to an extremely poor harvest of P. tenera and a revenue reduction of $108,000,000 compared to the year before (1999 to 2000) (28); R. setigera was recorded as one of the major constituent algae within the bloom (Y. Kawamura et al., personal communication; S. Oda et al., personal communication).
Viruses or virus-like particles (VLPs) have been found in more than 50 species in 12 of the 14 recognized classes of eucaryotic algae (34, 38, 41). Since the late 1970s, the isolation of more than 12 viruses that are infectious to marine eucaryotic microalgae has been reported. The successful isolation and maintenance of these microalgal viruses accelerated the studies on the roles of microalgal viruses in marine ecosystems, and evidence indicating a possible relationship between the viral infection and the dynamics of microalgae has gradually accumulated (1, 2, 11, 17, 20, 34). In contrast, the relationship between diatoms and viruses has scarcely been examined. While a finding of VLPs in diatom-like cells was reported by Proctor and Fuhrman (23), this was just a single account of a transmission electron microscopy study of a field material. On the other hand, although Suttle et al. (29, 30) found lytic activity of a virus-sized fraction concentrated from a seawater sample against the diatom Navicula sp. (order Pennales), the algicidal factor was later revealed to be bacterial (A. M. Chan, I. Kacsmarska, and C. A. Suttle, Abstr. Am. Soc. Limnol. Oceangr., p. 121, 1997). Thus, as far as we know, no direct evidence of viral infection in diatoms has been obtained to date.
In the present paper, the isolation and characterization of a novel virus infecting the bloom-forming diatom R. setigera (R. setigera RNA virus [RsRNAV]) is described.
MATERIALS AND METHODS
Algal cultures and growth conditions.
The algal strains used in this study are shown in Tables 1 and 2; all of them were maintained at the National Research Institute of Fisheries and Environment of Inland Sea, Hiroshima, Japan. Each algal strain was grown in modified SWM3 medium enriched with 2 nM Na2SeO3 (4, 10) under a 12-h-12-h light-dark cycle of ca. 110 μmol of photons m−2 s−1 with cool white fluorescent illumination at the temperatures shown in Table 1. All of the R. setigera strains were incubated at 15°C. In the present experiments, three strains of R. setigera (S2, S3, and RS0209A05) were used as hosts for RsRNAV strains (R. setigera S3 was lost during the experiments).
TABLE 1.
Algal strains tested in the present study
| Class | Species | Strain | Cultivation (°C) | Sensitivitya to:
|
|
|---|---|---|---|---|---|
| RsRNAV01 | RsRNA V06 | ||||
| Bacillariophyceae | Rhizosolenia setigera | S2b | 15 | + | + |
| S3b | 15 | + | NTc | ||
| RS0209A05 | 15 | + | + | ||
| Rhizosolenia imbricata | R106b | 15 | − | NT | |
| Eucampia zodiacus | a-1b | 15 | − | − | |
| EZ200101b | 15 | − | − | ||
| Skeletonema costatum | a-6b | 15 | − | − | |
| SC200112Ab | 15 | − | − | ||
| SK-1b | 15 | − | − | ||
| Ditylum brightwellii | Dityb | 15 | − | − | |
| Stephanopyxis palmeriana | SP199704b | 15 | − | − | |
| Chaetoceros curvisetum | CC200111Ab | 15 | − | NT | |
| Chaetoceros denticulatum | CDen200111Ab | 15 | − | NT | |
| Chaetoceros sociale | CS200111Ab | 15 | − | − | |
| Chaetoceros tortissimum | CT200112Ab | 15 | − | NT | |
| Chaetoceros debile | CDeb200111Ab | 15 | − | − | |
| Chaetoceros didymum | CDid200111Ab | 15 | − | − | |
| Chaetoceros affinis | a-6b | 15 | − | − | |
| Chaetoceros sp. | CM-4b | 15 | − | − | |
| CSP200101b | 15 | − | − | ||
| Thalassiosira rotula | TR200201b | 15 | − | − | |
| a-1b | 15 | − | − | ||
| HT-2b | 15 | − | − | ||
| Eustigmatophyceae | Nannochloropsis sp. | SFBB | 20 | − | − |
| Chlorophyceae | Oltmannsiellopsis viridis | Olt2-4 | 20 | − | − |
| Dinophyceae | Alexandrium catenella | ACNG | 15 | − | − |
| Gymnodinium catenatum | GC16-6 | 15 | − | − | |
| Gymnodinium mikimotoi | G303ax-2 | 20 | − | − | |
| Gymnodinium sanguinum | GSUR | 20 | − | − | |
| Heterocapsa circularisquama | HU9433-P | 20 | − | − | |
| HA92-1 | 20 | − | − | ||
| Heterocapsa triquetra | Ht | 20 | − | − | |
| Prorocentrum micans | 8304 | 20 | − | − | |
| Prorocentrum triestinum | Pt-7 | 20 | − | − | |
| Scrippsiella sp. | SCKR | 20 | − | − | |
| Euglenophyceae | Eutreptiella sp. | Eut-ax01 | 20 | − | − |
| Raphidophyceae | Chattonella antiqua | AR2 | 20 | − | − |
| Chattonella marina | CmUR976 | 20 | − | NT | |
| Chattonella ovata | CoV | 20 | − | − | |
| Chattonella verrculosa | M | 15 | − | − | |
| Fibrocapsa japonica | Fib-1 | 20 | − | − | |
| FE985 | 20 | − | − | ||
| Heterosigma akashiwo | H93616 | 20 | − | − | |
| KG95 | 20 | − | − | ||
+, algal lysis; −, no effect.
Algal strains used for screening of lytic viruses.
NT, not tested.
TABLE 2.
Intraspecies specificity of RsRNAV strains against R. setigera strains
| R. setigera strain | Sensitivitya to RsRNAV:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | |
| RS030121A01 | + | − | − | + | + | + | + | + | + |
| RS030121A02 | − | + | + | − | − | − | − | − | − |
| RS030121A03 | + | + | + | − | − | + | + | + | − |
| RS030121A04 | − | − | − | − | − | − | − | − | − |
| RS030121A05 | + | + | + | + | + | + | + | + | + |
| RS030121A06 | − | − | − | + | − | + | + | − | − |
| RS030121A07 | + | + | + | + | + | + | + | + | + |
| RS030121A08 | − | − | − | − | − | − | − | − | − |
| RS030121A09 | + | + | − | + | − | + | + | + | + |
| RS030121A10 | − | − | − | − | − | − | − | − | − |
| RS030121A11 | − | − | − | + | − | + | − | − | − |
| RS030121A12 | + | + | + | + | + | + | + | + | + |
| RS030121A13 | + | + | + | + | + | + | + | + | + |
| RS030121A14 | − | − | − | − | − | − | − | − | − |
| RS030121A15 | + | + | + | + | + | + | + | + | + |
| RS030121A16 | + | + | − | + | + | + | + | + | + |
| RS030121A17 | − | − | + | − | + | + | + | + | + |
| RS030121A18 | + | + | + | + | + | + | + | + | + |
| RS030121A19 | − | − | + | − | − | − | − | − | − |
| RS030121A20 | + | + | + | + | + | + | + | + | + |
| RS030121A21 | + | + | + | + | + | + | + | + | + |
| RS200111A | + | + | + | + | + | + | + | + | + |
| RS0209A05b | + | + | + | + | + | + | + | + | + |
| S2b | + | + | + | + | + | + | + | + | + |
| S15 | + | + | + | + | + | + | + | + | + |
| S3b | + | NTc | NT | NT | NT | + | NT | NT | NT |
+, algal lysis; −, no effect.
Typical strains mainly used as a host in the present study.
NT, not tested.
Clonal pathogens used in the experiments.
Surface water was collected off Oura Port in the Ariake Sea, Saga Prefecture, Japan, on 24 April 2002, when an unknown species of cryptophyta was dominant, and was sent to the laboratory within 24 h of sampling. The seawater sample was gently filtered through a 0.2-μm-pore-size polycarbonate membrane filter (Nuclepore). Aliquots (0.2 ml) of the filtrate were inoculated into exponentially growing cultures (0.6 ml) of the 22 diatom strains (Table 1) and were incubated at 15°C under the light conditions described above. Control cultures were inoculated with SWM3. In the R. setigera S3 culture inoculated with the filtrate, an apparent inhibition of algal growth was detected. In order to obtain more viral isolates, an aliquot (0.6 ml) of fresh R. setigera S3 culture was dispensed into 12 wells of a microplate (Costar 3513), and 0.2 ml of the filtered seawater sample was inoculated. After incubation of the wells under similar conditions, host cell lysis was observed in 10 of the 12 wells. From each well where algal lysis had occurred, a clonal pathogen was obtained through two cycles of the extinction dilution procedure (18, 31). Lysates in the most diluted wells of the second assay were sterilized by filtration through 0.1-μm-pore-size polycarbonate membrane filters and transferred to an exponentially growing culture of R. setigera S3. The resultant lysates were regarded as the clonal pathogen suspensions. Nine clonal and axenic pathogens were obtained (one was lost) and designated RsRNAV01 to RsRNAV09. For each clonal pathogen, serial transfers of a lysed culture to an exponentially growing culture of R. setigera were performed more than twice to verify its transferability.
An aliquot (5%, vol/vol) of each pathogen suspension was added to an exponentially growing culture of R. setigera S3. Algal growth was monitored by optical microscopy every day. Five days after inoculation, when cell lysis occurred, an aliquot of each lysate was collected and its titer was estimated by the extinction dilution method. Clonal pathogens RsRNAV01 and RsRNAV06, which had the highest yields among the nine pathogenic clones, were principally examined in the present experiments.
Transmission electron microscopy.
Duplicate R. setigera S3 cultures were inoculated with 0.5% (vol/vol) of a fresh RsRNAV01 suspension (∼107 infectious units ml−1). Control cultures were inoculated with SWM3. Subsamples were withdrawn at 0, 24, 48, 72, and 96 h after inoculation, fixed with 1% glutaraldehyde, and harvested by low-speed centrifugation (2,000 rpm, 10 min, 4°C, TOMY LC-100 rotor). The cell pellets were postfixed for 3 h in 2% osmic acid (in 0.1 M phosphate buffer [pH 7.2]), dehydrated in a graded ethanol series, and embedded in Quetol 653 resin (Nisshin Em Co., Ltd.). Thin sections were stained with 4% uranyl acetate and 3% lead citrate and observed at 80 kV with a JEOL JEM-1010 transmission electron microscope.
Algicidal pathogens negatively stained with uranyl acetate were also observed by transmission electron microscopy. Briefly, an algicidal pathogen suspension was mounted on a grid (no. 780111630; JEOL Datum Ltd.) for 30 s, and excess water was removed with filter paper (no. 1; Toyo Co., Ltd.); then 4% uranyl acetate was mounted on the grid for 10 s and the excess dye was removed with filter paper. After the grid was dried in a desiccator for 10 min, negatively stained pathogens were observed by transmission electron microscopy (JEOL JEM-1010) at an acceleration voltage of 80 kV. Particle diameters were estimated from the negatively stained images.
Epifluorescence microscopy.
Clonal pathogens RsRNAV01 and RsRNAV06 were observed at a magnification of ×1,000 with an Olympus BX50 epifluorescence microscope after staining with DAPI (4′,6-diamidino-2-phenylindole) or SYBR Gold (Molecular Probes) by a method described previously (3, 39).
Storage test.
An exponentially growing culture of R. setigera S3 was inoculated with RsRNAV01 and incubated for 5 days under the conditions given above. The titer of the resultant fresh lysate was then estimated by the extinction dilution method, and an aliquot of the lysate was kept at 4°C in the dark. Titration was also conducted after 17 days of storage to verify the stability of the pathogen.
Analysis of RsRNAV nucleic acid and protein.
Four hundred fifty milliliters of exponentially growing R. setigera S2 (7.8 × 103 ml−1) was inoculated with 22.5 ml of RsRNAV01 or RsRNAV06 (∼107 infectious units ml−1) and lysed. The resulting lysates were centrifuged at 4,500 × g for 10 min at 4°C, and then the supernatants were sequentially passed through 8.0-, 0.8-, and 0.2-μm-pore-size filters to remove cellular debris. Polyethylene glycol 6000 (Wako Co., Ltd.) was added to the filtrates to obtain a final concentration of 10% (wt/vol), and then the resulting suspension was stored at 4°C in the dark overnight. After centrifugation at 57,000 × g for 1.5 h, the viral pellet was washed with 10 mM phosphate buffer and again centrifuged at 217,000 × g for 4 h to collect the virus particles; they were then resuspended in 500 μl of TE buffer (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA in distilled water). The viral pellet was treated with proteinase K (1 mg ml−1; Nippon Gene Co., Ltd) and sarcosyl (1%; International Technologies, Inc.) in TE buffer at 55°C for 1.5 h. Nucleic acids were extracted with phenol-chloroform and digested for 1 h with RNase A (Nippon Gene Co., Ltd.) (final concentration, 0.1 μg μl−1) at 37 or 98°C or with DNase I (Promega Co., Ltd.) (final concentration, 0.2 μg μl−1) at 37°C. RNase A treatment at 37°C digests single-stranded RNA (ssRNA) but not double-stranded RNA (dsRNA), while dsRNA denatures into ssRNA at 98°C and thus it digestible with RNase A. Nucleic acid extraction mixtures held on ice without enzymatic treatment served as controls. A formaldehyde-agarose gel (1%; 15 × 20 cm) (Seakem Gold Agarose; BMA Inc.) was loaded with 20 μl of nucleic acid and electrophoresed at 50 V for 14.5 h. Nucleic acids were visualized by SYBR Green II staining (Molecular Probes, Inc.).
The virus suspension of each virus strain was mixed with a fourfold volume of sample buffer (62.5 mM Tris-HCl, 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate [SDS], 20% glycerol, and 0.005% bromophenol blue) and boiled for 5 min; the proteins were then separated by SDS-polyacrylamide gel electrophoresis (80- by 40- by 1.0-mm 10 to 20% gradient polyacrylamide gel; 150 V) with an XV Pantera system (DRC Co., Ltd.). Proteins were visualized by Coomassie brilliant blue staining. Protein molecular mass standards (DRC Co., Ltd) ranging from 6.5 to 200 kDa were used for size calibration.
Host range analysis.
The interspecies host specificities of the isolated pathogens RsRNAV01 and RsRNAV06 were tested by adding aliquots of 5% (vol/vol) fresh pathogen suspension to duplicate cultures of exponentially growing clonal algal strains belonging to families Bacillariophyceae, Chlorophyceae, Dinophyceae, Euglenophyceae, Eustigmatophyceae, and Raphidophyceae (Table 1). They were cultured under the conditions given above at the temperatures shown in Table 1. The growth and evidence of lysis of each algal culture were monitored by optical microscopy and compared to those of control cultures inoculated with SWM3. Cultures that were not lysed after 14 days were considered to be unsuitable hosts for the pathogen. To test the intraspecies host range of the clonal pathogens RsRNAV01 to -09, 22 additional R. setigera clonal strains were examined as potential hosts. Among the R. setigera strains shown in Table 2, strain S15 was isolated from Nomi Bay, Kochi Prefecture, Japan, and all of the others were from Ariake Sea, Fukuoka Prefecture, Japan. The sensitivity of each host strain was examined as described above.
One-step growth experiment.
In order to estimate the latent period and the burst size of RsRNAV06, one-step growth experiments were designed according to the methods given by Sandaa et al. (25). R. setigera S2 was inoculated with RsRNAV06 in the exponential and stationary growth phases at multiplicities of infection of 138 and 156, respectively. The algicidal effect was monitored by enumerating the healthy host cells by optical microscopy, and the density of RsRNAV06 was estimated by the extinction dilution method (18, 31). Incubation conditions were as described above.
RESULTS AND DISCUSSION
Characteristics of pathogens.
Nine clonal pathogens (RsRNAV01 to RsRNAV09) causing lysis of R. setigera S3 were successfully isolated. As the pathogens retained algicidal activity after filtration through a 0.1 μm-pore-size filter, they were easily made free from bacterial contamination. The algicidal activity was lost by treatment at 121°C for 15 min (data not shown). These data demonstrate that the algicidal factors were heat labile and smaller than 0.1 μm. The algicidal activity of each pathogen was serially transferable to exponentially growing R. setigera S3 cultures, which consistently resulted in lysis (data not shown). The densities of algicidal factors in the nine clonal pathogen suspensions were estimated at 3.50 × 107 to 3.85 × 108 ml−1, and RsRNAV01 and RsRNAV06, which had relatively high yields, were selected as typical clonal pathogens and further examined in the following experiments.
An RsRNAV01 suspension containing 3.01 × 108 (95% confidence interval, 1.25 × 108 to 7.26 × 108) infectious units ml−1 was subjected to the storage test. After 17 days of preservation at 4°C in the dark, the titer was 3.50 × 108 (95% confidence interval, 1.47 × 108 to 8.30 × 108) infectious units ml−1. These data show the high stability of RsRNAV.
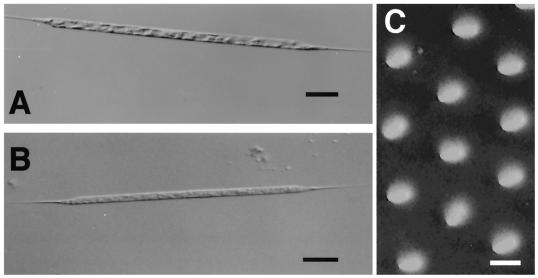
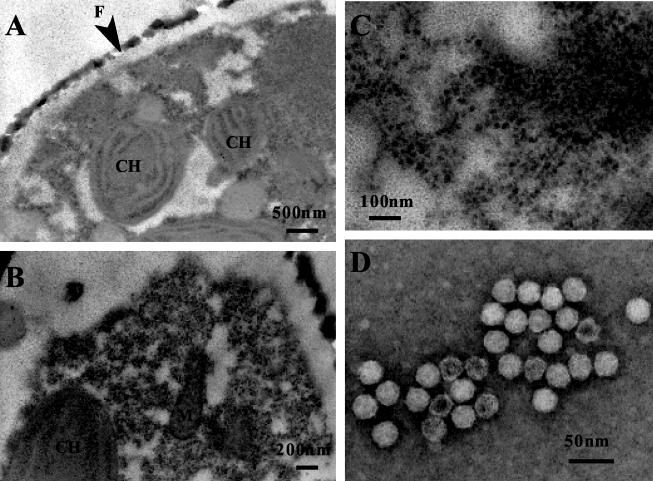
Cultures and cells of R. setigera lysed by the pathogens became pale, presumably due to the loss or degradation of photosynthetic pigments (Fig. 1). Although inoculation of the pathogens did not cause complete lysis of host cultures, the surviving cells did not regrow even when transferred to fresh SWM3 medium. Thin sections of healthy R. setigera S3 cells indicated that the cytoplasmic organization and the frustule were diagnostic of diatoms (Fig. 2A). In contrast, electron micrographs of R. setigera S3 cells inoculated with the pathogen RsRNAV01 revealed the presence of small VLPs in the cytoplasm (Fig. 2B and C). No trace of these particles was evident within healthy cells in the control cultures (Fig. 2A). Moreover, icosahedral VLPs were observed in culture lysates by negative staining (Fig. 2D).
FIG. 1.
Optical micrographs of an intact cell (A) and an RsRNAV01-infected cell (B) of R. setigera and a transmission micrograph of its frustule (C). Note that frustule pores of R. setigera are 91 ± 6 nm (n = 10; 80 to 98 nm) in length and 73 ± 6 nm (n = 10; 60 to 81 nm) in breadth (C). Bars, 50 μm (A and B) and 100 nm (C).
FIG. 2.
Transmission electron micrographs of R. setigera cultures. (A) Thin section of a healthy cell; (B) thin section of a cell 96 h after addition of the clonal pathogen RsRNAV01; (C) close-up of intracellular virus particles in panel B; (D) negatively stained virus particles in the culture lysate. CH, chloroplast; M, mitochondrion; F, frustule.
Because (i) the algicidal pathogen was transferable to a fresh algal culture, (ii) small VLPs were observed in the lysed culture, and (iii) the VLPs were not found in the healthy culture, fulfilling Koch's postulates, it was concluded that the VLPs observed within the infected cells and in the algal lysates were the pathogen of R. setigera, and they were both morphologically and physiologically considered lytic viruses; hence, the pathogenic particle was termed R. setigera virus (RsRNAV) after its host species.
Transmission electron microscopy observations revealed that virus particles appeared only in the cytoplasm. Negative-staining observations revealed that RsRNAV01 and RsRNAV06 were similar in appearance: they were icosahedral in shape; 32 ± 2 nm (n = 40, 28 to 36 nm) and 32 ± 1 nm (n = 40, 29 to 35 nm) in diameter, respectively; and lacked a tail and an outer membrane (Fig. 2D). Thus, RsRNAV is apparently distinct in size from other large viruses infectious to eucaryotic microalgae (>120 nm in diameter) that are included in the newly defined family Phycodnaviridae (38). So far, several small algal viruses distinct from the family Phycodnaviridae have been reported: Heterosigma akashiwo nuclear inclusion virus (HaNIV) (14), H. akashiwo RNA virus (HaRNAV) (32), Heterocapsa circularisquama RNA virus (HcRNAV) (37), and VLPs within algal cells (2, 38).
Genome and proteins.
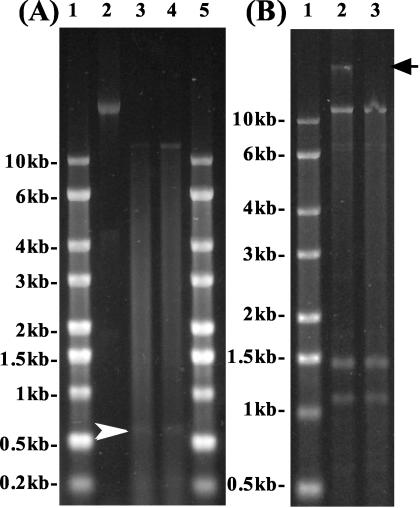
Denaturing gel electrophoresis revealed that the major nucleic acids extracted from RsRNAV01 and RsRNAV06 were 11.2 kb in length (Fig. 3A). They were sensitive to RNase A both at 37 and 98°C (data not shown) but not to DNase I (Fig. 3B). These data indicate that the RsRNAV genome is ssRNA, and they agree closely with the results of epifluorescence microscopy that RsRNAV particles were stainable with SYBR Gold but not with DAPI. As the viral RNAs were not retained by a poly(A) tail purification column but were recovered from the wash, the RsRNAV genome probably does not contain a poly(A) tail. Smaller RNA molecules of 0.6 kb (Fig. 3A) and 1.2 and 1.5 kb (Fig. 3B) were occasionally extracted from RsRNAV particles. Identification of the smaller RNA molecules has not been completed. For some other viruses, the viral genome consists of multiple ssRNAs (as in the case of the genus Bunyavirus [6]) or genomic RNAs of different lengths are packaged in separate virions (as in the case of the genus Bromovirus [24]). However, because the smaller RNA molecules were not always extracted, it is unlikely that these smaller RNAs are viral genome. The other possibilities are that (i) virions contain subgenomic RNAs as well as genomic RNA (as in the case of the genus Aureusvirus [15]) or (ii) defective interfering particle genomes appeared as smaller RNA bands (13). Partial sequencing of the RsRNAV06 genome is now under way (data not shown), and it shows some similarity (E value of 10E-32 to 10E-18) to HaRNAV (32), unidentified picornavirus-like viruses (5), unidentified Chinese clam virus (12), Strawberry mottle virus (36), and Taura syndrome virus (16), etc. Further characterization of the viral genome, however, is necessary both to identify the smaller RNA molecules and to determine the taxonomic position of RsRNAV.
FIG. 3.
(A) Nucleic acids isolated from RsRNAV01 (lane 3) and RsRNAV06 (lane 4). RNA molecular size markers are shown in lanes 1 and 5, and Sendai virus RNA (15.8 kb) is shown in lane 3 to estimate the lengths of viral RNAs. A smaller faint band (0.6 kb) is also observed (arrowhead). (B) Nucleic acids extracted from RsRNAV06 without (lane 2) or with (lane 3) DNase I treatment at 37°C for 1 h. No host DNA is found in lane 3 (arrow). RNA molecular size markers are shown in lane 1. Smaller RNA bands (1.5 and 1.2 kb) extracted from RsRNAV particles are also observed.
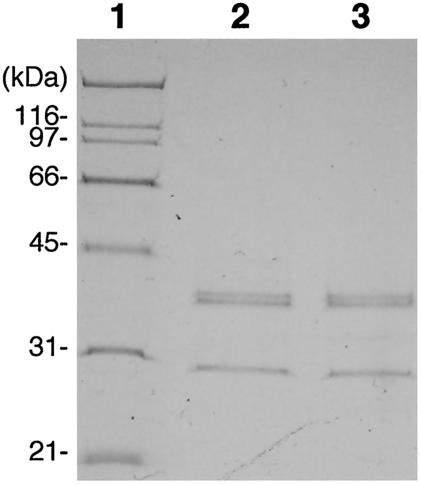
The size and number of structural proteins of the virus particles were determined by SDS-polyacrylamide gel electrophoresis. Both RsRNAV01 and RsRNAV06 contained at least three major polypeptides with molecular masses of 41.5, 41.0, and 29.5 kDa (Fig. 4). Two lower-abundance bands (155 and 69 kDa) were detected only by the silver staining method (data not shown).
FIG. 4.
Major structural proteins of RsRNAV. Proteins extracted from RsRNAV01 and RsRNAV06 were loaded in lanes 2 and 3. Molecular mass markers are shown in lane 1.
Host range.
The host ranges of the virus strains RsRNAV01 and RsRNAV06 were tested on 34 phytoplankton species, including 23 strains of diatoms isolated from the coastal waters of western Japan. RsRNAV01 and RsRNAV06 caused lysis only of R. setigera strains and not any of the other microalgal species tested (Table 1), showing that their infection specificity was high. Moreover, not all of the combinations between R. setigera strains and RsRNAV strains resulted in lysis (Table 2). Among the tested strains of R. setigera (except S3, which had been lost before the host range test), 12 were sensitive to all of the RsRNAV strains, but the other 9 and 4 strains were sensitive to only some and none of them, respectively. These data suggest that RsRNAV is likely not only species specific but also strain specific.
Based on the similarities between RsRNAV01 and RsRNAV06 in host range, genome, and proteins, they are considered to be very closely related to each other.
Viral replication.
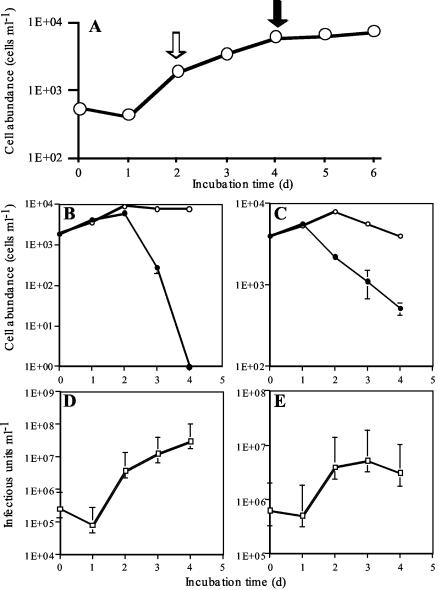
When viral inoculation was conducted at either the exponential or stationary phase of the host cultures (Fig. 5A), the increase in virus abundance was noticeable 2 days after inoculation (Fig. 5D and E). Thus, the latent period of RsRNAV06 was estimated to be 2 days. The decrease in host abundance was more obvious in the culture where viruses were inoculated at the exponential growth phase than in that where viruses were inoculated at the stationary phase (Fig. 5B). Considering that the multiplicity of infection was high enough to make all of the sensitive cells infected, it can be presumed that viral infection does not necessarily interrupt binary fissions all at once. The abundances of hosts and viruses at 1 to 3 days postinoculation were used to calculate the burst size in each experiment (Fig. 5B to E). When viral inoculation was conducted at the exponential and stationary phases of host cultures, the burst sizes were estimated at 3,100 and 1,010 infectious particles cell−1, respectively. A follow-up experiment gave almost similar results (2,120 and 960 infectious particles cell−1). These data support the idea that vigorously growing algal host cells are preferable for viral replication because of their higher biosynthesis activity (19). The burst size and the latent period of RsRNAV were comparable to those of HcRNAV109 (3,400 infectious units cell−1 and 24 to 48 h, respectively) (37). However, this kind of comparison should be interpreted with care, as these parameters of viral growth are affected by the physiological condition of the host cells and crystallization of virus particles can cause an underestimation, as the extinction dilution method was used for titration.
FIG. 5.
(A) Growth curve of R. setigera S2 used for the one-step growth experiments. (B and C) Changes in density of R. setigera S2 cells with (closed circles) or without (open circles) viral inoculation. (D and E) Changes in viral titer. RsRNAV06 inoculation was performed in the exponential growth phase (A [open arrow], B, and D) and the stationary phase (A [closed arrow], C, and E) at multiplicities of infection of 138 and 156, respectively. The error bars indicate standard deviations (B and C) or 95% confidence limits (D and E).
Implications.
Diatoms are among the most important groups of phytoplankton in the sea; they are global in distribution, contain a large number of species, and include various harmful bloom-forming species. The ecological significance of diatoms, especially as primary producers sustaining larval growth, has been well recognized (7, 40). In contrast, no direct evidence showing the relationship between diatoms and viruses has been reported, which has led to the idea that the cell covering of diatoms could contribute to reduce the probability of viral infection (41). However, our investigation demonstrates that viruses that infect and cause lysis of diatoms are also a component of the natural marine viral community. As frustule pores of R. setigera are larger than RsRNAV (Fig. 1C), they are also considered a possible route of viral infection. In the case of dinoflagellates, viruses infecting the harmful bloom-causing alga H. circularisquama (HcV and HcRNAV) were also isolated (35, 37); thus, it is likely that cell coverings such as a frustule, a theca, or an amphiesma do not necessarily prevent viral infection. The host-virus system obtained in the present study is expected to be important for understanding the relationship between diatoms and viruses in the natural marine environment.
A few RNA viruses infecting microalgae have been isolated: the dsRNA virus MpRNAV, infecting Micromonas pusilla (Prasinophyceae) (C. P. D. Brussaard et al., personal communication); the ssRNA virus HaRNAV, infecting H. akashiwo (32); and the ssRNA virus HcRNAV, infecting H. circularisquama (37). The finding of RsRNAV strengthens the idea that the diversity of microalgal viruses is higher than previously envisaged. Considering the high mutation rates of RNA viruses due to the lack of the proofreading and repair processes that increase the fidelity of RNA replication (9), their diversity and roles in aquatic environments are also of great interest.
Acknowledgments
This study was supported by the Industrial Technology Research Grant Program in 2001 to 2003 from the New Energy and Industrial Technology Development Organization of Japan (NEDO); the Society for Techno-innovation of Agriculture, Forestry and Fisheries (STAFF); and the Ministry of Agriculture, Forestry and Fisheries, Japan.
We thank T. Uchida (Hokkaido National Fisheries Research Institute), I. Imai (Kyoto University), S. Nagai and Y. Matsuyama (National Research Institute of Fisheries and Environment of Inland Sea), and R. A. Lewin (Scripps Institute of Oceanography) for providing some of the algal cultures used in this work and K. Hirota (Saga Prefectural Genkai Fisheries Research and Development Center) and S. Oda (Fukuoka Fisheries and Marine Technology Research Center) for providing the water samples. Thanks are also extended to T. Sakaguchi (Hiroshima University) for providing Sendai virus RNA and to K. Tarutani for critical reading of the manuscript and useful suggestions.
REFERENCES
- 1.Bratbak, G., J. K. Egge, and M. Heldal. 1993. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar. Ecol. Prog. Ser. 93:39-48. [Google Scholar]
- 2.Brussaard, C. P. D., R. S. Kempers, A. J. Kop, R. Riegman, and M. Heldal. 1996. Virus-like particles in a summer bloom of Emiliania huxleyi in the North Sea. Aquat. Microb. Ecol. 10:105-113. [Google Scholar]
- 3.Chen, F., J. R. Lu, B. J. Binder, Y. C. Liu, and R. E. Hodson. 2001. Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR Gold. Appl. Environ. Microbiol. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L. C. M., T. Edelstein, and J. McLachlan. 1969. Bonnemaisonia hamifera Hariot in nature and in culture. J. Phycol. 5:211-220. [DOI] [PubMed] [Google Scholar]
- 5.Culley, A. I., A. S. Lang, and C. A. Suttle. 2003. High diversity of unknown picorna-like viruses in the sea. Nature 424:1054-1057. [DOI] [PubMed] [Google Scholar]
- 6.Elliott, R. M., M. Bouloy, C. H. Calisher, R. Goldbach, J. F. Moyer, S. T. Nichol, R. Pettersson, A. Plyusnin, and C. S. Schmaljohn. 2000. Family Bunyaviridae, p. 599-621. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. Carstens, M. K. Estes, S. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 7.Guillard, R. R. L., and P. Kilham. 1977. The ecology of marine planktonic diatoms, p. 372-469. In D. Werner (ed.), The biology of diatoms. Botanical monographs, vol. 13. Blackwell Scientific Publications, Victoria, Australia.
- 8.Hasle, G. R., and G. A. Fryxell. 1995. Taxonomy of diatoms, p. 339-364. In G. M. Hallegraeff, D. M. Anderson, and A. D. Cembella (ed.), Manual on harmful marine microalgae. IOC manuals and guides no. 33. UNESCO, Paris, France.
- 9.Holland, J. J. 1998. The origin and evolution of viruses, p. 11-21. In B. Mahy and L. Collier (ed.), Virology. Topley and Wilson's microbiology and microbial infections, vol. 1, Oxford University Press, London, United Kingdom.
- 10.Itoh, K., and I. Imai. 1987. A guide for studies of red tide organisms, p. 122-130. Shuwa, Tokyo, Japan. (In Japanese.)
- 11.Jacquet, S., M. Heldal, D. Iglesias-Rodriguez, A. Larsen, W. Wilson, and G. Bratbak. 2002. Flow cytometric analysis of an Emiliania huxleyi bloom terminated by viral infection. Aquat. Microb. Ecol. 27:111-124. [Google Scholar]
- 12.Kingsley, D. H., G. K. Meade, and G. P. Richards. 2002. Detection of both hepatitis A virus and Norwalk-like virus in imported clams associated with food-borne illness. Appl. Environ. Microbiol. 68:3914-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuge, S., I. Saito, and A. Nomoto. 1986. Primary structure of poliovirus defective-interfering particle genomes and possible generation mechanisms of the particles. J. Mol. Biol. 192:473-487. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence, J. E., A. M. Chan, and C. A. Suttle. 2001. A novel virus (HaNIV) causes lysis of the toxic bloom-forming alga Heterosigma akashiwo (Raphidophyceae). J. Phycol. 37:216-222. [Google Scholar]
- 15.Lommel, S. A., G. P. Martelli, and M. Russo. 2000. Family Tombusviridae, p. 791-825. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. Carstens, M. K. Estes, S. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 16.Mari, J., B. T. Poulos, D. V. Lightner, and J. R. Bonami. 2002. Shrimp Taura syndrome virus: genomic characterization and similarity with members of the genus Cricket paralysis-like viruses. J. Gen. Virol. 83:915-926. [DOI] [PubMed] [Google Scholar]
- 17.Nagasaki, K., M. Ando, S. Itakura, I. Imai, and Y. Ishida. 1994. Viral mortality in the final stage of Heterosigma akashiwo (Raphidophyceae) red tide. J. Plankton Res. 16:1595-1599. [Google Scholar]
- 18.Nagasaki, K., and M. Yamaguchi. 1997. Isolation of a virus infectious to the harmful bloom causing microalga Heterosigma akashiwo (Raphidophyceae). Aquat. Microb. Ecol. 13:135-140. [Google Scholar]
- 19.Nagasaki, K., Y. Tomaru, K. Tarutani, N. Katanozaka, S. Yamanaka, H. Tanabe, and M. Yamaguchi. 2003. Growth characteristics and intraspecies host specificity of a large virus infecting the dinoflagellate Heterocapsa circularisquama. Appl. Environ. Microbiol. 69:2580-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagasaki, K., Y. Tomaru, K. Nakanishi, N. Hata, N. Katanozaka, and M. Yamaguchi. Possible relationship between dynamics of Heterocapsa circularisquama (Dinophyceae) and its viruses in Ago Bay, Japan, in summer 2001. Aquat. Microb. Ecol., in press.
- 21.Nishikawa, T., K. Miyahara, and S. Nagai. 2000. Effects of temperature and salinity on the growth of the giant diatom Coscinodiscus wailesii isolated from Harima-Nada, Seto Inland Sea, Japan. Nippon Suisan Gakkaishi 66:993-998. (In Japanese with English abstract.) [Google Scholar]
- 22.Nishikawa, T. 2002. Effects of temperature, salinity and irradiance on the growth of the diatom Eucampia zodiacus caused bleaching of seaweed Porphyra isolated from Harima-Nada, Seto Inland Sea, Japan. Nippon Suisan Gakkaishi 68:356-361. (In Japanese with English abstract.) [Google Scholar]
- 23.Proctor, L. M., and J. A. Fuhrman. 1991. Roles of viral infection in organic particle flux. Mar. Ecol. Prog. Ser. 69:133-142. [Google Scholar]
- 24.Roossinck, M. J., J. Bujarski, S. W. Ding, R. Hajimorad, K. Hanada, S. Scott, and M. Tousignant. 2000. Family Bromoviridae, p. 923-935. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. Carstens, M. K. Estes, S. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 25.Sandaa, R. A., M. Heldal, T. Castberg, R. Thyrhaug, and G. Bratbak. 2001. Isolation and characterization of two viruses with large genome size infecting Chrysochromlina ericina (Prymnesiophyceae) and Pyramimonas orientalis (Prasinophyceae). Virology 290:272-280. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki, K., and S. Uno. 1988. Comparison of the growth rate of six diatom species cultured with a red alga Porphyra tenera. Bull. Plankton Soc. Jpn. 35:57-65. (In Japanese with English abstract.) [Google Scholar]
- 27.Sasaki, K., and S. Uno. 1994. Growth interaction of diatom species in dialysis tube on the red tide. Bull. Plankton Soc. Jpn. 41:9-19. (In Japanese with English abstract.) [Google Scholar]
- 28.Sasaki, K., and H. Kito. 2003. Growth characteristics of Rhizosolenia imbricata Brightwell occurring in Ariake Sea. Bull. Plankton Soc. Jpn. 50:79-87. (In Japanese with English abstract.) [Google Scholar]
- 29.Suttle, C. A., A. M. Chan, and M. T. Cottrell. 1990. Infection of phytoplankton by viruses and reduction of primary productivity. Nature 347:467-469. [Google Scholar]
- 30.Suttle, C. A., A. M. Chan, and M. T. Cottrell. 1991. Use of ultrafiltration to isolate viruses from seawater which are pathogens to marine phytoplankton. Appl. Environ. Microbiol. 57:721-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suttle, C. A. 1993. Enumeration and isolation of viruses, p. 121-137. In P. F. Kemp, B. Sherr, E. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 32.Tai, V., J. E. Lawrence, A. S. Lang, A. M. Chan, A. I. Culley, and C. A. Suttle. 2003. Characterization of HaRNAV, a single-stranded RNA virus causing lysis of Heterosigma akashiwo (Raphidophyceae). J. Phycol. 39:343-352. [Google Scholar]
- 33.Takano, H. 1990. Rhizosolenia setigera Brightwell, p. 268-269. In Y. Fukuyo, H. Takano, M. Chihara, K. Matsuoka (ed.), Red tide organisms in Japan. Japan Fisheries Resource Conservation Association, Uchida Rokakuho, Tokyo, Japan.
- 34.Tarutani, K., K. Nagasaki, and M. Yamaguchi. 2000. Viral impacts on total abundance and clonal composition of the harmful bloom-forming phytoplankton Heterosigma akashiwo. Appl. Environ. Microbiol. 66:4916-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarutani, K., K. Nagasaki, S. Itakura, and M. Yamaguchi. 2001. Isolation of a virus infecting the novel shellfish-killing dinoflagellate Heterocapsa circularisquama. Aquat. Microb. Ecol. 23:103-111. [Google Scholar]
- 36.Thompson, J. R., G. Leone, J. L. Lindner, W. Jelkmann, and C. D. Schoen. 2002. Characterization and complete nucleotide sequence of Strawberry mottle virus: a tentative member of a new family of bipartite plant picorna-like viruses. J. Gen. Virol. 83:229-239. [DOI] [PubMed] [Google Scholar]
- 37.Tomaru, Y., N. Katanozaka, K. Nishida, Y. Shirai, K. Tarutani, M. Yamaguchi, and K. Nagasaki. Isolation and characterization of two distinct types of HcRNAV, a single-stranded RNA virus infecting the bivalve-killing microalga Heterocapsa circularisquama. Aquat. Microb. Ecol., in press.
- 38.Van Etten, J. L., L. C. Lane, and R. H. Meints. 1991. Viruses and viruslike particles of eukaryotic algae. Microbiol. Rev. 55:586-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinbauer, M. G., and C. A. Suttle. 1997. Comparison of epifluorescence and transmission electron microscopy for counting viruses in natural marine waters. Aquat. Microb. Ecol. 13:225-232. [Google Scholar]
- 40.Werner, D. 1977. Introduction with a note on taxonomy, p. 1-23. In D. Werner (ed.), The biology of diatoms. Botanical monographs, vol. 13. Blackwell Scientific Publications, Victoria, Australia.
- 41.Zingone, A. 1995. The role of viruses in the dynamics of phytoplankton blooms. Giorn. Bot. It. 129:415-423. [Google Scholar]