Abstract
It has been suggested that lumbar sympathectomy can reduce pain behavior, including mechanical allodynia and thermal hyperalgesia, caused by ligation of the spinal nerve. One well-characterized model, which involves application of nucleus pulposus to the spinal nerve and displacement of the adjacent nerve, shows behavioral changes in rats. However, there have been no previous reports regarding sympathectomy performed in this model. Disk incision and adjacent spinal nerve displacement were performed with (n=6) or without (n=6) sympathectomy. Sham surgery was also performed with (n=6) or without (n=6) sympathectomy. The animals were tested for 3 days before surgery and on days 1, 3, 7, 14, and 21 after surgery. Non-noxious mechanical thresholds were tested by determining the hind paw withdrawal response to von Frey hair stimulation of the plantar surface of the footpad using a touch stimulator. Thermal nociceptive thresholds were tested using a sensitive thermal-testing device. While rats in the disk incision with displacement surgery group showed allodynia and hyperalgesia after surgery on the experimental side, sympathectomized animals did not. No allodynia was observed in the sham groups. Sympathectomy seemed to prevent the pain behavioral changes caused by the combination of disk incision and nerve displacement.
Key words: Sympathectomy, Nucleus pulposus, Allodynia, Hyperalgesia, Behavior
Introduction
It has been reported that nucleus pulposus in the epidural space induces spinal nerve root damage, not only by mechanical but also by chemical mechanisms as nucleus pulposus is capable of producing proinflammatory cytokines [5, 14, 16, 17, 19, 21, 23, 29]. There have been a number of recent reports regarding the pain behavior due to lumbar disk herniation in rats [7, 18, 20, 24]. One well-characterized model involves L4/5 disk incision and/or displacement of the adjacent spinal nerve in rats. These studies indicated that the combination of chemical and mechanical damage results in behavioral changes, mechanical allodynia, thermal hyperalgesia, increased lifting of the hind paw on the operated side, and increased rotation of the head toward the operated side [18].
Tight and loose ligation with or without transection of the nerve are well-known neuropathic pain models in rats [1, 9]. These models show mechanical allodynia and thermal hyperalgesia. It has been reported that lumbar sympathectomy, which was performed in these pain models, can decrease the pain behavior [2, 8, 10]. Kim et al. reported the prevention of pain behavioral changes by sympathectomy prior to nerve ligation [10]. In contrast, it has also been reported that intraperitoneal injection of phentolamine hydrochloride, an alpha-adrenoceptor blocker, or surgical sympathectomy does not reverse either allodynia or hyperalgesia in this model [27, 28]. The reasons for the discrepancies in these reports are not yet clear. The effects of sympathectomy in neuropathic pain models remain unknown.
Many researchers have performed sympathectomy in ligation models. However, there have been no previous reports about the effects of sympathectomy performed in nucleus pulposus-induced nerve damage models, which involve not only mechanical but also chemical factors. In the present study, the effects of surgical sympathectomy were studied in a rat model involving incision of the L4/5 disk and displacement of the adjacent spinal nerve root and dorsal root ganglion (DRG).
Materials and methods
All animal procedures were carried out in accordance with the “Principles of Laboratory Animal Care” (NIH publication No.86-28, rev. 1985) and were approved by the local “Animal Research Ethics Committee.” Female Sprague–Dawley rats (n=24) weighing 200–250 g were used. The animals were housed under a 12-h light–dark schedule (lights on starting at 6:00 a.m.) with free access to food (B&K Rat/Mouse Standard, BeeKay Feeds & Beddings, Sollentuna, Stockholm, Sweden) and tap water in an animal room maintained at a constant temperature of 21°C and humidity of 50%. The rats were anesthetized by intraperitoneal injection of 0.5°ml of hypnorm:dormicum:saline (1:1:2).
A dorsal longitudinal incision was made over the lumbar spine, and the left L4–5 facet joint capsule was exposed microscopically. One day before this dorsal incision, a midline abdominal longitudinal incision was made.
Sham exposure (n=6)
The left L4–5 facet joint was removed, and the L4 DRG and L5 nerve root, including the L4–5 intervertebral disk, were visualized. The spinal muscles were sutured and the skin was closed with metal clips.
Disk incision + spinal nerve displacement (n=6)
After the same exposure as in the sham series, the L4–5 intervertebral disk was incised, and 0.3 ml of air was injected into the center of the disk using 1-ml syringe with a 27-gauge needle. As the intradiskal pressure was increased by the injection, the nucleus pulposus escaped from the disk. The nucleus pulposus was spread gently onto the adjacent L4 DRG and the L5 spinal nerve root. A 25-gauge needle was placed between the L4 DRG and the pedicle of the L4 vertebra to displace the L4 DRG and the L5 spinal nerve root. The L4 DRG was displaced gently to the former location of the center of the DRG by the needle. The needle was then fixed in this position by securing it into the L4 vertebral body. The details of this surgical method were described previously [18, 20, 24].
Sham exposure with sympathectomy (n=6)
One day before the same exposure as in the sham series, the internal organs and fat tissue were retracted to approach the aorta, the vena cava, and the left psoas major muscle. The left paravertebral sympathetic trunk, including the sympathetic chain to the right side, was visualized by gently retracting the psoas major muscle, and resected from L2 to L6 level under a microscope.
Disk incision + spinal nerve displacement with sympathectomy (n=6)
One day before the same surgery as performed in the disk incision with nerve displacement series, the rats were placed in the supine position, and sympathectomy was performed in the same manner as described earlier.
Mechanical stimulation
The animals were tested for 3 days before and on days 1, 3, 7, 14, and 21 after spine surgery. The non-noxious mechanical threshold was tested by determining the hind paw withdrawal response to von Frey hair stimulation of the plantar surface of the footpad. A touch stimulator (37400 Dynamic Plantar Aesthesiometer, Ugo Basile, Varese, Italy) was used for the stimulation. One of the authors tested the rats in a blind manner. Rats received stimulation of their footpads with a straight stainless steel filament 0.5 mm in diameter, for a total of three applications with intervals of at least 5 min. The filament was touched to the plantar surface and began to exert an upward force below the threshold of sensation. The force was increased until a stop signal, consisting of either the animal removing the paw or the point at which greatest preset force (50 g) was met, was attained. The force was set to increase constantly from 0 to 50 g over a period of 20 s. The gram force of the filament causing the positive response was recorded digitally. These values per paw in each test were averaged to determine the threshold. A difference score in percentage was calculated by dividing the threshold of the experimental side by that of the control side to compare the disk incision and nerve displacement series with those that underwent sympathectomy.
Thermal stimulation
The animals were tested for 3 days before and on days 1, 3, 7, 14, and 21 after surgery. The thermal nociceptive threshold was measured using a sensitive thermal testing device (7370 Plantar Test, Ugo Basile). This technique is based on the measurement of latency of removal of the foot from a radiant heat source focused to a diameter of 4 mm on the plantar surface of the footpad [3, 4]. Rats were acclimated to being placed on a glass plate for 15 min. After the acclimation period, an infrared source placed under the glass floor was positioned directly beneath the hind paw. When the rat recognized the stimulation and withdrew its paw, the sudden drop of reflected radiation switched off the infrared source and stopped the reaction timer; reaction time is shown in seconds. Rats were stimulated for a total of three applications at intervals of at least 5 min. By performing the test separately on each foot, the latency to removal of the experimental foot was compared with that of the control foot to study the degree of hyperalgesia on the experimental side. These values per paw in each test were averaged to determine the threshold. The difference score in percentage was calculated by dividing the recorded time of the experimental side by that of the control side to compare the disk incision and nerve displacement series with those that underwent sympathectomy.
Statistical analysis
The differences in the gram force and response delay between the experimental and control sides were compared statistically for each day by ANOVA. A P value of less than 0.05 was considered statistically significant.
Results
Mechanical stimulation
Animals in all experimental groups showed stable conditions before surgery. There were no significant differences between the experimental and control sides before surgery.
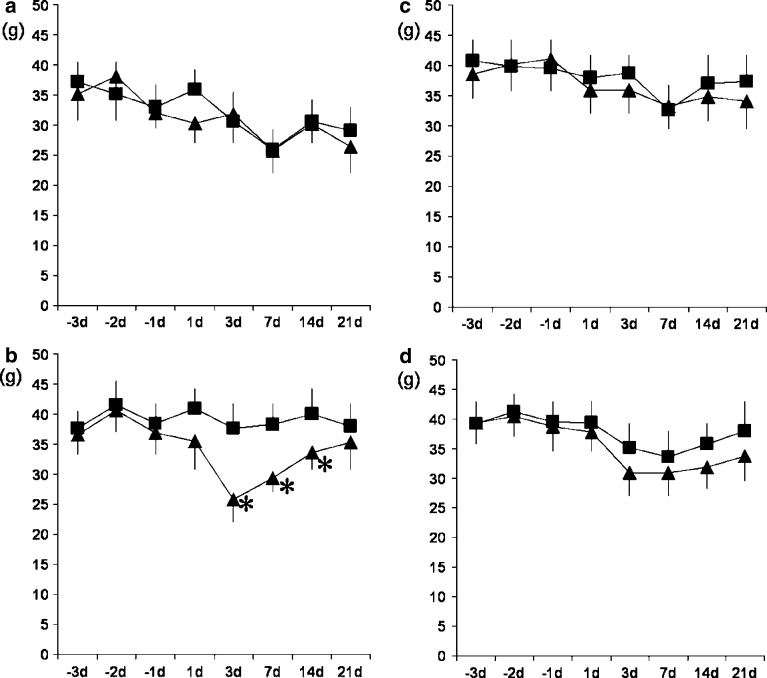
In the sham series, there were no statistically significant differences between the experimental and control sides after surgery (Fig. 1a). Reductions were observed at 3, 7, and 14 days after surgery on those that recovered on day 21 after surgery in the experimental side of the rats in the disk incision with nerve displacement surgery group (Fig. 1b). The maximum reduction was observed at 3 days after surgery. In the sham with sympathectomy series, there were no statistically significant differences between the experimental and control sides after surgery (Fig. 1c). Although the experimental side showed lower values as compared with the control side after surgery in the disk incision and displacement surgery with sympathectomy series, the differences were not statistically significant (Fig. 1d).
Fig. 1.
Responses to von Frey mechanical stimulation of the footpads on the experimental and control sides after L4/5 disk incision with adjacent nerve displacement (n=12), and sham surgery (n=12). Half of the rats underwent sympathectomy 1 day before surgery, that is, each group consisted of six rats. All animals received 3 days of baseline testing before surgery. a Responses of the sham surgery without sympathectomy group. b Responses of the disk incision and nerve displacement without sympathectomy group. There were significant differences between the experimental and control sides on days 3, 7, and 14 after surgery (P<0.05). c Responses of the sham surgery with sympathectomy group. d Responses of the disk incision and nerve displacement with sympathectomy group. Although the experimental side showed a lower value than the control side on the graph, there were no significant differences before and after surgery. Filled square control side, filled triangle experimental side
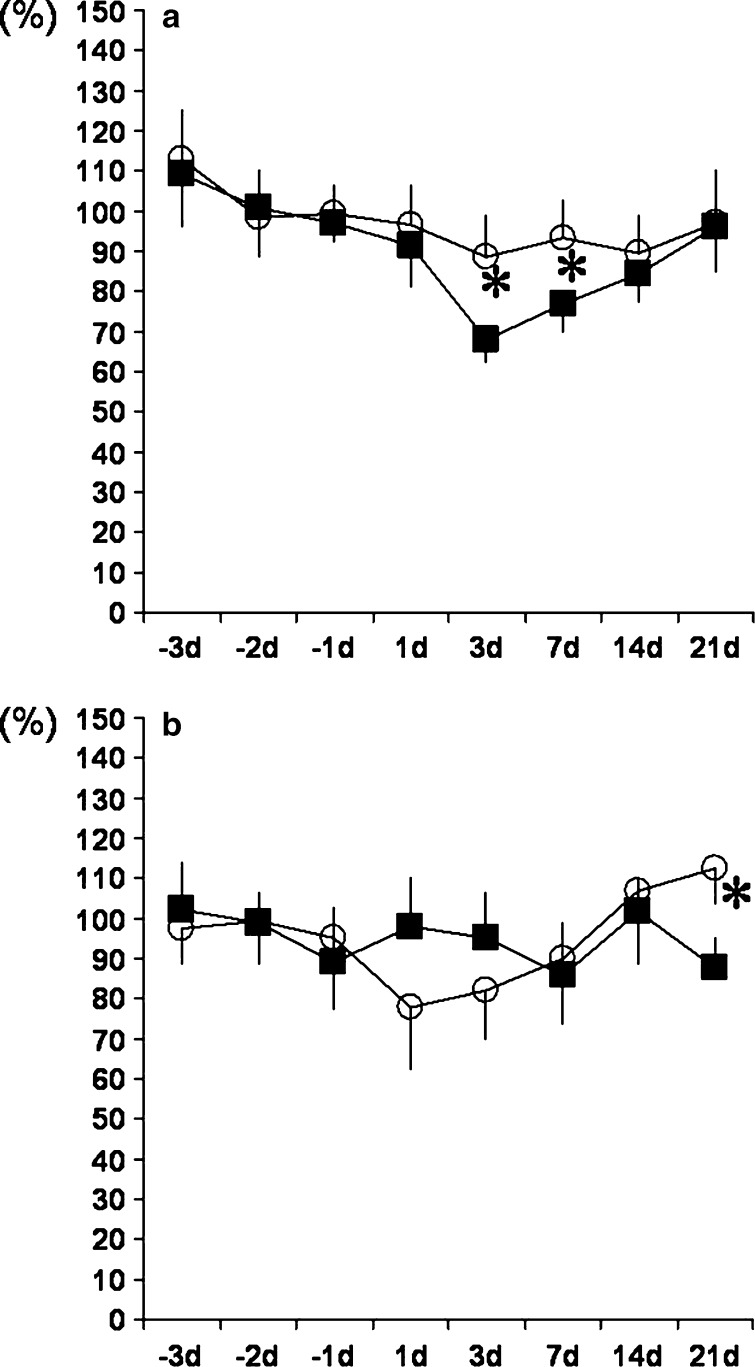
The results of difference scores in percentage showed reductions at 3 and 7 days after surgery in the disk incision and nerve displacement surgery series (Fig. 3a). On the other hand, no reductions were observed in the disk incision and nerve displacement surgery with sympathectomy series. The differences between the two series were statistically significant on days 3 and 7 after spine surgery.
Fig. 3.
Difference scores in percentage calculated by dividing the threshold of the experimental side by that of the control side in the L4/5 disk incision and adjacent nerve displacement with and without sympathectomy series. a Mechanical stimulation. The reductions caused by the combination of disk incision and nerve displacement on days 3 and 7 after spine surgery were significantly prevented by sympathectomy (P<0.05). b Thermal stimulation. The reduction caused by the combination of disk incision and nerve displacement on day 21 after spine surgery was significantly prevented by sympathectomy (P<0.05). Filled square disk incision + nerve displacement group, open circle disk incision + nerve displacement with sympathectomy group
Thermal stimulation
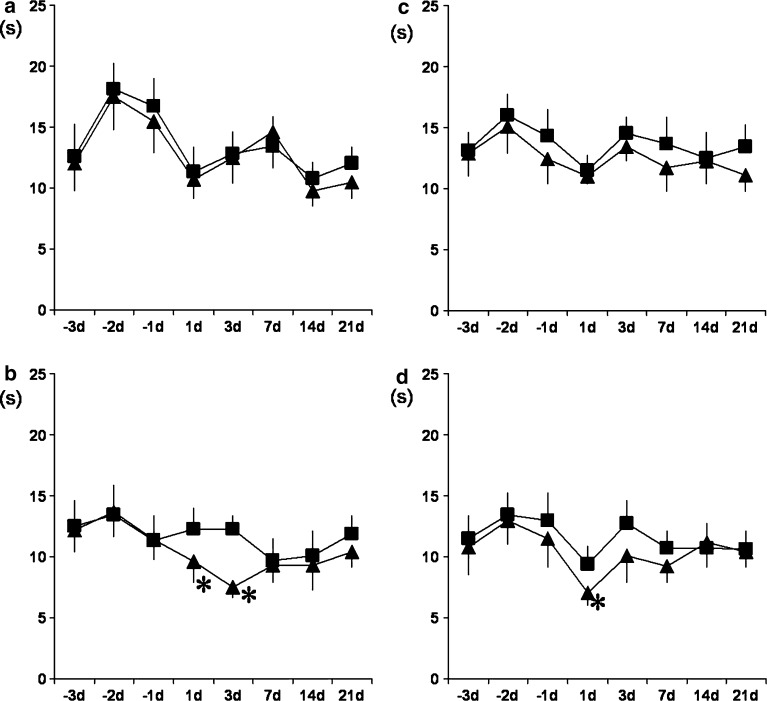
There were no significant differences between the experimental and control sides in any of the groups before surgery. In the sham series, there were no statistically significant differences between the experimental and control sides after spine surgery (Fig. 2a). The experimental side showed decreased values as compared with the control side on days 1 and 3 after surgery in the disk incision with nerve displacement surgery series (Fig. 2b). There were no statistically significant differences between the experimental and control sides after surgery in the sham with sympathectomy series (Fig. 2c). In the disk incision and nerve displacement surgery with sympathectomy series, a reduction was observed 1 day after spine surgery (Fig. 2d).
Fig. 2.
Responses to thermal stimulation of the footpads on the experimental and control sides after L4/5 disk incision with adjacent nerve displacement (n=12), and sham surgery (n=12). Half of the rats underwent sympathectomy 1 day before surgery, that is, each group consisted of six rats. All animals received 3 days of baseline testing before surgery. a Responses of the sham surgery without sympathectomy group. b Responses of the disk incision and nerve displacement without sympathectomy group. There were significant differences between the experimental and control sides on days 1 and 3 after surgery (P<0.05). c Responses of the sham surgery with sympathectomy group. d Responses of the disk incision and nerve displacement with sympathectomy group. There were significant differences between the experimental and control sides on day 1 after spine surgery (P<0.05). Filled square control side, filled triangle experimental side
The difference scores in percentage were reduced on days 7 and 21 after surgery in the disk incision and nerve displacement surgery series (Fig. 3b). The difference between the two series was statistically significant on day 21 after spine surgery.
Discussion
There have been a number of studies of sympathectomy performed employing spinal nerve ligation models [2, 8, 10]. In these studies, surgical sympathectomy performed at least 1 week after spinal nerve ligation reversed the pain behavioral changes. Surgical sympathectomy also prevented the changes when sympathectomy was performed prior to spinal nerve ligation. The sympathetic trunk was usually exposed by a transperitoneal or dorsolateral, retroperitoneal approach in these previous studies. A dorsolateral, retroperitoneal approach may have some advantages to treat rami communicantes. However, this approach is too invasive for behavior studies as it requires the psoas major muscle to be separated. In some previous studies, ipsilateral or bilateral sympathectomy was performed for one-side nerve injury models. Peterson and Norvell [25] suggested that the contralateral sympathetic nerve innervates a small portion of the hind limb. On the other hand, it was reported that no apparent behavioral changes occurred with regard to mechanical or thermal stimuli in the contralateral foot following ipsilateral nerve injury with bilateral sympathectomy [10]. Clinically, sympathetic ganglion block or sympathectomy is usually performed on only one side. Thus, we selected a transabdominal approach, which allowed visualization of the sympathetic trunk by separating the fat tissue and slightly retracting the psoas major muscle, and performed only ipsilateral sympathectomy. Although there are some differences in the animal model of neuropathic pain and sympathectomy protocol between the present study and those reported previously, the results of the present study indicated the effects of sympathectomy.
Olmarker and Myers [18] reported that a combination of disk puncture and nerve displacement induced allodynia and hyperalgesia in rats although disk incision without nerve displacement, or nerve displacement without disk incision, did not induce significant changes in thresholds for mechanical or thermal stimulation as compared to sham-operated animals. In their study, a statistically significant reduction was observed on days 2, 4, 16, and 18 for mechanical stimulation, and on days from 2 to 12 for thermal stimulation. This study demonstrated obvious changes in signs of focal pain behavior only if disk incision and nerve displacement were combined. Spontaneous behavior has also been studied using a combination of disk incision and nerve displacement in rats [20, 24]. According to these reports, the non-treated showed increased signs of focal pain behavior during the first 7 postoperative days. Another picture of increased immobility and decreased locomotion was seen in the combination group, possibly indicating more generalized pain, at day 21 after surgery. Treatment with infliximab significantly reduced these behaviors. In the present study, mechanical allodynia was significantly prevented on day 3 and 7 with sympathectomy, and thermal hyperalgesia was prevented on day 21 with sympathectomy. Considering these data, thermal hyperalgesia may relate with generalized pain described in the spontaneous behavior studies.
It has also been reported that treatment with the tumor necrosis factor alpha (TNF)-inhibitor, infliximab, significantly reduced these behaviors [20]. It is well known that nucleus pulposus is capable of producing proinflammatory cytokines, such as TNF, interleukin-1 (IL-1), and IL-6 [5, 6, 14, 17, 26]. These previous observations suggested that sensitization of the nerve tissue might be induced either by mechanical or chemical factors. Kuslich et al. [13] performed disk herniation surgery under progressive local anesthesia in clinical cases. They reported that mechanical stimulation of the intact spinal nerve root was not painful but induced discomfort, while stimulation of the spinal nerve root exposed to the herniated disk reproduced symptomatic radicular pain. These reports indicated that a combination of disk incision and displacement of the adjacent spinal nerve may be a suitable model in which to study disk herniation because of the chemical factors involved.
It has been reported that mechanical compression induces intraneural edema of nerve roots [12, 22]. Such intraneural edema is associated with a reduction in nerve blood flow [15]. Yabuki et al. [30] harvested autologous nucleus pulposus and applied it to the nerve root just proximal to the DRG, with continuous monitoring of the blood flow using a laser Doppler flow probe. They reported that application of nucleus pulposus to the nerve root decreased blood flow in the dorsal root ganglia. On the other hand, Kinoshita and Monafo [11] performed sympathectomy with simultaneous measurement of regional blood flow in the sciatic nerve by monitoring the distribution of 14C-butanol, and reported that the blood flow was elevated after sympathectomy by 47% in rats. These observations suggested that the reduced DRG blood flow by compression and application of nucleus pulposus may have been recovered by sympathectomy in the present study.
In conclusion, the results of the present study suggested that sympathectomy may prevent pain behavioral changes, such as allodynia and hyperalgesia, in the combined disk incision and displacement of the adjacent spinal nerve model in rats. These observations may be important in understanding the pathophysiology and the treatment of sciatica caused by disk herniation.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (8685), the Swedish Medical Society, the Gothenburg Medical Society, the Greta och Einar Asker Research Foundation, and the Inga Britt and Arne Lundberg Research Foundation. The authors thank Karin Larsson, B.Sc. and Mrs. Marina Halvarsson for experimental assistance.
References
- 1.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 3.Galbraith JA, Mrosko BJ, Myers RR. A system to measure thermal nociception. J Neurosci Methods. 1993;49:63–68. doi: 10.1016/0165-0270(93)90109-5. [DOI] [PubMed] [Google Scholar]
- 4.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi T, Kikuchi S, Shubayev V, Myers RR. Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology: molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, III, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101–2107. doi: 10.1097/00007632-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Chung JM. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci Lett. 1991;134:131–134. doi: 10.1016/0304-3940(91)90524-W. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita Y, Monafo WW. Guanethidine chemical sympathectomy: spinal cord and sciatic nerve blood flow. Am J Physiol. 1993;265:1155–1159. doi: 10.1152/ajpheart.1993.265.4.H1155. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Yoshizawa H, Hachiya Y, Ukai T, Morita T. Vasogenic edema induced by compression injury to the spinal nerve root: distribution of intravenously injected protein tracers and gadolinium-enhanced magnetic resonance imaging. Spine. 1993;18:1410–1424. [PubMed] [Google Scholar]
- 13.Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–187. [PubMed] [Google Scholar]
- 14.Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K. Distribution and appearance of tumor necrosis factor alpha in the dorsal root ganglion exposed to experimental disc herniation in rats. Spine. 2004;29:2235–2241. doi: 10.1097/01.brs.0000142223.30453.e5. [DOI] [PubMed] [Google Scholar]
- 15.Myers RR, Mizisin AP, Powell HC, Lampert PW. Reduced nerve blood flow in hexachlorophene neuropathy. J Neuropathol Exp Neurol. 1982;41:391–399. doi: 10.1097/00005072-198207000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Olmarker K, Brisby H, Yabuki S, Nordborg C, Rydevik B. The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine. 1997;22:471–475. doi: 10.1097/00007632-199703010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Olmarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998;78:99–105. doi: 10.1016/S0304-3959(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 19.Olmarker K, Nordborg C, Larsson K, Rydevik B. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine. 1996;21:411–414. doi: 10.1097/00007632-199602150-00002. [DOI] [PubMed] [Google Scholar]
- 20.Olmarker K, Nutu M, Størkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine. 2003;28:1635–1642. doi: 10.1097/00007632-200308010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. Spine. 1989;14:569–573. [PubMed] [Google Scholar]
- 23.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. [PubMed] [Google Scholar]
- 24.Olmarker K, Størkson R, Berge O. Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. spine. 2002;27:1312–1317. doi: 10.1097/00007632-200206150-00013. [DOI] [PubMed] [Google Scholar]
- 25.Peterson DF, Norvell JE. Sympathetic postganglionic pathways to the hind-limb of the rat: a fluorescent histochemical study. J Auton Nerv Syst. 1985;13:171–174. doi: 10.1016/0165-1838(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 26.Rand N, Reichert F, Floman Y. Murine nucleus pulposus-derived cells secrete interleukins-1-β, -6, and -10 and Granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598–2601. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ringkamp M, Eschenfelder S, Grethel EJ, Habler HJ, Meyer RA, Janig W, Raja SN. Lumbar sympathectomy failed to reverse mechanical allodynia- and hyperalgesia-like behavior in rats with L5 spinal nerve injury. Pain. 1999;79:143–153. doi: 10.1016/S0304-3959(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 28.Ringkamp M, Grethel EJ, Choi Y, Meyer RA, Raja SN. Mechanical hyperalgesia after spinal nerve ligation in rat is not reversed by intraplantar or systemic administration of adrenergic antagonists. Pain. 1999;79:135–141. doi: 10.1016/S0304-3959(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Yabuki S, Kikuchi S, Olmarker K, Myers RR. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517–2523. doi: 10.1097/00007632-199812010-00006. [DOI] [PubMed] [Google Scholar]