Abstract
Trunk stability requires muscle stiffness associated with appropriate timing and magnitude of activation of muscles. Abnormality of muscle function has been implicated as possible cause or consequence of back pain. This experimental study compared trunk muscle activation and responses to transient force perturbations in persons with and without self-reported history of low back pain. The objective was to determine whether or not history of back pain was associated with (1) altered anticipatory preactivation of trunk muscles or altered likelihood of muscular response to a transient force perturbation and (2) altered muscle activation patterns during a ramped effort. Twenty-one subjects who reported having back pain (LBP group) and twenty-three reporting no recent back pain (NLBP group) were tested while each subject stood in an apparatus with the pelvis immobilized. They performed ‘ramped-effort’ tests (to a voluntary maximum effort), and force perturbation tests. Resistance was provided by a horizontal cable from the thorax to one of five anchorage points on a wall track to the subject’s right at angles of 0°, 45°, 90°, 135° and 180° to the forward direction. In the perturbation tests, subjects first pulled against the cable to generate an effort nominally 15% or 30% of their maximum extension effort. The effort and the EMG activity of five right/left pairs of trunk muscles were recorded, and muscle responses were detected. In the ramped-effort tests the gradient of the EMG–effort relationship provided a measure of each muscle’s activation. On average, the LBP group subjects activated their dorsal muscles more than the NLBP group subjects in a maximum effort task when the EMG values were normalized for the maximum EMG, but this finding may have resulted from lesser maximum effort generated by LBP subjects. Greater muscle preactivation was recorded in the LBP group than the NLBP group just prior to the perturbation. The likelihood of muscle responses to perturbations was not significantly different between the two groups. The findings were consistent with the hypothesis that LBP subjects employed muscle activation in a quasi-static task and preactivation prior to a perturbation in an attempt to stiffen and stabilize the trunk. However, interpretation of the findings was complicated by the fact that LBP subjects generated lesser efforts, and it was not known whether this resulted from anatomical differences (e.g., muscle atrophy) or reduced motivation (e.g., pain avoidance).
Keywords: Muscle activation, Perturbation, Back pain, Electromyography, Trunk stability
Introduction
Muscular function in the trunk is complex, since there are numerous vertebrae that can each move in six degrees of freedom relative to their neighbors, and an even greater number of muscles that can be activated to control force equilibrium and motion of those degrees of freedom. In addition, the spine must be stabilized against possible buckling by the stiffness of the muscles and motion segments [2] and by central nervous system mediated response to actual perturbations. The restoration of equilibrium after a perturbation can be achieved by active adjustment of muscle tensions, but with inherent neuromuscular delays [30]. Alternatively, small perturbations might be accommodated without such active responses, provided there is sufficient anticipatory muscular stiffness and damping in the trunk. Coactivation of antagonistic muscles is a part of a strategy that can increase the muscular stiffness and hence stability [7], but at the cost of increased spinal loads [2, 10, 16, 17].
Low back pain (LBP) is thought to be associated with altered muscle recruitment patterns, either as a predisposing cause of the pain, or as a secondary response to pain. Three general aspects of altered muscle recruitment patterns in people with back pain have been investigated: (1) altered muscle activation patterns, especially coactivation, in a static task such as pulling against a fixed object or slow lifting [1, 3, 19, 20, 28], (2) altered muscle reflex latency times in response to a sudden perturbation [26] or dropped weight [18, 32], quick release of trunk loading [27], or moving support platform [10], (3) altered muscle activation pattern in anticipation of an unexpected or voluntary perturbation [14, 25].
It is possible that instability (buckling) of the spine is responsible for sudden onset of episodes of LBP. In this supposed mechanism, spinal buckling causes large localized local tissue deformations and associated painful tissue damage. The degree of stability of the trunk with a given external loading, and a known muscle activation pattern can be quantified with the help of models that analyze the potential energy of the trunk [5, 7]. Experimentally, buckling instability cannot be induced by a perturbation, but the amount of trunk excursions (stiffness) [4, 8, 9] and/or the magnitude and timing of muscular response to a perturbation [18, 32] can be recorded. Persons with LBP might respond differently to the anticipation of the perturbation, or to the actual perturbation.
In an earlier study [29] that was intended to investigate spinal stability, we developed a paradigm in which a full sine wave force perturbation was delivered through a cable to a subject who was already pulling against the cable and the existence of a detectable muscular response was recorded. The initial effort (the generated force prior to the perturbation) was termed the ‘preload’. Since the sine-wave perturbation was ‘symmetrical’ (equal positive and negative forces) we proposed that the existence of a muscle response indicated a real or perceived potential spinal instability. Healthy subjects produced a detectable increase in trunk muscle activation (response) in fewer than 15% of trials, and there were fewer responses with increased preload. This was thought to result from increased muscular preactivation stiffening the trunk. Therefore, it was expected that subjects with a history of LBP might demonstrate an altered pattern of muscle responses to perturbation, either because of different degree of muscle preactivation, or because of altered ‘gain’ in the control of muscular responses.
This paper reports a study that investigated whether or not the pattern of trunk muscle activation differed between subjects with and without a history of self-reported LBP, in a quasi-static (ramped effort) force-generating task, and in response to a perturbation. Two alternate possibilities concerning expected differences in response to the perturbation among people with LBP were considered: In the first, those with LBP would employ greater muscle preactivation to stabilize the trunk and therefore be less likely to activate those muscles in response to the perturbation. In the second, they would preactivate their muscles less (as a pain avoidance strategy) and hence be more likely to respond to the perturbation. Both possibilities seemed plausible, since both the muscle preactivation and the response might be painful. By contrast, in a quasi-static task, it was expected that subjects with LBP would endeavor to adopt a ‘pain avoidance’ pattern of muscle activation that would minimize the potentially painful tissue forces.
Comparison of muscle activation in people with and without back pain is complicated by the fact that people with back pain have been found to generate lesser force in maximum effort tasks [21]. It is not known whether this difference is due to differing motivation (associated with pain or fear or apprehension of pain), or whether it results from those subjects having lesser force generating capacity (smaller or weaker muscles, perhaps as a result of disuse atrophy). In EMG studies, normalization by the magnitude of the maximum recorded EMG signal is normally used to compensate for differences in the instrumentation, and to facilitate within-individual comparisons. However, for between-individual comparisons of activation levels it depends on the assumption that the maximum EMG corresponds to true physiological maximum effort.
The purpose of this investigation was to test the hypothesis that (1) the level of muscle activation (in the ramped effort task) and (2) preactivation (prior to a perturbation) and/or (3) the likelihood of a detectable response to a force perturbation would differ in LBP subjects, compared with a group without any history of back pain. Normalization by both the force generated (effort) and by the maximum observed EMG signal magnitude was used to compensate for differences in both effort and EMG–muscle force relationships.
Methods
Twenty-three subjects who reported no recent (prior year) history of back pain were compared to a group of twenty-one subjects who reported current LBP, (Table 1). Subjects were studied after they signed the informed consent form that had been approved by the Institutional Committee on Human Research. Trunk muscle activation was recorded during steadily increasing isometric efforts to each subject’s maximum voluntary effort, and their muscle preactivation and muscle responses to a transient force perturbation were recorded with two different magnitudes of preload.
Table 1.
Details of the subjects studied
| Age (years) | Height (m) | Body weight (kg) | ||
|---|---|---|---|---|
| LBP | Female (n=10) | 34.2 (10.4) | 1.63 (0.08) | 64.7 (11.2) |
| Male (n=11) | 28.4 (8.4) | 1.79 (0.04) | 76.9 (9.5) | |
| No LBP | Female (n=8) | 33.5 (13.2) | 1.63 (0.06) | 60.7 (10.6) |
| Male (n=15) | 30.3 (9.1) | 1.68 (0.46) | 81.8 (14.2) |
Mean values (with SD in parentheses) are presented
Subjects responded to announcements of the study posted in public places, in medical facilities and in a local newspaper. They were recruited into the study if they were between 18 years and 60 years of age. Those in the LBP group were determined in a structured interview to have a history of episodic LBP (one or more episode lasting more than 3 days and less than 4 weeks), and on the day of recruitment reported LBP greater than 3 on a 0–10 range visual-analog scale. Subjects were excluded from the LBP group if they had: (1) an objectively diagnosed cause of their pain (herniated nucleus pulposus, tumor, fracture, stenosis, or neuropathy evidenced by symptoms radiating below the knee); (2) prior spine surgery; (3) deformity: symptoms or treatment attributed to scoliosis or spondylolisthesis; or (4) pending legal action associated with their LBP.
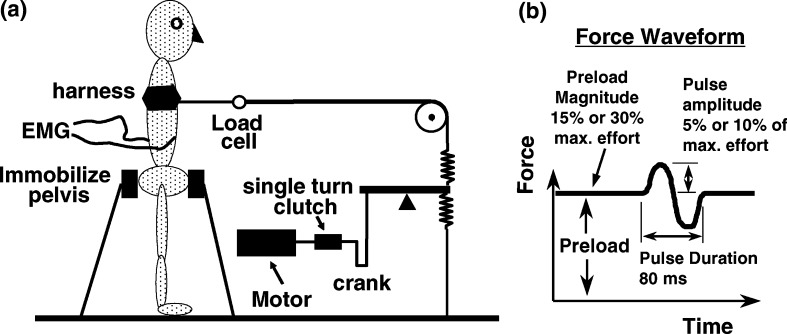
For testing, the subjects first had surface EMG electrodes applied at ten locations on the trunk (described below). Then they stood in an apparatus [29] with the pelvis effectively immobilized by a support structure with pads pressing on the regions over the anterior superior iliac spines and the sacrum (Fig. 1). A harness around the subject’s thorax was connected via a cable and pulley to the system for applying a force perturbation of variable (and controlled) amplitude and duration (Fig. 1a). Prior to the perturbation, subjects pulled against the cable to generate the predetermined preload. The cable was aligned approximately horizontally and at the level of the T-12 vertebra. The pulley was attached to one of five anchorage points on a wall track surrounding the subject at angles of 0°, 45°, 90°, 135° and 180° to the anterior direction. The mechanical system for generating the force perturbation (Fig. 1b) consisted of an electric motor driving an eccentric-crank lever system via a single turn electromagnetic clutch, activated by the experimenter pushing a button. This produced a single full sine-wave displacement of the lever arm attached to the two springs in line with the cable connected to the harness around the subject. The amplitude of the sinusoidal displacement and the stiffness of the springs determined the amplitude of the force perturbation superimposed on the preload efforts. Here, ‘effort’ refers to the measured external force in the cable.
Fig. 1.
Diagram showing a the arrangement of the apparatus relative to the subject, and b the waveform of the force perturbation generated by the single turn of the motor
Initially, the cable was anchored to the wall at 0° (extension effort) and subjects generated a timed ramped effort test up to their maximum effort in 5 s with a further 5 s for gradual release of the load. A computer screen in front of the subject displayed a vertical bar whose height was proportional to the effort generated, and with a mark to indicate the prior maximum effort. Three trials were performed to help subjects to learn how to achieve a maximum effort. The maximum achieved was used as the basis for determining the preload effort and perturbation amplitude in the perturbation experiments.
At each of the five test angles (the sequence of angles was randomly selected) subjects first performed three ramped maximum effort tests. Then, they were instructed to generate a preload of nominal magnitudes 15% and 30% of the maximum effort recorded in the extension efforts, by pulling against the cable. The computer display with a target mark was used to help subjects maintain the desired steady-state preload effort. The subjects were instructed to maintain a normal erect posture, symmetrically oriented with the apparatus during all tests. A single full sine-wave force perturbation pulse (nominal amplitude 5% or 10% of maximum effort, nominal duration 80 ms) was triggered by the investigator without warning at a random time between 5 s and 10 s after the subject reached the desired steady-state preload effort. They were expecting the force perturbation and were instructed to maintain the target effort until after the perturbation (i.e., not to react actively to it); then they were allowed to relax. The subjects had experienced the force perturbations in a practice session prior to the recorded trials. At each angle there were four test conditions (two preload efforts, two pulse amplitudes) which were randomly presented. Three repeated trials of each test condition were made sequentially. The total time of the testing session was about 3 h, and the typical duration of a sustained effort was about 15 s. Subjects could rest between trials and while the load direction and perturbation parameters were altered, and were given a rest period of about 10 min after half of the testing protocol was completed.
Bipolar EMG electrodes (Delsys Inc., Type DE-02.3, Boston, MA, USA) recorded signals from five right and left pairs of muscles (rectus abdominis, internal and external obliques, longissimus, iliocostalis). The Delsys electrodes have 10×1 mm silver-bar electrodes with 10-mm spacing; their single differential amplifiers have a gain of 1,000, bandwidth 20–450 Hz, 92 dB (typical) common mode rejection ratio, and 1012 Ω input impedance. A ground electrode was placed over the lateral epicondyle of the elbow. EMG and load cell signals were recorded digitally at 2,048 Hz.
The electrodes placements were: rectus abdominis—30 mm lateral to the midline at the level of the umbilicus, aligned vertically; external oblique—halfway between the iliac crest and the 12th rib along the mid-axillary line, aligned at an 80° angle to the horizontal; internal oblique 20-mm medial and superior to the anterior superior iliac spine, aligned vertically; longissimus—30 mm lateral to the midpoint of the spinous process of L-3, aligned vertically; iliocostalis—60 mm lateral to the midpoint of the spinous process of L-3, aligned vertically. Suspect data resulting from technical problems such as loose electrodes, or EKG artifacts were excluded from further statistical analyses by a dual process of visual inspection, and identification of outliers. Overall, 1.3% of recordings were excluded, with no evident predominant rate of exclusion by electrode location.
For the ramped-effort tests, the EMG signals were passed through an RMS filter with a moving window having a width of 250 ms. Then a linear regression analysis was performed for the increasing-effort part of the recording between the RMS-EMG signal and the force (effort) generated. Muscle activation was obtained from the gradient of the EMG–effort relationship, normalized in each of two ways. In the first, the gradient was divided by the maximum EMG value recorded for that muscle and subject in all the ramped-effort tests, and multiplied by the value of the maximum effort for that testing angle, hence, it was nondimensional. In the second normalization, the gradient was divided by the maximum EMG value for that muscle, without normalization by effort (hence, it had units of kN−1). Thus, in both normalizations there was division by the maximum EMG to account for differences in electrode and amplifier gain, etc. Only the first normalization accounted for differences in effort. The source of differences in maximum effort was unknown. If it was due to anatomical differences (e.g., in muscle size) then normalization by the maximum effort would provide better comparison between different individuals’ muscle recruitment patterns. If the different effort was due to differences in subject motivation, then normalization by maximum effort would not be appropriate for measuring relative muscle activation.
The muscle preactivation was recorded while subjects were maintaining the preload just prior to the perturbations. This preactivation was quantified by averaging the EMG signal magnitude in the window 25–150 ms prior to the onset of the perturbation and it was expressed (nondimensionally) as a proportion of the maximum EMG value for that muscle obtained from all the ramped-effort tests of the corresponding subject.
For the perturbation tests, EMG signals were first bandpass filtered by a 10–100 Hz Chebyshev type II filter with no lag, and rectified. The filtration was intended to reduce any EKG or motion artifact and high frequency noise contamination of the signals. A 25 ms moving average of the rectified EMG signal was then calculated. The onset of the force perturbation was first identified from the load cell recording by detecting the time at which there was a significant increase in the effort–time slope. A 25–150 ms time window after the force perturbation was examined to detect any short (reflexive) and medium (automatic) latency muscle responses to the perturbation. Two different methods of response identification were employed, which are as follows:
Shewhart method [12]
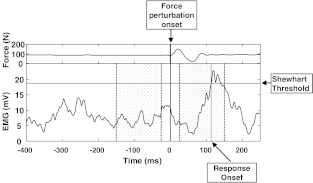
A muscle response to force perturbation was considered to occur if the processed EMG signal exceeded a threshold of 3 standard deviations (SDs) above the baseline (preactivation) EMG signal. If a response was detected, a value of one was assigned, otherwise zero was assigned [29]. The latency was measured as the time from the start of the force perturbation to onset of the EMG response, and then was used to select responses between 25 ms and 150 ms after the beginning of the perturbation for inclusion in the analyses (Fig. 2).
Fig. 2.
Sample recording of an EMG signal from a perturbation experiment. The upper panel shows the force recording, that defined the force perturbation onset time (that defined zero time). Relative to this time, pre-perturbation and post-perturbation ‘windows’ (shaded) were defined, and used to calculate the MEMGD. The EMG activity prior to the perturbation defined the Shewhart threshold used to identify the EMG response onset that occured here 113 ms after the perturbation onset
Mean EMG difference (MEMGD) method [29]
The difference between the mean EMG signal in a 25–150 ms window after the perturbation and a 25–150 ms window prior to perturbation was computed for each EMG signal. A value of zero was assigned if the difference was negative, and unity if positive. The observed numbers of responses were examined relative to the 50% rate expected by chance if there was no true increase is muscle activity. This detection method is not influenced by the differences in the SD of the baseline EMG signal between experimental conditions that could potentially produce detection bias with the Shewhart method.
Antagonistic/agonistic activation ratios
The coactivation pattern for individual muscles was evaluated by two methods: (1) Flexion-extension measure: the mean activation (or preactivation) was calculated at the angle where the muscle was considered to be antagonist (activation averaged over the three repeat trials) and was expressed as a proportion of the activation (or preactivation) when it was considered to be an agonist. For dorsal muscles, this was the ratio of activation at 180° (antagonist) and 0° (agonist) effort angles, and for abdominal muscles, the ratio at the 0° (antagonist) and 180° (agonist) effort angles. (2) Lateral bend measure: the right muscle activation (or preactivation) was expressed as a proportion of the left muscle activation (or preactivation) at the 90° effort angle.
Statistical analyses
To analyze the likelihood of muscle responses, a dichotomous response was recorded for the Shewhart and MEMGD methods for each of three trials at each combination of experimental conditions. The estimated muscle response frequency was the average over the three trials. An arcsine square root transformation was applied to these frequencies to improve compliance with assumptions made in the subsequent analysis of variance (ANOVA). ANOVA was also used to evaluate the significance of group-wise differences between muscle activation and preactivation. In all analyses, a probability less than 0.05 was considered statistically significant.
Results
The maximum efforts generated in the maximum effort trials, averaged over subjects and angles, was 575 N for the NLBP group and 403 N for the NLBP group, which was a significant difference (P<0.01). For the NLBP group, the highest value of maximum effort averaged over subjects (585 N) was observed at 180° (flexion effort), and the lowest value (566 N) was observed at 0° (extension effort). Conversely, for the LBP group, the greatest value (463 N) was observed at 0°, and the lowest maximum effort (337 N) was observed at 180°.
Muscle activation in ramped efforts and preactivation prior to perturbations
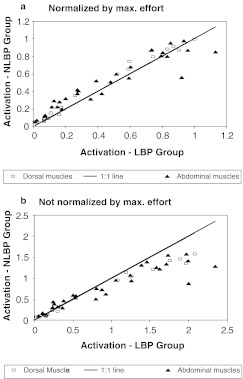
When activation was measured by the EMG–effort slope normalized by both the maximum effort and maximum EMG, the subjects in the LBP group were observed to activate their dorsal muscles on average less than those in the NLBP group in the ramped efforts (Fig. 3a). LBP subjects activated their dorsal muscles less than those in the NLBP group in 15 of 20 dorsal muscle/angle permutations and in 23 of 30 abdominal muscle/angle permutations. The difference between groups was significant for five of the ten muscles. In contrast, when the activation was normalized by maximum EMG only, the opposite trend was found (Fig. 3b): LBP subjects activated their dorsal muscles more than NLBP subjects in 18 of 20 muscle/angle permutations and activated their abdominal muscles more in 18 of 30 muscle/angle permutations, indicating that on average the subjects in the LBP group employed greater muscle activation. The difference between groups was significant for five of the ten muscles. This contrasting finding associated with normalization method was consistent with the fact that the groups differed in the maximum efforts generated.
Fig. 3.
Relationship between groupwise averages of the muscle activation in ramped-maximum-effort tests. Each point on the graphs represents the mean for one muscle/angle permutation. aUpper: For activation normalized by both maximum EMG and maximum effort: 15/20 of points for dorsal muscles (squares) and 23/30 points for the abdominal muscles (triangles) lie to the left of the 1:1 (equality) line, indicating that on average the subjects in the LBP group employed lesser muscle activation. bLower: Activation normalized by maximum EMG only: 18/20 points for dorsal muscles (squares) and 18/30 points for abdominal muscles (triangles) lie to the right of the 1:1 (equality) line, indicating that on average the subjects in the LBP group employed greater muscle activation
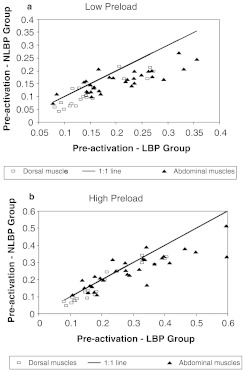
In the perturbation tests, the subjects in the LBP group were observed to preactivate their dorsal muscles on average more than those in the NLBP group prior to the perturbation (Fig. 4). Averaged over all muscles and trials, the mean preactivation (as measured by the EMG as a proportion of the maximum EMG in ramped efforts) was 0.22 in the LBP group, and 0.18 in the NLBP group (P<0.01). In the low-preload condition, the LBP group on average preactivated their dorsal muscles more in 17 of 20 muscle/angle permutations, and for 26 of the 30 abdominal muscle/angle permutations (Fig. 4a). For the high-preload tests this difference between groups was observed in 17 of 20 dorsal muscle/angle permutations, and in 20 of 30 abdominal muscle/angle permutations (Fig. 4b). The difference between groups was significant in nine of ten muscles at low preload, and in seven of ten muscles at high preload.
Fig. 4.
Relationship between groupwise averages of the muscle preactivation in perturbation tests. Each point on the graph represents the mean for one muscle/angle permutation. aUpper: For trials with low preload, where 17 of 20 points for dorsal muscles (squares) and 26 of 30 abdominal muscles (triangles) lie to the right of the 1:1 (equality) line, indicating that on average the subjects in the LBP group employed greater muscle preactivation. bLower: For trials with high preload, where 17 of 20 points for dorsal muscles (squares) and 20 of 30 abdominal muscles (triangles) lie to the right of the 1:1 (equality) line, indicating that on average the subjects in the LBP group employed greater muscle preactivation
Variation of muscle activation with angle
When the LBP and NLBP groups were compared, there was no clear pattern of differences in the antagonist/agonist activation ratios between groups in the ramped-effort task. For the preactivation (prior to perturbation) coactivation ratios the LBP group had greater ratios in eight of ten muscles by the flexion-extension measure, and in four of the five muscle pairs by the lateral bend measure. However, in individual muscle comparisons, none of the group differences were significantly different.
There were differences among muscles for both the activation and preactivation antagonistic/agonistic ratios. For the flexion-extension measure of coactivation, the activation ratios (in the ramped efforts) averaged 0.097 for dorsal muscles, 0.492 for oblique abdominals, and 0.099 for the rectus abdominis (averaged over both LBP and NLBP groups). For preactivation (prior to perturbation) the ratios averaged 0.388 for dorsal muscles, 0.576 for oblique abdominals, and 0.499 for the rectus abdominis (again averaged over both groups). These values also indicate that the ratios were greater for all muscles for preactivation (perturbation task) compared to activation (maximum effort task).
A similar pattern was observed for the lateral bend measure of coactivation. Here, the ratios in the ramped efforts averaged 0.291 for dorsal muscles, 0.440 for oblique abdominals, and 0.759 for the rectus abdominis (averaged over both LBP and NLBP groups). The ratios for preactivation (prior to the perturbation) averaged 0.889 for dorsal muscles, 0.941 for oblique abdominals, and 1.25 for the rectus abdominis (averaged over both LBP and NLBP groups). Again, the ratios were greater for all muscles for preactivation compared to activation.
Likelihood of response to perturbation
When the LBP and NLBP groups were compared, the overall numbers of muscle responses detected by the Shewhart method averaged 3.5% in the LBP group and 4.3% in the NLBP group (NS, P=0.7). Using the MEMGD method, the overall average was 0.545 in the LBP group, and 0.548 in the NLBP group (NS, P=0.8). Thus, both measures indicated a slightly (not significantly) lesser likelihood of muscular responses in the LBP group. When the group differences were examined for the high- and low-preload conditions, and in each muscle and angle permutation, no trends were evident to suggest any differences between the groups. Significant differences were only found in four Shewhart and five MEMGD higher-order interactions involving specific combinations of the five angles, two preload magnitudes, and/or perturbation magnitude. The significant differences in the MEMGD all indicated higher response rates of the right iliocostalis in the NLBP group, but these did not appear as significant differences in the Shewhart measure of responses. There was no consistency in findings from these two measures of the likelihood of a perturbation response in the analyses of higher-order interactions.
Muscle responses to the perturbations (averaged for both the NLBP and the LBP groups) were detected more frequently in the low-preload condition (overall average 5.3% by the Shewhart method) than in the high-preload condition (overall average 2.5% by Shewhart) (P<0.01). Differences were significant for all individual muscles. This difference was also evident in the average MEMGD values (0.58 and 0.52, respectively) (P<0.01), significant for all individual muscles except left iliocostalis and right rectus. Also, the responses were more frequent for high than low perturbation magnitudes (5.3% compared to 2.5% by the Shewhart method) (P<0.01). Similar effects were reported previously [29].
The responses were detected more frequently on average among the right-side muscles (6.11% of low-preload trials and 3.08% of high-preload trials) than left-side muscles (4.42% of low-preload trials and 1.95% of high-preload trials). The right-side-muscles were considered to be antagonistic with respect to the preload and perturbation at angles of 45°, 90° and 135°. Dorsal muscles were found to have increasing numbers of responses with angle (more responses when the muscles were antagonistic with respect to the preload and perturbation direction), and the opposite angle trend (decreasing number of responses with angle) was observed for the oblique abdominal muscles (not for the rectus abdominis). There was no evidence of differences in response rates between individual muscles, or anatomical groupings of muscles (dorsal versus abdominal).
Discussion
The findings of this study are consistent with the notion that persons with a history of LBP prepared for the perturbation by generating greater muscle preactivation than the NLBP group subjects. However, the findings for the ramped-effort test were less clear, depending on whether EMG–effort gradients were normalized by the effort generated. The maximum efforts were less in the LBP group, but it was not known whether this was due to anatomical differences (e.g., weaker atrophied muscles) or motivational differences. If it was the former, then this was compensated by normalization by both the effort and the activation by maximum effort generated—this led to a finding of lesser activation in the LBP group (Fig. 3a). If differences were due to motivational differences then normalization by maximum effort would not be appropriate, leading to a finding of greater activation in the LBP subjects (Fig. 3b).
Motivational differences would likely be due to actual pain, or fear of pain. It should be noted that reduction in muscle force does not necessarily imply lesser tissue loads. For example, if shear force in an intervertebral disc was painful, additional muscle activation might conversely be required in a specific task to minimize this force.
The antagonistic muscle activity measured in the LBP subjects during the ramped effort was not different from that of the NLBP subjects, a finding that was independent of normalization method. But in preparing for a force perturbation, the LBP subjects employed a greater level of muscle antagonist activity. Lesser antagonistic activation is thought to be a strategy to minimize loading of pain sensitized tissues, whereas greater antagonism is thought to stabilize the spine. The greater preactivation and muscle antagonism prior to the perturbations might be part of a strategy to stiffen the trunk and reduce the need for subsequent responses to the perturbations, although the LBP group subjects apparently did not succeed in reducing the number of detected responses.
van Dieën et al. [31] reviewed published literature on trunk muscle activation with respect to two different hypotheses as to possible differences between subjects with and without back pain. In the ‘pain–spasm–pain’ hypothesis a vicious cycle of muscular spasm and pain develops. In the ‘pain-adaptation’ hypothesis there is increased antagonistic activation of muscles that slows motion and hence, guards against the exacerbation of existing pain. Their review indicated that the available data did not consistently support either hypothesis, and as an alternative the authors proposed that the observed differences were consistent with the notion that persons with back pain adopt strategies that enhance the stability of the spine. The present findings are compatible with those reviewed by van Dieën et al. [31] in that little difference was observed between LBP and NLBP groups in a quasi-static task, but there was a tendency of greater muscle preactivation and activation in a perturbation experiment. The LBP subjects had been expected to be apprehensive of the perturbation, and to preactivate their trunk muscles more in anticipation of it.
On average, muscle responses for both groups were significantly less frequent at high-preload than the low-preload condition. This is consistent with the biomechanical concept that increased muscle activation stiffens the trunk and reduces the likelihood of a response to the perturbation. However, this effect was evidently not of sufficient magnitude to reduce the likelihood of a muscular response when the LBP subjects preactivated their muscles to a greater extent in anticipation of the perturbation. The perturbation experiment was designed not to require a response by the subjects, so the observed responses were very small in magnitude, and infrequent, which made their accurate detection difficult. The credibility of the response findings is supported by a general agreement between the findings obtained by the Shewhart and MEMGD methods.
Radebold et al. [27] reported that subjects with LBP, compared to NLBP subjects, demonstrated delayed muscle responses and maintained activation of both agonist and antagonist muscles for a longer period after a perturbation caused by the sudden release of a preload. Other studies employing loading perturbations have also documented alterations in neuromuscular control for subjects with LBP [18, 32]. In response to horizontal support surface perturbations, subjects with chronic LBP [15], and episodic LBP [11] have been found to demonstrate a decrease in the magnitude of center of mass displacement and a delayed onset of net center of pressure displacement. The LBP subjects also demonstrated large trunk torque responses that peak later and that have a faster rate of torque development compared to control subjects [15]. These findings also suggest that subjects with LBP employ a strategy of pre-stiffening prior to the anticipated perturbation. In studies that use quiet standing tasks, subjects with LBP demonstrated more postural sway, shifted their center of pressure more posteriorly, and were less able to balance on one foot with eyes closed compared to control subjects [24]. A direction-specific (medial–lateral) increase in postural sway under increased task complexity or removal of vision was reported for LBP subjects [22]. Studies examining postural control patterns of trunk muscles during voluntary arm raising tasks suggest that muscle activation magnitudes and latencies can be altered by the induction of pain, and in subjects preoccupied with nonpainful attention-demanding tasks or stressful tasks [13, 23].
The LBP subjects in the present study were selected as those having a history of episodic back pain (and current pain), while most other published studies selected chronic pain patients for study, precluding direct comparisons. The LBP group studied here was expected to include subjects at risk for spinal or trunk instability.
Despite evidence that anticipatory and automatic postural coordination is altered with LBP, it is still not clear how differences in muscle activation relate to LBP and what are the implications for LBP intervention [6]. Currently, it is not known whether abnormal muscle function can cause or contribute to LBP. The data from the perturbation part of this study lend support to the ‘pain-adaptation’ hypothesis, which proposes that there is increased antagonistic activation of muscles that slows motion and hence, guards against the exacerbation of existing pain. The lesser maximum efforts in the ramped-effort task appear more compatible with a pain-avoidance hypothesis, although if lesser motivation were the reason for lesser effort our data suggest that the LBP subjects employed relatively greater muscle activation in this task. This is because the EMG–effort gradients not normalized by effort (Fig. 3b) show greater activation in the LBP subjects, and this normalization (by maximum EMG only) demonstrates differing muscle activation per unit effort produced. However, the results of this study may be best explained by the hypothesis that subjects with LBP have an altered central set, based on apprehension, anticipation, prior experience, or other factors yet to be identified. This, then, influences automatic postural coordination, such as anticipatory postural adjustments in preparation for voluntary arm movement [23], automatic postural responses in response to surface translations [11] as well as tonic muscular activity.
Conclusions
In this comparison of persons with and without history of LBP, findings were consistent with the hypothesis that LBP subjects employed muscle activation in a quasi-static task and preactivation prior to a perturbation compatible with an attempt to stiffen and stabilize the trunk.
Persons with a self-reported history of back pain (LBP group) employed muscle activation patterns with greater muscle activation in static (ramped effort) tasks, compared to a group without back pain (NLBP group), if it were assumed that maximum efforts were less in the LBP group because of motivational differences.
The LBP group subjects had greater muscle preactivation prior to a perturbation than the NLBP group.
The LBP muscle activation and preactivation patterns were apparently an attempt to stiffen and stabilize the trunk, although there was no evidence of lesser likelihood of a response to the perturbation.
Acknowledgements
This work was supported by NIH grants R01 AR 44119 and K01 HD 01194. Richard M. Single performed some of the statistical analyses. The work was done after review and approval of human subject procedures by the Institutional Committee on Human Research, and experiments were in compliance with USA law.
References
- 1.Alexiev AR. Some differences of the electromyographic erector spinae activity between normal subjects and low back pain patients during the generation of isometric trunk torque. Electromyogr Clin Neurophysiol. 1994;34(8):495–499. [PubMed] [Google Scholar]
- 2.Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl. 1986;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- 3.Cassisi JE, Robinson ME, O’Conner P, MacMillan M. Trunk strength and lumbar paraspinal muscle activity during isometric exercise in chronic low-back pain patients and controls. Spine. 1993;18(2):245–251. doi: 10.1097/00007632-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Chiang J, Potvin JR. The in vivo dynamic response of the human spine to rapid lateral bend perturbation: effects of preload and step input magnitude. Spine. 2001;26(13):1457–1464. doi: 10.1097/00007632-200107010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Cholewicki J, Panjabi MM, Khachatryan A. Stabilizing function of trunk flexor-extensor muscles around: a neutral spine posture. Spine. 1997;22(19):2207–2212. doi: 10.1097/00007632-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ebenbichler GR, Oddsson LI, Kollmitzer J, Erim Z. Sensory-motor control of the lower back: implications for rehabilitation. Med Sci Sports Exerc. 2001;33(11):1889–1898. doi: 10.1097/00005768-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Gardner-Morse MG, Stokes IAF. The effects of abdominal muscle coactivation on lumbar spine stability. Spine. 1998;23(1):86–92. doi: 10.1097/00007632-199801010-00019. [DOI] [PubMed] [Google Scholar]
- 8.Gardner-Morse MG, Stokes IAF. Trunk stiffness increases with steady-state effort. J Biomech. 2000;34(4):457–463. doi: 10.1016/S0021-9290(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 9.Granata KP, Wilson SE. Trunk posture and spinal stability. Clin Biomech. 2001;16(8):650–659. doi: 10.1016/s0268-0033(01)00064-x. [DOI] [PubMed] [Google Scholar]
- 10.Granata KP, Marras WS. The influence of trunk muscle coactivity on dynamic spinal loads. Spine. 1995;20(8):913–919. doi: 10.1097/00007632-199504150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Henry SM, Hitt JR, Jones SL, Bunn JY (2004) Altered postural responses with episodic, recurrent low back pain. American Physical Therapy Association combined sections meeting, Feb 2004, Nashville Tennessee
- 12.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101(6):511–519. doi: 10.1016/S0921-884X(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 13.Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res. 2003;151(2):262–271. doi: 10.1007/s00221-003-1457-x. [DOI] [PubMed] [Google Scholar]
- 14.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80(9):1005–1012. doi: 10.1016/S0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 15.Jones SL, Raasch CC, Hitt JR, Henry SM, Bunn JY (2004) Persons with chronic recurrent low back pain exhibit altered neuromuscular patterns in response to postural perturbations. American Physical Therapy Association annual meeting, June 2004, Chicago
- 16.Lavender SA, Tsuang YH, Andersson GB, Hafezi A, Shin CC. Trunk muscle coactivation: the effects of moment direction and moment magnitude. J Orthop Res. 1992;10:691–700. doi: 10.1002/jor.1100100511. [DOI] [PubMed] [Google Scholar]
- 17.Lavender SA, Tsuang YH, Hafezi A, Andersson GB, Chaffin DB, Hughes RE. Coactivation of the trunk muscles during asymmetric loading of the torso. Hum Factors. 1992;34(2):239–247. doi: 10.1177/001872089203400209. [DOI] [PubMed] [Google Scholar]
- 18.Magnusson ML, Aleksiev A, Wilder DG, Pope MH, Spratt K, Lee SH, Goel VK, Weinstein JN. Unexpected load and asymmetric posture as etiologic factors in low back pain. Eur Spine J. 1996;5(1):23–35. doi: 10.1007/BF00307824. [DOI] [PubMed] [Google Scholar]
- 19.Marras WS, Ferguson SA, Burr D, Davis KG, Gupta P. Spine loading in patients with low back pain during asymmetric lifting exertions. Spine J. 2004;4(1):64–75. doi: 10.1016/S1529-9430(03)00424-8. [DOI] [PubMed] [Google Scholar]
- 20.Marras WS, Davis KG, Ferguson SA, Lucas BR, Gupta P. Spine loading characteristics of patients with low back pain compared with asymptomatic individuals. Spine. 2001;26(23):2566–2574. doi: 10.1097/00007632-200112010-00009. [DOI] [PubMed] [Google Scholar]
- 21.McNeill T, Warwick D, Andersson G, Schultz A. Trunk strengths in attempted flexion, extension, and lateral bending in healthy subjects and patients with low-back disorders. Spine. 1980;5(6):529–538. doi: 10.1097/00007632-198011000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mientjes MI, Frank JS. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech. 1999;14(10):710–716. doi: 10.1016/S0268-0033(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 23.Moseley GL, Nicholas MK, Hodges PW. Pain differs from non-painful attention-demanding or stressful tasks in its effect on postural control patterns of trunk muscles. Exp Brain Res. 2004;156(1):64–71. doi: 10.1007/s00221-003-1766-0. [DOI] [PubMed] [Google Scholar]
- 24.Nies N, Sinnott PL. Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine. 1991;16(3):325–330. doi: 10.1097/00007632-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Oddsson LI, De Luca CJ. Activation imbalances in lumbar spine muscles in the presence of chronic low back pain. J Appl Physiol. 2003;94(4):1410–1420. doi: 10.1152/japplphysiol.01183.2001. [DOI] [PubMed] [Google Scholar]
- 26.Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26(7):724–730. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25(8):947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Robinson ME, Cassisi JE, O’Connor PD, MacMillan M. Lumbar iEMG during isotonic exercise: chronic low back pain patients versus controls. J Spinal Disord. 1992;5(1):8–15. doi: 10.1097/00002517-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Stokes IAF, Gardner-Morse M, Henry S, Badger GJ. Trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine. 2000;25(15):1957–1964. doi: 10.1097/00007632-200008010-00015. [DOI] [PubMed] [Google Scholar]
- 30.Thelen DG, Schultz AB, Ashton-Miller JA. Quantitative interpretation of lumbar muscle myoelectric signals during rapid cyclic attempted trunk flexions and extensions. J Biomech. 1994;27(2):157–167. doi: 10.1016/0021-9290(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 31.Dieën JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13(4):333–351. doi: 10.1016/S1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilder DG, Aleksiev AR, Magnusson ML, Pope MH, Spratt KF, Goel VK. Muscular response to sudden load. A tool to evaluate fatigue and rehabilitation. Spine. 1996;21(22):2628–2639. doi: 10.1097/00007632-199611150-00013. [DOI] [PubMed] [Google Scholar]