Abstract
We investigated back muscle fatigue and endurance in patients with lumbar disc herniation before and after surgery, and established the degree of association between perceived fatigue and objectively measured fatigue. Additionally, the relationships between muscle fatigue and endurance time on the one hand, and activity, participation, self-efficacy and health on the other, were investigated to clarify the grades of association between these factors. Forty-three consecutive patients with lumbar disc herniation were tested before surgery and 4 weeks after surgery. The protocol comprised an isometric endurance test (modified Sørensen’s test) with concomitant measures of electromyography, and Borg ratings of pain and fatigue. To measure activity, participation, self-efficacy and health, the patients also filled in questionnaires. Results showed a post-operatively significant improvement in lumbar muscle fatigue expressed as a flatter L5 slope for the men. No significant improvement was found for endurance times or for Borg ratings. Endurance time correlated with questionnaire answers on physical activity, the Roland–Morris, the Oswestry, self-efficacy and some items of the SF-36 with correlation coefficients ranging from 0.52 to 0.91. The L5 slope correlated with the Roland–Morris, the Oswestry and some items of the SF-36 only in women with correlation coefficients between 0.53 and 0.77. We conclude that the effects of surgery reduced muscle fatigue for the men. There is an association between muscle fatigue and endurance with activity limitations, participation restrictions, self-efficacy and health in patients undergoing surgery for lumbar disc herniation.
Keywords: Electromyography, Endurance, Lumbar disc herniation, Muscle fatigue, Subjective factors
Introduction
Some studies have shown that patients with low back pain are more easily fatigued in their back muscles than people without back pain [4, 29, 54, 61]. As a result of fatigue, the endurance time to back muscle exhaustion during a sub-maximal isometric holding test becomes shorter, which might be a risk factor for low back pain [1, 5, 36]. Measures of back muscle fatigue and endurance are either objective, i.e. endurance time and electromyography (EMG), or subjective, i.e. ratings by the person experiencing the fatigue.
When muscles fatigue, the median frequency (MF) of the EMG power spectrum shifts to lower frequencies, due to altered muscle fibre recruitment and other changes in the contractile apparatus [2, 14]. Electromyography is regarded as an objective reflection of muscle fatigue. Even though muscle fatigue seems to be mostly peripheral, in healthy subjects as well as in patients experiencing back pain, a central activation deficit due to pain and lack of motivation cannot be excluded [7]. Psychosocial factors influence physical factors. Beliefs regarding self-efficacy affect the outcome of physical performance tests [18]. Fear-avoidance and self-efficacy beliefs interact to reduce physical activity, and might contribute to activity limitations [8, 33]. Correspondingly, the main effects of therapies for low back pain might not be due to a reversal of physical weakness but to altered perception of pain and activity limitations [42].
When measuring subjective experience of lumbar muscle fatigue, patients experiencing low back pain rate their fatigue higher than those do without back pain [59]. Subjective ratings also correspond well with the EMG measures and endurance time-to-force-failure in healthy subjects [10]. On the other hand, there has been a low correlation between the EMG and subjective ratings in protocols with short contraction times requiring a high level of effort. In these kinds of protocol, the former experience of physical effort is important and therefore the subjective rating probably reflects an additional aspect of fatigue [12, 15]. Thus, psychological aspects of muscle fatigue seem to affect performance outcomes. For this reason, subjects’ experience of fatigue together with assessment and other personal factors such as self-efficacy beliefs add valuable information to the objective measures when assessing lumbar muscle fatigue.
Patients with pain due to lumbar disc herniation mostly suffer from sciatica but they can also have pain from the lower back [63] due to algogenic substances from the disc [51], as well as ischaemia or altered use of back muscles [25]. They also report activity limitations and low-health status [52]. Treatment for lumbar disc herniation includes conservative intervention and surgery [23, 27]. Impaired trunk muscle performance due to surgery has been reported as decreased trunk strength and lifting ability. Restricted lumbar motion has also been found [22, 45]. Patients with lumbar disc herniation have shorter endurance times and lower MF slopes than healthy subjects before surgery, which improved with post-operative rehabilitation [13]. Patients reportedly benefit from intensive post-operative training programmes [9, 30, 37].
The aim of the current study was to investigate back muscle fatigue and endurance in patients with lumbar disc herniation before and after surgery and to establish the degree of association between perceived fatigue and objectively measured fatigue. In addition, we investigated the relation between muscle fatigue and endurance versus activity, participation, self-efficacy and health to clarify the grade of association between these factors.
Subjects and methods
Subjects
Patients
Forty-three patients (27 men and 16 women) with lumbar disc herniation who fulfilled standard clinical criteria to be treated surgically with a microdiscectomy participated consecutively in this prospective study. The patients underwent surgery in the spring of 2000 or the autumn of 2001 by one of four spinal surgeons, skilled at performing microdiscectomy. Inclusion criteria were: a disc herniation at the spinal levels L4–L5 (n = 16) or L5–S1 (n = 27) diagnosed with magnetic resonance imaging (MRI), clinical findings in accordance with the radiological findings, ability to read and write Swedish and age, below 65 years. Patients with previous back surgery, multiple disc herniations, spinal stenosis, spondylolysis, spondylolistheses or other spinal deficits were excluded. All patients gave their informed consent and the study was approved by the Local Ethics Committee at the Karolinska Hospital.
Patients’ physical characteristics and pain parameters are presented in Table 1. The current pain duration was defined as the last episode of pain, which led to surgery. Twenty-one patients had experienced back pain before the present episode (15 men (10–240 mo.), six women (11–240 mo.)). Twenty-two patients had been on sick leave before the operation (10 men (1–36 mo.), 12 women (0.5–16 mo.)) and 14 patients had physiotherapy treatment regularly before surgery (10 men (1–4 mo.), four women (2–14 mo.)). No significant difference was present between men and women regarding age, BMI, pain duration, time for sick leave and physiotherapy. To further characterise the patients, they underwent a clinical examination by a physiotherapist including inspection, functional tests, straight-leg-raising test, manual testing for muscle weakness and palpation.
Table 1.
Patients’ physical characteristics (mean and SD), current pain duration, and straight leg-raising test (SLR)
| Variable | All patients (n = 43) | Men (n = 27) | Women (n = 16) | p-value |
|---|---|---|---|---|
| Age (years) | 42.2 (11.0) | 42.2 (11.5) | 42.2 (10.4) | 0.999 |
| Weight (kg) | 77.9 (12.3) | 82.7 (9.5) | 69.7 (12.3) | <0.001*** |
| Height (m) | 1.76 (0.09) | 1.80 (0.06) | 1.68 (0.06) | <0.001*** |
| BMI (kg/m2) | 25.2 (3.4) | 25.5 (3.4) | 24.7 (3.4) | 0.444 |
| Pain duration (mo.) | 8.4 (4.7) | 8.2 (4.7) | 8.8 (4.7) | 0.692 |
| SLR (°) | 44 (21) | 51 (19) | 33 (20) | 0.005** |
The level of significant difference between men and women is presented
The patients in the current study were compared to healthy subjects from a former study where the same test protocol was used [10].
Physical activity
The patient’s level of physical activity was self-rated using a six-grade scale (the more physically active, the higher the score) [20]. For men, the median (25th–75th percentile) physical activity level was 3 (2–3), and for the women 2.5 (2–3). No significant difference was present between men and women for the physical activity scores.
Dropouts
Thirty-six of the 43 patients completed all tests. On entry to the study, two women were not able to do the endurance test due to severe back pain and leg pain in the test position, both presenting with positive crossed Lasegue’s sign. However, they were successfully tested on the retest 4 weeks after surgery. On this retest, two women and one man could not do the endurance test due to back pain. All three had endurance times shorter than 60 s before surgery. Two patients did not show up to the post-operative test due to hospital treatment at inpatient clinics, one woman for post-operative infection and one man for severe dizziness.
Testing procedure
The assessments described below were carried out by one of the authors (Å D), 2 weeks to 1 day before surgery and 4 weeks after surgery. A one-year and a two-year follow-up with the same assessments will be presented in a separate article.
Muscle fatigue test
A modified Sørensen’s test, an isometric trunk holding test to exhaustion, was performed [10]. The subjects lay prone on a bench with the lower extremities tightly secured with straps at hips, knees and ankles. The modification was to have the hips flexed 40°. The original Sørensen’s test is performed with straight hips [5]. During the test, the patients held the upper trunk horizontal and unsupported for as long as possible. They were unaware of the time that passed and no verbal encouragement was given. Endurance times measured were rounded down to the closest 5 s, but times less than 15 s were rounded down to the closest second.
Surface EMG
During the modified Sørensen’s test, surface EMG was used for recordings of the rate of muscle fatigue as measured by MF shifts. After cleaning the skin of the lower back thoroughly with alcohol, four pairs of self-adhesive EMG surface electrodes (Blue Sensor N-00-S, Medicotest) were applied in the middle of the erector spine muscle belly bilaterally at L1 and L5, respectively. The centre-to-centre distance was 20 mm. A ground electrode (Blue Sensor N-00-VL Medicotest) was placed at the left, lateral malleolus. The raw EMG signals were recorded at a sampling rate of 1,000 Hz, band-pass filtered (10–800 Hz), analogue-to-digital converted and stored on a computer. Ideally, the bandwidth upper value should not exceed 500 Hz. The aliasing effect, due to excessive bandwidth, was 1 Hz for frequencies over 200 Hz, and did not affect the results. A Hanning window was used prior to applying a fast Fourier transform algorithm for every 1-s period, and the MF was drawn from the power spectrum.
Borg CR-10 scale
The Borg CR-10 scale [50] was used to assess back muscle fatigue and back pain and leg pain, respectively. Assessments were made before the test started in the test position, resting with supported trunk. During the modified Sørensen’s test, fatigue was assessed every 15 s and back pain and leg pain every 30 s.
Questionnaires measuring activity, participation, self-efficacy and health
The patients filled out four questionnaires: (1) the Oswestry disability questionnaire [19], (2) the Roland–Morris disability questionnaire [28, 53], (3) the Self-efficacy scale [18] and (4) the generic medical outcomes study short form 36-item questionnaire (SF-36) [55].
Oswestry and Roland–Morris are designed to evaluate patients with low back pain and are among the most used questionnaires for the patient category [3, 31]. The SF-36 and Roland–Morris have shown high correlation to symptoms and clinical findings in patients with sciatica, indicating that the questionnaire is valid for this patient category [52]. The Self-efficacy scale is a strong predictor of isokinetic strength in patients with back pain [18].
Data analysis
EMG
A mean of the MF during the initial 5 s (MF initial), a mean of the 5 s at the end (MF end), the MF decrease (MF initial—MF end), an MF slope of the linear regression over time normalised to the initial MF (MF slope), were calculated. An ANOVA was used to check for differences between electrode sites for the MF slope and the MF initial values. Since there was no difference between right and left at group level, the mean for each spinal level was used. This increases reliability [32]. An analysis of the difference between affected side and unaffected side normalised to the mean of the sides was also done. Affected side was first defined as the side of the surgical incision, then the side of the sciatica and then the side of the lumbar scoliosis. None of the approaches showed any side differences.
The EMG disturbances were present in four cases, affecting random channels and leading to exclusion. In those four cases, the existing right- or left-side value was used instead of the calculated mean. The frequency reduction at the time of Borg ratings 3, 5 and 7 in relation to the total reduction was also calculated, which is similar to analysis of healthy subjects done in an earlier study [10]. The mean 5 s of the MF at Borg ratings 3, 5 and 7, respectively, was therefore calculated. A mean of the four electrode sites was used.
Endurance time
A similar approach was followed with endurance time as with MF. The fixed endurance time at Borg ratings 3, 5 and 7, respectively, was divided by the subject’s total endurance time.
Borg CR-10 scale
The ratings before the test started and the ratings at the end of the test and the ratings of fatigue at 1–3 min were used in the analyses.
Questionnaires
For the Roland–Morris, the ‘yes’ answers were counted (maximum 24). The ten questions of the Oswestry were: pain-intensity, personal care, lifting, walking, sitting, standing, sleeping, sexual life, social life and travelling. For the Self-efficacy scale, a time estimate (eight categories) of the patient’s beliefs in performing physical activities was assessed. The questions, were: walking, running, carrying, standing, bicycling, sitting in an armchair, sitting at a table and working bent forwards. The questions of the SF-36 were re-coded, transformed and analysed according to instructions from the inventors. The scores of eight sub-scales were calculated: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health. For the Self-efficacy scale and the SF-36, the higher the score, the higher the activity level; for the Oswestry and the Roland–Morris, the higher the score, the more limited the activity. One author has addressed a risk of invalidating measurement results by using sum scores of questionnaires [57, 58]. Both sum scores of each questionnaire and results of each questionnaire item are therefore presented.
Statistics
For statistical analysis, the SPSS computer software was used, significance level 5%. The statistical hypothesis was that no changes were present between before and after the surgery. The accompanying statistical tests for each variable were:
An ANOVA with repeated measures and with men and women as between-subject factor was used for each of the variables MF initial, MF end, MF slope and endurance time since these data were considered continuous. The Roland–Morris was considered continuous, and plotting all the data from the questionnaire indicated that was normally distributed. The items of the questionnaires Oswestry, Self-efficacy scale and SF-36, were considered to be ordered categorical data. When sum scores were used, the data were already treated as continuous and an ANOVA was used.
A Friedman ANOVA test was used for each of the variables Borg ratings of fatigue and pain and for each questionnaire item.
A McNemar test was done for the clinical examination since the data was nominal.
Spearman’s correlation coefficients were used to describe correlation. Correlation coefficients (absolute values) ranging from 0.00 to 0.25 represented little if any correlation, 0.26–0.49 represented low correlation, 0.50–0.69 represented moderate correlation, 0.70–0.89 represented high correlation and 0.90–1.00 represented very high correlation [49].
Results
Short-term outcome of surgery
The clinical examination revealed significantly fewer men with: radiating leg pain, lumbar scoliosis, limping and reduced strength in one lower extremity compared to the other 4 weeks after surgery. For the women, only the radiating leg pain was significantly less after surgery. In both men and women, the perceived leg pain at the end of the endurance test was significantly reduced after surgery (Table 2). Significant improvement after surgery was present for the men for the perceived leg pain before the test started and for women for the perceived back pain at the same point. The perceived fatigue did not improve with surgery. The scores from the questionnaires improved significantly after surgery compared to before, and the women scored significantly worse than the men both before and after surgery (Table 3). The Oswestry questionnaire scores for the men improved significantly on eight items of ten, the exceptions being “lifting” and “sitting”. For women, six items of ten improved significantly, with “lifting”, “sitting”, “sexual life” and “travelling” as the exceptions. Scores on the Self-efficacy scale for men improved significantly on five items of eight: “walking”, “standing”, “bicycling”, “sitting in an armchair” and “sitting at a table”. For the women, four items of eight improved: “running”, “standing”, “sitting in an armchair” and “sitting at a table”. All the SF-36 sub-scales for men improved except “role-physical” and “general health”. For women, only “physical-functioning” and “mental-health” improved significantly.
Table 2.
Subjective measures: Borg ratings of leg pain and back pain initially and at the end of the endurance contraction before and 4 weeks after surgery, for men and women separately (median and 25:e-75: e percentile)
| Borg rating | Men | Women | ||||
|---|---|---|---|---|---|---|
| Before (n = 27) | After (n = 25) | p-value | Before (n = 14) | After (n = 12) | p-value | |
| Initial leg pain | 1 (0–2) | 0 (0–0.5) | 0.005** | 1.25 (0–2) | 0 (0–0.6) | 0.157 |
| Initial back pain | 0 (0–1) | 0 (0–0.5) | 0.248 | 1.5 (0.5–2) | 0 (0–0) | 0.034* |
| End leg pain | 5 (3–7) | 0.5 (0–3) | < 0.001*** | 5 (2–8) | 2.75 (0–4.5) | 0.008** |
| End back pain | 3 (2–6) | 3 (1–6) | 0.827 | 2.85 (0. 5–7) | 2.25 (0.5–4.5) | 0.739 |
Table 3.
Mean (SD) sum scores of the questionnaires Roland-Morris (possible range 0–24), Oswestry (% of possible score) and self-efficacy scale (% of possible score)
| Before surgery | After surgery | ANOVA | |||
|---|---|---|---|---|---|
| psurgery | pgender | psurgeryXgender | |||
| Roland–Morris | |||||
| Men | 10.8 (5.0) | 6.4 (3.3) | < 0.001*** | < 0.001*** | 0.882 |
| Women | 16.5 (3.3) | 11.7 (6.1) | |||
| Oswestry | |||||
| Men | 36 (15) | 18 (12) | 0.001*** | 0.002 | 0.905 |
| Women | 50 (14) | 31 (22) | |||
| Self-efficacy | |||||
| Men | 56 (16) | 72 (16) | < 0.001*** | 0.005 | 0.595 |
| Women | 44 (16) | 56 (21) | |||
Electromyography
The MF slope
The L5 MF slopes were flatter for the men after surgery (Table 4). For the L1 MF slopes, no differences were found. The female patients had significantly steeper slopes than the healthy women did. One woman with 8-s endurance time had extreme slope values of the L1 and L5 and was therefore excluded from the ANOVA. No technical disturbances were seen in the EMG for this patient.
Table 4.
Comparison between (1) patients before and after surgery (men n = 25, women n = 11) (ANOVA) (2) healthy subjects (men n = 25, women n = 25) from a previous study using the same fatigue-testing procedure [10] versus the patients (t-test), for the MF slope (% s−1), initial (Hz) and end (Hz) and endurance time
| Patients | ANOVA | Healthy subjects | t-test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | psurgery | pgender | psurgeryXgender | ppost-hoc | pbefore-healthy | pafter-healthy | ||
| L1 slope a | |||||||||
| All | −0.11 (0.12) | −0.14 (0.07) | |||||||
| Men | −0.13 (0.06) | −0.13 (0.06) | 0.155 | 0.621 | 0.160 | −0.13 (0.06) | 0.983 | 0.994 | |
| Women | −0.08 (0.22) | −0.16 (0.09) | −0.09 (0.05) | 0.127 | 0.213 | ||||
| L5 slope a | |||||||||
| All | −0.20 (0.13) | −0.18 (0.13) | |||||||
| Men | −0.19 (0.06) | −0.16 (0.08) | 0.630 | 0.370 | 0.027* | 0.045* | −0.17 (0.07) | 0.219 | 0.950 |
| Women | −0.20 (0.15) | −0.23 (0.21) | 0.265 | −0.12 (0.06) | 0.047* | 0.021* | |||
| L1 initial | |||||||||
| All | 58 (7.4) | 54 (7.3) | |||||||
| Men | 60 (7.2) | 56 (7.4) | < 0.001*** | 0.006* | 0.672 | 62 (12) | 0.344 | < 0.001*** | |
| Women | 53 (6.2) | 49 (4.3) | 67 (11) | < 0.001*** | < 0.001*** | ||||
| L1 end | |||||||||
| All | 45 (7.1) | 42 (6.9) | |||||||
| Men | 45 (6.1) | 42 (6.6) | < 0.001*** | 0.952 | 0.271 | 37 (9.0) | < 0.001*** | 0.021* | |
| Women | 46 (9.2) | 40 (7.8) | 45 (9.7) | 0.761 | 0.235 | ||||
| L5 initial | |||||||||
| All | 76 (18) | 66 (13) | |||||||
| Men | 82 (15) | 69 (13) | 0.002* | < 0.001*** | 0.010** | < 0.001*** | 86 (17) | 0.398 | < 0.001*** |
| Women | 60 (12) | 59 (12) | 0.731 | 89 (15) | < 0.001*** | < 0.001*** | |||
| L5 end | |||||||||
| All | 50 (9.5) | 47 (9.5) | |||||||
| Men | 52 (10) | 47 (9.8) | 0.154 | 0.466 | 0.124 | 41 (14) | 0.003** | 0.088 | |
| Women | 47 (6.4) | 47 (9.3) | 52 (13) | 0.235 | 0.277 | ||||
| Endurance | |||||||||
| All | 175 (72) | 187 (86) | |||||||
| Men | 201 (58) | 216 (78) | 0.337 | < 0.001*** | 0.622 | 385 (127) | < 0.001*** | < 0.001*** | |
| Women | 117 (68) | 121 (70) | 380 (144) | < 0.001*** | < 0.001*** | ||||
a For the L1 and L5 slope one more woman was excluded due to too extreme values
Initial and end median frequencies
Comparing the initial MF before and after surgery revealed a significant difference at both L1 and L5 levels (Table 4). End MF differed only at L1 level. There was a significant influence of gender for the initial MF at both L1 and L5 levels with an interaction effect showing that only the men decreased in initial MF at L5 after surgery. However, there was no gender influence on end MF values. Compared to the healthy men, the male patients had significantly higher end MF.
Relative change in MF at Borg ratings 3, 5 and 7
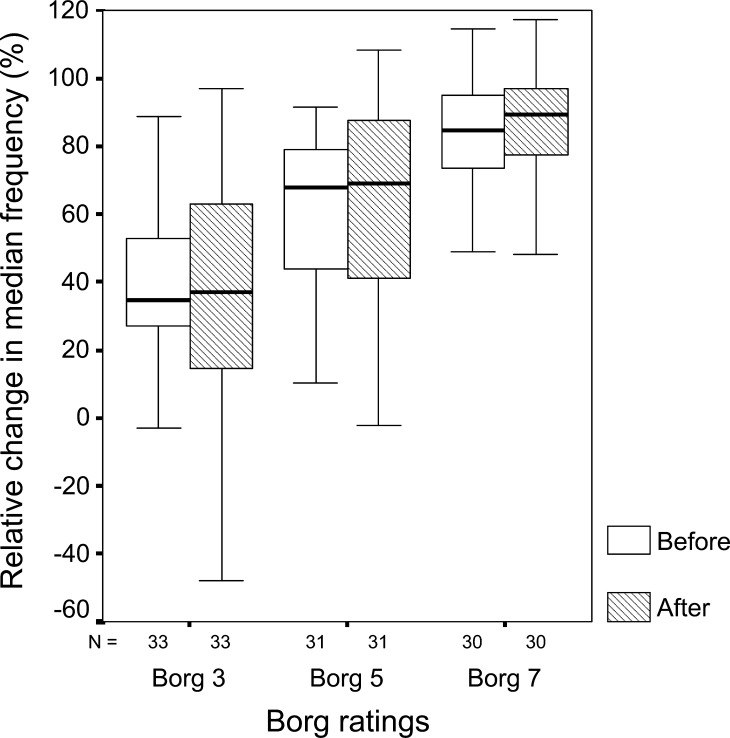
The relative MF at Borg ratings 3, 5 and 7 were 35, 68 and 85% before surgery and 37, 69 and 89% after surgery (Fig. 1).
Fig. 1.
Box-plot of percentage of MF change at Borg ratings of perceived fatigue 3 (moderate), 5 (strong) and 7 (very strong) for patients before and after surgery. N number of subjects in each box. The boxes represent the interquartile range, which contains 50% of the values. The whiskers show the extremes. The lines across the boxes indicate the median
Endurance time
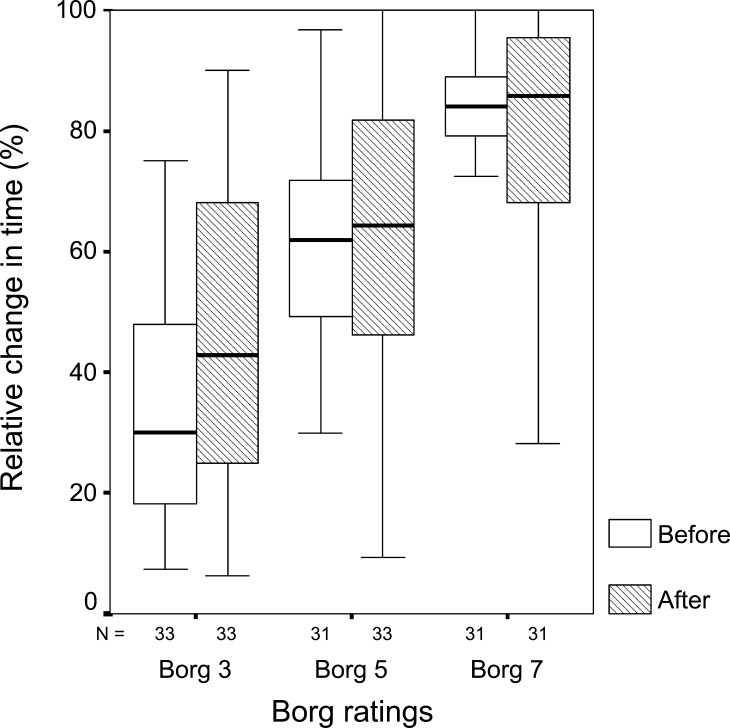
No significant difference was revealed between endurance times before and 4 weeks after surgery. However, there was a difference between men and women, with women’s endurance times being significantly shorter both before and after surgery (Table 4). Before surgery, termination of the test was due to fatigue for 29 patients, back pain or leg pain for nine patients and other reasons for three patients (n = 41). After surgery, termination was due to fatigue for 35 patients, back pain or leg pain for 1 and other reasons for 2 (n = 37). Compared to the healthy subjects, the patients had significantly shorter endurance times (Table 4). The relative mean endurance times at Borg ratings 3, 5 and 7 were 30, 62 and 84% before surgery and 43, 64 and 85% after surgery (Fig. 2).
Fig. 2.
Box-plot of percentage of total time passed at Borg ratings of perceived fatigue 3 (moderate), 5 (strong) and 7 (very strong) for patients before and after surgery. N number of subjects in each box. The boxes represent the interquartile range which contains 50% of the values. The whiskers show the extremes. The lines across the boxes indicate the median
Correlation between endurance time and EMG slope versus Borg ratings of muscle fatigue
For the patients, moderate-to-high correlation was found between Borg scale ratings at 2–3 min and endurance time (Table 5). Correlation coefficients between Borg ratings versus slope and endurance time versus slope were low.
Table 5.
Spearman correlation coefficients for Borg ratings of fatigue at 1, 2 and 3 min versus endurance time, L1 and L5 slope, L1 and L5 decrease for patients before and after surgery
| Borg 1 min | Borg 2 min | Borg 3 min | Endurance | |
|---|---|---|---|---|
| Endurance time | ||||
| Before surgery | −0.36 | −0.62 | −0.61 | |
| After surgery | −0.55 | −0.68 | −0.72 | |
| L1 slope | ||||
| Before surgery | −0.24 | −0.03 | −0.22 | 0.02 |
| After surgery | −0.37 | −0.22 | −0.21 | 0.35 |
| L5 slope | ||||
| Before surgery | −0.28 | −0.17 | 0.03 | 0.41 |
| After surgery | −0.18 | −0.13 | −0.34 | 0.26 |
Correlation coefficients ≥0.50 are in bold. The number of subjects were before surgery 1 min (n = 37), 2 min (n = 32), 3 min (n = 21), after surgery 1 min (n = 36), 2 min (n = 30), 3 min (n = 21)
Correlation of questionnaire scores with endurance time and MF slope
Generally, for all the questionnaires, the correlation between the questionnaire scores with the endurance time and MF slopes at L5 was best for the women after surgery (Table 6). The correlation coefficients were generally better for the L5 slope than the L1 slope. Women with long endurance times scored high on physical activity and self-efficacy and low on the Roland–Morris and Oswestry questionnaires. Women with steep slopes scored high on the Roland–Morris and Oswestry questionnaires. Before surgery, men with long endurance times scored high physical activity and low on the Roland–Morris questionnaire. Men had low correlation between L5 slope and the questionnaire answers.
Table 6.
Spearman correlation coefficients for endurance time and L5 slope versus physical activity, Roland–Morris disability questionnaire, Oswestry disability questionnaire, self-efficacy scale and SF-36 before and 4 weeks after surgery for men (n = 25) and women (endurance time n = 11, L5 slope n = 10)
| Men endurance time |
L5 slope | Women endurance time |
L5 slope | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Physical activity | 0.53 | 0.42 | 0.04 | −0.02 | 0.70 | 0.71 | 0.27 | 0.39 |
| Roland–Morris | −0.54 | −0.36 | −0.38 | −0.03 | −0.60 | −0.91 | −0.02 | −0.77 |
| Oswestry: | −0.34 | −0.38 | 0.16 | 0.09 | −0.58 | −0.88 | −0.27 | −0.53 |
| Self-efficacy scale: | 0.14 | 0.37 | 0.15 | −0.01 | 0.52 | 0.83 | −0.22 | 0.38 |
| SF-36 | ||||||||
| Physical functioning | 0.28 | 0.32 | 0.26 | −0.22 | 0.62 | 0.73 | 0.12 | 0.36 |
| Role-physical | 0.17 | 0.66 | 0.24 | −0.01 | 0.30 | 0.77 | −0.17 | 0.56 |
| Body pain | 0.24 | 0.52 | 0.32 | −0.02 | 0.10 | 0.78 | −0.42 | 0.86 |
| General health | 0.25 | 0.05 | 0.07 | 0.23 | 0.58 | 0.39 | 0.33 | 0.40 |
| Vitality | 0.42 | 0.28 | 0.10 | 0.14 | 0.19 | 0.53 | 0.29 | 0.64 |
| Social functioning | 0.15 | 0.18 | 0.19 | −0.10 | 0.61 | 0.62 | −0.39 | 0.53 |
| Role emotional | 0.28 | 0.35 | −0.33 | −0.22 | 0.09 | 0.59 | −0.08 | 0.47 |
| Mental health | 0.46 | 0.05 | −0.11 | 0.05 | −0.08 | 0.39 | 0.24 | 0.57 |
Correlation coefficients ≥ 0.50 are in bold. Significant correlation was | r| > 0.41 for men and | r| > 0.60 for women
Discussion
The current study showed that male patients with a lumbar disc herniation were less fatigued (flatter L5 slope) in back extensor muscles four weeks after surgery than before. However, muscle fatigue did not improve to such an extent that muscle endurance time and rated fatigue improved. After surgery, the subjects improved significantly on the ratings of leg pain, clinical examination and the questionnaires measuring activity, participation, self-efficacy and health. The endurance time was moderately to highly correlated to physical activity, Roland–Morris, Oswestry and self-efficacy scale. The L5 slope was moderately correlated to the Roland–Morris and the Oswestry for women only.
One limitation of the current study was that the number of subjects was chosen for 80% statistical power for the whole group. Our sample size was large enough to detect differences of more than 0.02% s−1 for the L5 slope, 4 or 8 Hz for initial MF at the L1 and L5 levels, respectively, and above 30 s for the endurance time if such occurred. These values were taken from earlier studies [10, 11]. Men and women were presented separately even though a gender comparison was not the aim of the study because the men were in significantly better physical condition (straight leg-raising test and endurance time) than the women. We did no statistical comparison apart from subjects’ characteristics and the interaction effect in the ANOVA. This was because of the design limitation with too few women, resulting in insufficient statistical power. There were 63% men and 37% women, which resembles the gender distribution in other studies using consecutive selection of patients with lumbar disc herniation [22, 64].
The gender aspect was, however, important for the current study. Back extensor fatigue improved with surgery for the men only. The reason might have been a significantly lower initial MF only seen for men at the L5. The EMG changes found in both men and women could, however, partly be an effect of the surgical approach, including detachment of erector spinae from the spinous processes and laminae. During a lumbar discectomy, there is a risk of muscle injury [22], which might lead to altered muscle function.
The men also improved significantly on more physical examination tests and more questionnaire items than the women. The women had significantly shorter endurance times than the men both before and after surgery. Earlier studies indicate the opposite for healthy, i.e. that healthy men’s endurance times are shorter than, or equal to, healthy women’s [10, 39]. In patients with low back pain, an association between physical activity and back muscle endurance is reported [48]. Due to the disc herniation, both our men and our women decreased their level of physical activity significantly. Prolonged periods of low physical activity are associated with muscle atrophy [26] and an atrophied back muscle is susceptible to fatigue. Muscle fibre composition might be one cause. Of the patients with low back pain, men have a lower I:II fibre-size ratio than women, which is similar to the differences for healthy subjects [40, 60]. But compared to healthy subjects, low back pain patients have a lower proportion of type I fibres accompanied by a higher proportion of type II [43, 41]. The female patients of the current study could be expected to have lower proportion of type II due to gender and type I due to the disc herniation resulting in reduction of both types compared to the men, giving shorter endurance times than for men and may be also their lower initial MF. It is also possible that some changes reached significance in men but not in the women because of the larger numbers of men.
On entry to the study, the female patients had significantly worse physical status, i.e. lower endurance and rated more ability limitation than men. No significant difference was found for pain duration before surgery. This combination of findings has also been found for patients with low back pain without sciatica [44]. The women might have responded to their disc herniation with more activity limitations than the men since reduced physical capacity was most prominent in the women. These gender differences might be explained by the fact that women are at greater risk of pain-related activity limitations than men, have a stronger pain-response through health-related activities [62] and “pain-catastrophize” to a greater extent [56].
The different approaches for the endurance times longer or shorter than 15 s might be of concern, especially for short measures exceeding 15 s. There were six measures under 1 min. Of those, four measures were under 15 s (7, 8, 13 and 15 s, respectively) and two was 25 s. For one 25 s, there would be an error of 8%. We do not think that this would influence the relationship between endurance time and other parameters on a group level.
That endurance time and Borg rating were correlated shows that the endurance time depends on the subject’s perception of fatigue. Endurance time is influenced by psychological factors, which might affect the subject’s motivation [38]. The slope, does not depend on the endurance time and therefore not affected by motivation, which explains its low correlation with Borg ratings for the current patients. In a review, Gandevia [21] suggested a definition of muscle fatigue “any exercise-induced reduction in the ability of a muscle to generate force or power; it has peripheral and central causes”. We therefore believe that a combination of subjective and objective measures is needed to fully assess muscle fatigue.
When the patients rated five on the Borg scale the percentage of total endurance time passed was about 60% and the percentage of MF decreased about 70%. Clinically, patients do not have to perform to their endurance limit but the endurance time to Borg rating five could be used. The patients’ relative endurance time and MF were 10–20% higher than the healthy subjects’ from our former study were [10]. This might be explained by some of the healthy subjects rating ten for several minutes before ending the test. When the patients reached ten, they were probably at their performance limit. Individual coping strategies are important for the development of pain in the neck and back and for the rehabilitation of patients with chronic pain [34, 35]. A person’s reaction to a pain stimulus depends not only on earlier experience of pain but also on physical activity. Analogously, it seems reasonable to assume that subjective ratings depend on earlier experience of physical exertion and of coping with them. Motivation along with tolerance of discomfort is very important [17]. A low tolerance of discomfort may lead to low motivation and therefore a higher fatigue rating on the Borg scale.
The interaction between endurance time with activities and participation was evinced in moderate-to-high correlation coefficients for some questionnaire answers. The L5 slope was moderately correlated to activity limitations only in women after surgery. Earlier studies have shown the relationship between impairments, activity limitations and participation restrictions to be weak for patients with low back pain [16, 24, 44, 46, 47]. Also, the specific impairments fatigue and endurance have low correlation coefficients with the Oswestry questionnaire and the Roland–Morris questionnaire [16, 24, 44]. The moderate-to-high correlation between endurance time and some activity limitations and participation restrictions for the present patients might be because only patients with lumbar disc herniation were investigated. For many patients, the leg pain was more bothersome than the back pain. Clinically, patients with lumbar disc herniation are therefore not comparable with other patients with low back pain. Patients developing chronic low back pain are more affected by psychosocial factors, especially coping strategies than other patients with low back pain [6]. Also, the pathogenesis and treatment could influence their situation. Our patients had a clear diagnosis and were about to receive treatment to “cure” their pain “overnight”. Hence, they might have had a positive attitude, which influenced both their endurance performance and their questionnaire scores.
Conclusions
We conclude the main findings of the current study to be that:
Back muscle fatigue improved with surgery for men with lumbar disc herniation.
Both subjective and objective measures are needed to fully describe the back muscle fatigue.
A moderate-to-high correlation between endurance time and activity limitations exists in patients with lumbar disc herniation.
References
- 1.Adams MA, Mannion AF, Dolan P. Personal risk factors for first-time low back pain. Spine. 1999;24:2497–2505. doi: 10.1097/00007632-199912010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Basmajian JV, De Luca CJ. Muscles alive. Baltimore: Williams& Wilkins; 1985. [Google Scholar]
- 3.Beurskens AJ, Vet HC, Köke AJ, Heijden GJ, Knipschild PG. Measuring the functional status of patients with low back pain. Spine. 1995;20:1017–1028. doi: 10.1097/00007632-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Biedermann HJ, Shanks GL, Forrest WJ, Inglis J. Power spectrum analyses of electromyographic activity. Spine. 1991;16:1179–1184. [PubMed] [Google Scholar]
- 5.Biering-Sørensen F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine. 1984;9:106–119. doi: 10.1097/00007632-198403000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Burton AK, Tillotson KM, Main CJ, Hollis S. Psychosocial predictors of outcome in acute and subchronic low back trouble. Spine. 1995;20:722–728. doi: 10.1097/00007632-199503150-00014. [DOI] [PubMed] [Google Scholar]
- 7.Cooper RG, Stokes MJ, Sweet C, Taylor RJ, Jayson MIV. Increased central drive during fatiguing contractions of the paraspinal muscles in patients with chronic low back pain. Spine. 1993;18:610–616. doi: 10.1097/00007632-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Crombez G, Vlaeyen JWS, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339. doi: 10.1016/S0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 9.Danielsen JM, Johnsen R, Kibsgaard SK, Hellevik E. Early aggressive exercise for postoperative rehabilitation after discectomy. Spine. 2000;25:1015–1020. doi: 10.1097/00007632-200004150-00017. [DOI] [PubMed] [Google Scholar]
- 10.Dedering Å, Németh G, Harms-Ringdahl K. Correlation between electromyographic spectral changes and subjective assessment of lumbar muscle fatigue in subjects without pain from the lower back. Clin Biomech. 1999;14:103–111. doi: 10.1016/S0268-0033(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 11.Dedering Å, Roosaf Hjelmsäter M, Elfving B, Harms-Ringdahl K, Németh G. Between days reliability of subjective and objective assessments of lumbar muscle fatigue. J Electromyogr Kinesiol. 2000;10:151–158. doi: 10.1016/S1050-6411(00)00009-2. [DOI] [PubMed] [Google Scholar]
- 12.Dedering Å, Oddsson LIE, Harms-Ringdahl K, Németh G. Electromyography and ratings of lumbar muscle fatigue using a four-level staircase protocol. Clin Biomech. 2002;17:171–176. doi: 10.1016/S0268-0033(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 13.Dolan P, Greenfield K, Nelson RJ, Nelson IW. Can exercise therapy improve the outcome of microdiscectomy. Spine. 2000;25:1523–1532. doi: 10.1097/00007632-200006150-00011. [DOI] [PubMed] [Google Scholar]
- 14.De Luca CJ. Use of surface EMG signal for performance evaluation of back muscles. Muscle Nerve. 1993;16:210–216. doi: 10.1002/mus.880160216. [DOI] [PubMed] [Google Scholar]
- 15.Elfving B, Németh G, Arvidsson I. EMG spectral parameters and subjective rating of back muscle fatigue in healthy men and women. Scand J Rehabil Med. 2000;32:117–123. doi: 10.1080/003655000750045460. [DOI] [PubMed] [Google Scholar]
- 16.Elfving B, Dedering Å, Németh G. Lumbar muscle fatigue and recovery in patients with long-term low back trouble-electromyography and health related factors. Clin Biomech. 2003;18:619–630. doi: 10.1016/S0268-0033(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 17.Enoka RM. Mechanisms of muscle fatigue: central factors and task dependency. J Electromyogr Kinesiol. 1995;5:141–149. doi: 10.1016/1050-6411(95)00010-W. [DOI] [PubMed] [Google Scholar]
- 18.Estlander A-M, Vanharanta H, Moneta GB, Kaivanto K. Anthropometric variables, self-efficacy beliefs, and pain and disability ratings on the isokinetic performance of low back pain patients. Spine. 1994;19:941–947. doi: 10.1097/00007632-199404150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Fairbank JCT, Davies JB, Couper J, O’brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 20.Frändin K, Grimby G. Assessment of physical activity, fitness and performance in 76-year-olds. Scand J Med Sci Sports. 1994;4:41–46. [Google Scholar]
- 21.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 22.Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023–1028. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- 23.Gibson AJN, Grant IC, Wadell G. The cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine. 1999;24:1820–1832. doi: 10.1097/00007632-199909010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Grönblad M, Hurri HO, Kouri J-P. Relationships between spinal mobility, physical performance tests, pain intensity and disability assessments in chronic low back pain patients. Scand J Rehabil Med. 1997;29:17–24. [PubMed] [Google Scholar]
- 25.Haig AJ, Weismann G, Haugh LD, Pope MH, Grobler LJ. Prospective evidence for change in paraspinal muscle activity after herniated nucleus pulposus. Spine. 1993;18:926–930. doi: 10.1097/00007632-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman RM, Wheeler KJ, Deyo RA. Surgery for herniated lumbar discs: A literature synthesis. J Gen Int Med. 1993;8:487–496. doi: 10.1007/BF02600110. [DOI] [PubMed] [Google Scholar]
- 28.Johansson E, Lindberg P. Subacute and chronic low back pain. Reliability and validity of a Swedish version of the Roland and Morris disability questionnaire. Scand J Rehabil Med. 1998;30:139–143. doi: 10.1080/003655098444066. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen K, Nicolaisen T. Trunk extensor endurance: determination and relation to low-back trouble. Ergonomics. 1987;30:259–267. doi: 10.1080/00140138708969704. [DOI] [PubMed] [Google Scholar]
- 30.Kjellby-Wendt G, Styf JR. Early active training after lumbar discectomy. Spine. 1998;23:2345–2351. doi: 10.1097/00007632-199811010-00019. [DOI] [PubMed] [Google Scholar]
- 31.Kopec JA, Esdaile JM. Functional disability scales for back pain. Spine. 1995;20:1943–1949. doi: 10.1097/00007632-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Koumantakis GA, Arnall F, Cooper RG, Oldham JA. Paraspinal muscle EMG fatigue testing with two methods in healthy volunteers. Reliability in the context of clinical applications. Clin Biomech. 2001;16:263–266. doi: 10.1016/S0268-0033(00)00113-3. [DOI] [PubMed] [Google Scholar]
- 33.Lackner JM, Carosella AM. The relative influence of perceived pain control, anxiety, and functional self-efficacy on spinal funtion among patients with chronic low back pain. Spine. 1999;24:2254–2261. doi: 10.1097/00007632-199911010-00014. [DOI] [PubMed] [Google Scholar]
- 34.Linton SJ, Andersson T. Can chronic disability be prevented? A randomized clinical trial of a cognitive-behavior intervention and two forms of information for patients with spinal pain. Spine. 2000;25:2825–2831. doi: 10.1097/00007632-200011010-00017. [DOI] [PubMed] [Google Scholar]
- 35.Linton SJ, Ryberg M. A cognitive-behavioral group intervention as prevention for persistent neck and back pain in a non-patient population: a randomized controlled trial. Pain. 2001;90:83–90. doi: 10.1016/S0304-3959(00)00390-0. [DOI] [PubMed] [Google Scholar]
- 36.Luoto S, Heliövaara M, Hurri HO, Alaranta HT. Static back endurance and the risk of low-back pain. Clin Biomech. 1995;10:323–324. doi: 10.1016/0268-0033(95)00002-3. [DOI] [PubMed] [Google Scholar]
- 37.Manniche C, Skall HF, Braendholt L, Christensen BH, Christophersen L, Ellegaard B, Heilbuth A, Ingerslev M, Jörgensen OE, Larsen E, Lorentzen L, Nielsen CJ, Nielsen H, Windelin M. Clinical trial of postoperative dynamic back exercises after first lumbar diskectomy. Spine. 1993;18:92–97. doi: 10.1097/00007632-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Mannion AF, Dolan P, Adams MA. Psychological questionnaires: Do abnormal scores precede or follow first-time low back pain. Spine. 1996;21:2603–2611. doi: 10.1097/00007632-199611150-00010. [DOI] [PubMed] [Google Scholar]
- 39.Mannion AF, Connolly B, Wood K, Dolan P. The use of surface EMG power spectral analysis in the evaluation of back muscle function. J Rehabil Res Dev. 1997;34:427–439. [PubMed] [Google Scholar]
- 40.Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat. 1997;190:505–513. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mannion AF, Weber BR, Dvorak J, Grob D, Müntener M. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Othop Res. 1997;15:881–887. doi: 10.1002/jor.1100150614. [DOI] [PubMed] [Google Scholar]
- 42.Mannion AF, Müntener M, Taimela S, Dvorak J. A randomized clinical trial of three active therapies for chronic low back pain. Spine. 1999;24:2435–2448. doi: 10.1097/00007632-199912010-00004. [DOI] [PubMed] [Google Scholar]
- 43.Mannion AF, Käser L, Weber BR, Rhyner A, Dvorak J, Müntener M. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J. 2000;9:273–281. doi: 10.1007/s005860000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannion AF, Junge A, Taimela S, Müntener M, Käser L, Dvorak J. Active therapy for chronic low back pain. Part. 3 Factors influencing self-rated disability and its change following therapy. Spine. 2001;26:920–929. doi: 10.1097/00007632-200104150-00015. [DOI] [PubMed] [Google Scholar]
- 45.Mayer TG, Mooney V, Gatchel R, Barnes D, Terry A, Smith S, Mayer H (1989) Quantifying postoperative deficits of physical funtion following spinal surgery. Clin Orthop 147–157 [PubMed]
- 46.McGregor AH, Doré CJ, McCarthy ID, Hughes SP. Are subjective clinical findings and objective clinical tests related to the motion charcteristics of low back pain subjects. J Sports Phys Ther. 1998;28:370–377. doi: 10.2519/jospt.1998.28.6.370. [DOI] [PubMed] [Google Scholar]
- 47.Michel A, Kohlmann T, Raspe H. The association between clinical findings on physical examination and self-reported severity in back pain. Spine. 1997;22:296–303. doi: 10.1097/00007632-199702010-00013. [DOI] [PubMed] [Google Scholar]
- 48.Moffroid MT, Reid S, Henry SM, Haugh LD, Ricamato A. Some endurance measures in persons with chronic low back pain. J Orthop Sports Phys Ther. 1994;20:81–87. doi: 10.2519/jospt.1994.20.2.81. [DOI] [PubMed] [Google Scholar]
- 49.Munro B. Statistical methods for health care research. Philadelphia: JB Lippincott; 1997. [Google Scholar]
- 50.Noble BJ, Borg GAV, Jacobs I, Ceci R, Kaiser P. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc. 1983;15:523–528. [PubMed] [Google Scholar]
- 51.Olmarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998;78:99–105. doi: 10.1016/S0304-3959(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 52.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899–1909. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Roland M, Morris R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Roy SH, De Luca CJ, Casavant DA. Lumbar muscle fatigue and chronic lower back pain. Spine. 1989;14:992–1001. doi: 10.1097/00007632-198909000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan M, Karlsson J, Ware J. The Swedish SF-36 health survey-I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41:1358. doi: 10.1016/0277-9536(95)00125-Q. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspective on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Svensson E. Construction of a single global scale for multi-item assessments of the same variable. Stat Med. 2001;20:3831–3846. doi: 10.1002/sim.1148. [DOI] [PubMed] [Google Scholar]
- 58.Svensson E. Guidelines to statistical evaluation of data from rating scales and questionnaires. J Rehab Med. 2001;33:47–48. doi: 10.1080/165019701300006542. [DOI] [PubMed] [Google Scholar]
- 59.Taimela S, Kankaanpää M, Airaksinen O. A submaximal back extension endurance test utilising subjective perception of low back fatigue. Scand J Rehabil Med. 1998;30:107–112. doi: 10.1080/003655098444219. [DOI] [PubMed] [Google Scholar]
- 60.Thorstensson A, Carlsson H. Fibre types in human lumbar muscles. Acta Physiol Scand. 1987;131:195–202. doi: 10.1111/j.1748-1716.1987.tb08226.x. [DOI] [PubMed] [Google Scholar]
- 61.Tsuboi T, Satou T, Egawa K, Izumi Y, Miyazaki M. Spectral analysis of electromyogram in lumbar muscles: fatigue induced endurance contraction. Eur J Appl Physiol. 1994;69:361–366. doi: 10.1007/BF00392044. [DOI] [PubMed] [Google Scholar]
- 62.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 63.Vroomen PCAJ, Krom MCTFM, Knottnerus JA. Diagnostic value of history and physical examination in patients suspected of sciatica due to disc herniation: a systematic review. J Neurol. 1999;246:899–906. doi: 10.1007/s004150050480. [DOI] [PubMed] [Google Scholar]
- 64.Woertgen C, Rothoerl RD, Breme K, Altmeppen J, Holzschuh M, Brawanski A. Variability of outcome after lumbar disc surgery. Spine. 1999;24:807–811. doi: 10.1097/00007632-199904150-00013. [DOI] [PubMed] [Google Scholar]