Abstract
Degenerated intervertebral disc has lost its normal architecture, and there are changes both in the nuclear and annular parts of the disc. Changes in cell shape, especially in the annulus fibrosus, have been reported. During degeneration the cells become more rounded, chondrocyte-like, whereas in the normal condition annular cells are more spindle shaped. These chondrocyte-like cells, often forming clusters, affect extracellular matrix turnover. In previous studies transforming growth factor β (TGFβ) −1 and −2, basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) have been highlighted in herniated intervertebral disc tissue. In the present study the same growth factors are analysed immunohistochemically in degenerated intervertebral disc tissue. Disc material was obtained from 16 discs operated for painful degenerative disc disease. Discs were classified according to the Dallas Discogram Description. Different disc regions were analysed in parallel. As normal control disc tissue material from eight organ donors was used. Polyclonal antibodies against different growth factors and TGFβ receptor type II were used, and the immunoreaction was detected by the avidin biotin complex method. All studied degenerated discs showed immunoreactivity for TGFβ receptor type II and bFGF. Fifteen of 16 discs were immunopositive for TGFβ-1 and −2, respectively, and none showed immunoreaction for PDGF. Immunopositivity was located in blood vessels and in disc cells. In the nucleus pulposus the immunoreaction was located almost exclusively in chondrocyte-like disc cells, whereas in the annular region this reaction was either in chondrocyte-like disc cells, often forming clusters, or in fibroblast-like disc cells. Chondrocyte-like disc cells were especially prevalent in the posterior disrupted area. In the anterior area of the annulus fibrosus the distribution was more even between these two cell types. bFGF was expressed in the anterior annulus fibrosus more often in chondrocyte-like disc cells than in fibroblast-like disc cells. Control discs showed cellular immunopositivity for only TGFβ-1 and −2 and TGFβ receptor type II . We suggest that growth factors create a cascade in intervertebral disc tissue, where they act and participate in cellular remodelling from the normal resting stage via disc degeneration to disc herniation.
Keywords: Intervertebral disc, TGF-β, FGF, PDGF
Introduction
Intervertebral disc degeneration is a complex process characterised by biochemical and structural changes in both the nucleus pulposus and annulus fibrosus. The distinction between normal ageing and degeneration is important, but difficult to distinguish either morphologically or biomechanically. Different types of annular defects and tears, rim lesions, concentric tears and radiating clefts, can be observed in conjunction with the disc degeneration process [3].
To image degenerated discs, discography and magnetic resonance imaging (MRI) have been used. In some comparative studies MRI seemed to be more accurate [4, 21]. On the other hand MRI signal intensity depends strongly on the water content of the disc tissue sample [30] and thus the early stages of degeneration which do not affect the water content may not be detected by MRI [30].
Discography offers a sensitive evaluation of disc morphology and provides, compared to MRI, additional information about intradiscal pressure condition and pain provocation [3].
The relationship between intradiscal pressure and the morphological patterns in discography was first observed by Nachemson [11]. During discography typical pain reproduction has been noticed to associate with annular tears extending to the outer annulus [16]. None of the discograms showing normal morphology reproduced the patients’ typical pain [16].
Type-I collagen has been abundantly located as a ring in the outer zone and in the outer lamellas of the inner zone of the annulus fibrosus [22]. Type-II collagen was present in the inner annulus, but not in the outer zone. In degenerated annulus fibrosus collagen biosynthesis has been noticed to be increased, but due to the faster turnover the total content of collagen remained unchanged [8]. The reinsertion of stimulated nucleus pulposus cells in an experimental animal model retarded disc degeneration [15]. It delayed the formation of cell clusters of chondrocyte-like cells, the destruction of disc architecture, and production of type-II collagen. Oegema et al have noted that in degenerated discs fibronectin content was elevated suggesting disc cell response to altered environment [14]. Fibronectin was frequently present as fragments capable of stimulating cells to produce metalloproteases and cytokines [14].
Growth factors are proteins regulating cell growth and the turnover of extracellular matrix components. We have previously demonstrated basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and transforming growth factorβ-1 and −2 and their receptor type II in human herniated intervertebral disc tissue [26–28]. In the present study we have focused on these same growth factors targeting disc degeneration in the absence of herniation.
Materials and methods
We obtained 16 discs from 12 patients operated for painful degenerative disc disease at the Texas Back Institute in Plano, TX, USA. The patients had undergone previous spine operations, mainly posterior fusions. The disc samples were convenience samples and the location of the sample within the disc prior to removal was judged clinically by the operating surgeon. Patients were operated by anterior fusion. Preoperative evaluation of degeneration, pain and possible annular tears were classified by the Dallas Discogram Description [20]. After removal, tissue representing different disc regions was immediately frozen in liquid nitrogen in the operating theatre. Anterior annulus fibrosus, nucleus pulposus and posterior annulus fibrosus were stored at −70°C in a deep-freeze. Eight micrometer-thick cryostat sections were fixed in ice-cold acetone. Eight normal control discs were obtained from five organ donors. Morphologically, all control discs lacked signs of degeneration, such as fissures and annulus fibrosus and nucleus pulposus could clearly be distinguished with well demarcated annular lamellae, corresponding to Thompson grades I and II [24]. The samples were collected 1 h post-mortem in various hospitals in Finland. In all cases the cause of death was unrelated to the spine. The control disc material was treated identically to the surgical samples. From frozen discs representative samples of various disc regions (anterior/posterior annulus fibrosus, nucleus pulposus) were cut with an electric saw. The control disc samples were then stored and processed further as described above.
All immunoreactions were detected using an avidin biotin complex-(ABC-) peroxidase staining method (Vectastain Elite, Vector Laboratories, Burlingame, CA, USA). As chromogen substrate we used AEC (immunoreaction shown in red). Tissue sections were counterstained by haematoxylin.
Antibodies
Polyclonal anti-human TGF-β-1, TGF-β-2 and TGF-β receptor type II antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used at the dilutions 1:200,1:50 and 1:100 respectively. Polyclonal bovine bFGF antibody (R & D Systems Inc., Minneapolis, MN, USA) was used at the dilution 1:500 and polyclonal PDGF antibody (R & D Systems Inc) was used at the dilution 1:100. For all antibodies (TGFβ-1, −2, TGFβ receptor type II, PDGF and bFGF) preincubation with the corresponding antigen (1:10) was done [26–28]. Sections were also stained omitting the primary antibody.
Samples were classified as being immunopositive if more than 20 immunopositive cells were noted. If only a few scattered immunopositive cells were found the total disc sample was classified as being immunonegative, as well as if there was a total lack of immunoreaction. All samples were independently examined blinded to their origin by two observers.
Statistical analysis
Statistical analysis was done using the Sigma Stat Version 1.0 (Jandel Scientific GmbH, Erlerath, Germany) software program. Groups were compared using the Fisher exact test. The level of statistical significance was set at P<0.05.
Results
Clinical data of the patients are described in Table 1. The age of the patients varied from 29 years to 63 years (mean 44.8 years). According to the Dallas Discogram Description the degree of degeneration was 3 in five discs and 2 in seven discs. In one disc the degree of degeneration was 1. Discography data were missing in three discs. The age of the control donors varied from 28 years to 53 years (mean 43 years ; Table 2).
Table 1.
Clinical data of the patients operated for degenerative disc disease
| Patient | Gender | Age | DDD grade | Level | Discography result |
|---|---|---|---|---|---|
| 1. | M | 40 | Deg 2 Leak 4 Pain R |
L4–5 | Posterior rupture |
| 2. | F | 29 | Deg 3 Leak 3 Pain R |
L5-S1 | Both anterior and posterior rupture |
| 3. | M | 63 | - | L2–3 | Data missing |
| 4. | M | 44 | Deg 2 Leak 2 Pain D |
L4–5 | Anterior rupture |
| 5. | F | 31 | Deg 2 Leak 3 Pain R |
L5-S1 | Posterior rupture |
| Deg 2 Leak 3 Pain S |
L4–5 | Posterior rupture | |||
| 6. | M | 60 | Deg 2 Leak 4 Pain S |
L5-S1 | Posterior rupture |
| Deg 2 Leak 3 Pain D |
L4-L5 | Posterior rupture | |||
| 7. | M | 42 | Deg 3 Leak 3 Pain |
L5-S1 | Data missing |
| Deg 2 Leak 3 Pain |
L4-L5 | Posterior rupture | |||
| 8. | F | 47 | Deg 3 Leak 3 Pain |
L5-S1 | Posterior rupture |
| 9. | M | 44 | – | L4-L5 | Posterolateral rupture |
| 10. | F | 64 | – | L3-L4 | Data missing |
| 11. | F | 33 | Deg 1 Leak 4 Pain S |
L4-L5 | Posterior rupture |
| 12 | M | 41 | Deg 3 Leak 3 Pain S |
L3–4 | Posterior rupture |
| Deg 3 Leak 3 Pain S |
L4-L5 | Totally fissured |
Dallas Discogram Description [20] Deg Degree of disc degeneration (scale: 0–3), Leak degree of disc rupture (scale: 0–4), Pain provocation of pain (D dissimilar pain, S similar pain, R pain reproduction)
Table 2.
Clinical data of control disc patients (organ donors)
| Patient | Gender | Age | Disc level |
|---|---|---|---|
| 1. | F | 53 | L3–4 |
| 2. | M | 45 | L1–2 |
| 3. | M | 45 | L3–4 |
| 4. | F | 41 | L3–4 |
| 5. | F | 31 | L2–3 |
Immunopositivity of TGFβ −1 and −2 was detected in 15 of 16 degenerated discs, whereas TGFβ receptor type-II immunopositivity was noted in all degenerated discs. All degenerated discs showed positive immunoreaction for bFGF, but somewhat surprisingly none of the degenerated discs were PDGF immunopositive (Fig. 1a).
Fig. 1.
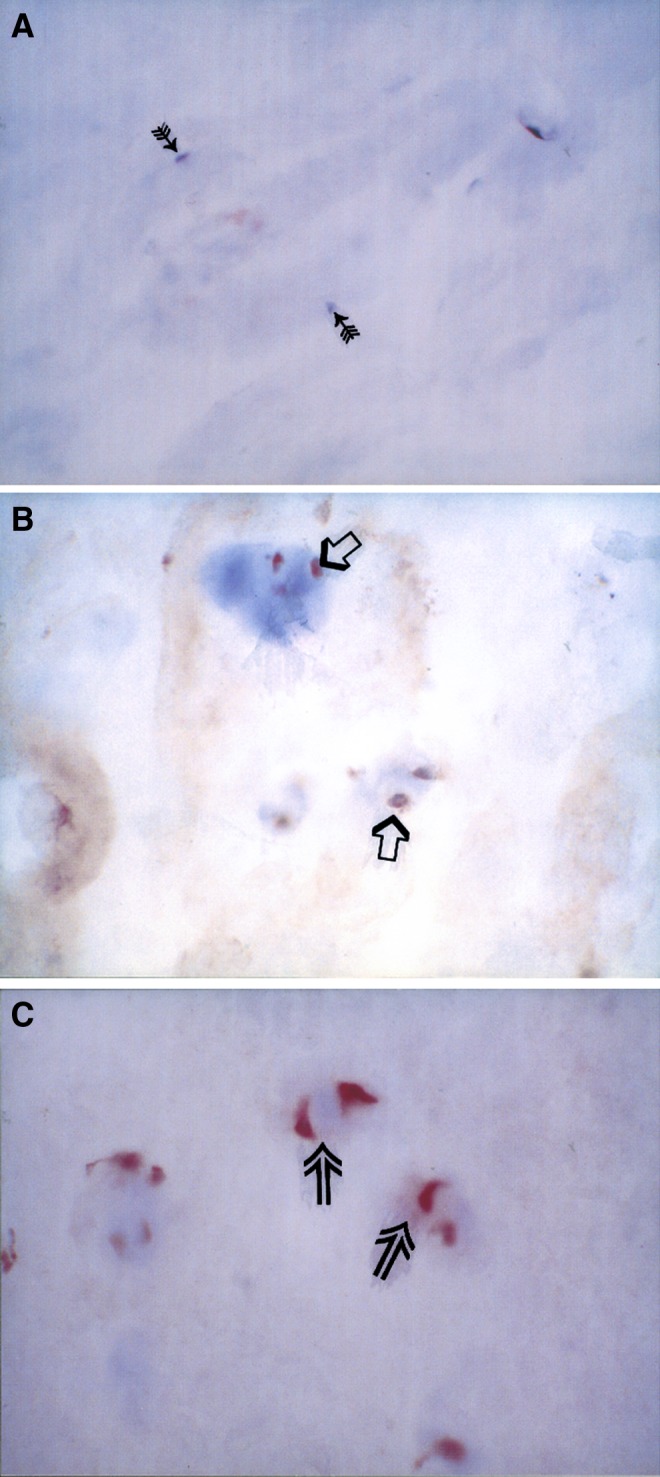
In all figures the ABC-peroxidase immunostaining method was used. The used AEC chromogen shows the specific immunoreaction in red. All the used antibodies were of polyclonal type and as counterstain we used hematoxylin.
a Platelet-derived growth factor (PDGF) immunostaining in posterior annulus fibrosus from a 40 year old male patient. Note the total lack of positive immunoreaction. Arrows mark pale nuclei of disc cells. Original magnification×370. b Transforming growth factor (TGFβ-1) immunopositive chondrocyte-like disc cells (open arrows) in anterior annulus fibrosus. The surgical sample was obtained from a 42-year-old male patient operated for painful degenerative disc disease. The operation level was L5-S1. Original magnification×370. c The TGFβ-receptor type II immunopositivity (open arrows) in cluster of chondrocyte-like posterior annulus fibrosus disc cells from a 40-year-old male patient. The operation level was L4–5. Original magnification×370
Immunoreaction was present either in fibroblast-like disc cells, mainly seen in the annular area of the discs, or in chondrocyte-like disc cells (Fig. 1b, c), scattered more evenly throughout the discs. Chondrocyte-like disc cells were especially present in the nucleus pulposus. Fibroblast-like disc cells were spindle-shaped, often forming lines, whereas chondrocyte-like disc cells were more rounded, often forming cell clusters (Fig. 1c). Immunopositive (TGFβ-1 and −2, and TGFβ receptor type II and bFGF) blood vessels were also detected.
Detailed results of the studied degenerative disc samples are presented in Tables 3 and 4. Control discs appeared normal according to conventional histological staining. Furthermore, control discs did not show any immunoreactivity for PDGF or bFGF. TGFβ-I, -II and TGFβ receptor type II immunoreactivity was, however, noted .
Table 3.
Immunostaining results for transforming growth factor (TGF) β-1 and −2, TGFβ receptor type II, basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) in degenerated intervertebral discs studied
| Patient | Area | TGFβ −1 | TGFβ −2 | TGFβ receptor type II | BFGF | PDGF |
|---|---|---|---|---|---|---|
| 1 | Ant ann | – | – | DC | DC | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | DC | DC | DC | DC | – | |
| 2 | Ant ann | F | DC | DC | .. | .. |
| Nucleus | F,DC | F,DC | F,DC | .. | .. | |
| Post ann | F | F | .. | .. | .. | |
| 3 | Ant ann | F | F | F | – | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | DC | DC | F | – | – | |
| 4 | Ant ann | – | – | – | DC | – |
| Nucleus | – | – | – | DC | – | |
| Post ann | – | – | F | DC | – | |
| 5 | Ant ann | – | DC | – | DC | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | DC | DC | DC | DC | – | |
| Ant ann | – | DC | DC | DC | – | |
| Nucleus | F | DC | DC | DC | – | |
| Post ann | F | DC | DC | DC | – | |
| 6 | Ant ann | F | F | F | F,DC | – |
| Nucleus | F | F | F | F,DC | – | |
| Post ann | – | DC | – | – | – | |
| Ant ann | F | F | F | F,DC | – | |
| Nucleus | DC | DC | DC | DC | – | |
| Patient | Area | TGFβ-I | TGFβ-II | TGFβ receptor type II | BFGF | PDGF |
| 6 | Post ann | DC | F | F | DC | – |
| 7 | Ant ann | – | DC | DC | – | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | DC | DC | DC | .. | – | |
| Ant ann | F | F | F,DC | F,DC | – | |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | F,DC | F,DC | F,DC | DC | – | |
| 8. | Ant ann | F | F | F,DC | F,DC | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | – | DC | DC | – | – | |
| 9 | Ant ann | – | F | DC | DC | – |
| Nucleus | DC | DC | DC | DC | – | |
| 10. | Ant ann | DC | – | F | .. | .. |
| Nucleus | F,DC | – | DC | .. | .. | |
| Post ann | DC | DC | DC | DC | – | |
| 11 | Ant ann | DC | DC | F | DC | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | .. | DC | – | DC | – | |
| 12. | Ant ann | DC | DC | DC | DC | – |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | DC | DC | DC | DC | – | |
| Ant ann | F | F | F | DC | – | |
| Nucleus | DC | DC | DC | DC | – | |
| Post ann | – | – | DC | DC | – |
DC chondrocyte-like disc cell immunopositivity, F fibroblast-like disc cell immunopositivity, – no immunoreaction, .. data not available, Ant ann anterior annulus fibrosus, Post ann posterior annulus fibrosus, Nucleus nucleus pulposus
Table 4.
Summary of immunostaining results for TGF β-1 and −2, TGFβ receptor type II and bFGF in degenerated intervertebral disc tissue samples obtained from various regions of the disc
| Disc area | Growth | Location of immunoreaction | ||
|---|---|---|---|---|
| DC | F | No immunoreactivity | ||
| Anterior annulus | TGFβ-1 | 3/16 | 7/16 | 6/16 |
| Posterior annulus | 8/15 | 3/15 | 4/15 | |
| Nucleus | 13/16 | 4/16 | 1/16 | |
| Anterior annulus | TGFβ-2 | 6/16 | 7/16 | 3/16 |
| Posterior annulus | 11/15 | 2/15 | 2/15 | |
| Nucleus | 13/16 | 2/16 | 2/16 | |
| Anterior annulus | TGFβ receptor type II | 8/16 | 8/16 | 1/16 |
| Posterior annulus | 9/14 | 4/14 | 2/14 | |
| Nucleus | 14/16 | 2/16 | 1/16 | |
| Anterior annulus | bFGF | 12/14 | 4/14 | 2/14 |
| Posterior annulus | 10/13 | 0/13 | 3/13 | |
| Nucleus | 14/14 | 1/14 | 0/14 | |
DC chondrocyte-like disc cell, F fibroblast-like disc cell
Sections that were stained omitting the primary antibody did not show any immunopositivity. Antigen-preabsorbed immunoreactions were also totally negative.
As can be deduced from Table 4, in the anterior annulus the prevalence of bFGF immunopositivity in chondrocyte-like disc cells was particularly high (present in 85,7% of samples). In the posterior annulus fibrosus and nucleus pulposus all growth factors, with the exception of PDGF which was totally absent from all degenerated discs, were highly prevalent in chondrocyte-like disc cells (present in 53.3–100% of samples). The prevalence of growth factor immunopositivity in fibroblast-like disc cells was lower (present in 0–50% of samples), with the highest prevalence in anterior annulus (present in 31.5–50% of samples).
Statistical differences in immunoreactivity with respect to disc region and disc cell type are shown in Table 5. In the nucleus pulposus immunopositivity was almost exclusively located in chondrocyte-like disc cells. In the posterior annulus fibrosus, which was often disrupted, statistically significant immunopositivity in chondrocyte-like disc cells was noted for bFGF, TGFβ −2 and TGFβ receptor type II (P=0.0169, P=0.0025 and P=0.0183 respectively). Furthermore, bFGF and TGFβ-2 immunopositivity was more often located in chondrocyte-like disc cells than in fibroblast-like disc cells (P=0.0001 and P=0.0092 respectively). In the anterior annulus fibrosus only for growth factor bFGF (P=0.0063) immunopositivity in chondrocyte-like disc cells predominated. Furthermore, only in the anterior annulus fibrosus bFGF immunopositivy in chondrocyte-like disc cells was detected more often than such immunoreactivity for TGFβ-1 and −2. In addition, only in the anterior annulus fibrosus TGFβ receptor type II was located both in chondrocyte-like and fibroblast-like disc cells (Table 5).
Table 5.
Statistically significant differences in TGF β-1, −2 and TGF βreceptor type II, and bFGF immunopositivity in different disc regions and disc cell types. (Fisher Exact Test, the level of statistical significance p<0.05).
| Disc region | Statistically significant difference in immunoreactivity | ||
|---|---|---|---|
| Anterior annulus fibrosus | Cell type | ||
| DC | bFGF versus TGFβ −1 | P=0.0007 | |
| DC | bFGF versus TGFβ −2 | P=0.0106 | |
| DC | bFGF versus no immunoreaction | P=0.0004 | |
| DC | TGFβrec versus no immunoreaction | P=0.0155 | |
| F | TGFβrec versus no immunoreaction | P=0.0155 | |
| Antibody | Localization of immunoreaction | ||
| bFGF | DC versus F | P=0.0063 | |
| Posterior annulus fibrosus | Cell type | ||
| DC | bFGF versus no immunoreaction | P=0.0169 | |
| DC | TGFβ-2 versus no immunoreaction | P=0.0025 | |
| DC | TGFβrec versus no immunoreaction | P=0.0183 | |
| Antibody | Localization of immunoreaction | ||
| bFGF | DC versus F | P=0.0001 | |
| TGFβ-2 | DC versus F | P=0.0092 | |
| Nucleus pulposus | Cell type | ||
| DC | bFGF versus no immunoreaction | P<0.0001 | |
| DC | TGFβ-1 versus no immunoreaction | P<0.0001 | |
| DC | TGFβ-2 versus no immunoreaction | P=0.0002 | |
| DC | TGFβrec versus no immunoreaction | P<0.0001 | |
| Antibody | Localization of immunoreaction | ||
| bFGF | DC versus F | P<0.0001 | |
| TGFβ-1 | DC versus F | P=0.0038 | |
| TGFβ-2 | DC versus F | P<0.0001 | |
| TGFβrec | DC versus F | P<0.0001 | |
DC chondrocyte-like disc cell, F fibroblast-like disc cell, bFGF basic fibroblast growth factor, TGFβ-1 transforming growth factor β-1, TGFβ-2 transforming growth factor β-2, TGFβrec transforming growth factor β receptor type II
Discussion
Degenerated disc has lost its normal architecture. The shape of the annulus cells changes markedly with degeneration: a healthy disc contains spindle-shaped cells, whereas in degenerated discs cells are more rounded and are surrounded by unusual accumulations of extracellular matrix components [6, 18]. In the present study growth factor immunopositivity was noted in spindle shaped (fibroblast-like) as well as rounded (chondrocyte-like) disc cells. With respect to different areas of the disc, immunopositivity in chondrocyte-like disc cells was highly prevalent for most growth factors in the posterior annulus, and particularly in the nucleus pulposus. Compared with the other growth factors, bFGF immunopositivity was highly prevalent in chondrocyte-like disc cells particularly in the anterior annulus. Interestingly, PDGF immunopositivity was, however, absent from all disc regions. Thus in degenerated discs, there may be some regional differences with respect to the expression of growth factors. Growth factor expression is particularly prevalent in the rounded chondrocyte-like disc cells. In the anterior annulus the prevalence of growth factor immunopositivity is somewhat more even between chondrocyte-like and fibrocyte-like disc cells. But also in this region immunopositivity for bFGF was more prevalent in the chondrocyte-like disc cells. The observed growth factor immunoreactivity in disc cells suggests that these cells may be actively regulating extracellular matrix component turnover.
Discography showed disruption of the intervertebral discs, especially in the posterior region. The immunoreactivity for growth factors was detected throughout the discs, not only in the disrupted areas. Furthermore, the fact that immunopositivity was observed more often in chondrocyte-like, than fibroblast-like, disc cells may suggest that the former disc cell type is more metabolically active participating in the cellular remodelling of disc degeneration. We have in previous studies located these same growth factors in herniated intervertebral disc tissue [26–28]. In herniated disc tissue the predominant immunopositive disc cell type was the chondrocyte-like disc cell [26–28]. Fibroblast-like disc cell immunopositivity was rare in herniations. These findings may suggest a step-by-step change in cell type from normal disc tissue to pathological processes; i.e. disc degeneration and disc herniation.
In this study and in our earlier studies on herniated disc tissue [26–28] TGFβ-1, −2 and TGFβ receptor type II were the only growth factors observed in control discs. Some studies have suggested that this growth factor is absent in control disc tissue [7, 23]. However, in a recent biochemical tissue culture study on growth factors and inflammatory mediators in patients undergoing surgery for scoliosis, lumbar radiculopathy and discogenic pain, production of TGFβ-1 was demonstrated both in control (scoliosis) and degenerate human disc tissues [2]. In the same study Burke et al. [2] demonstrated the production of bFGF in control (scoliotic) as well as degenerated human nucleus pulposus in vitro. However, in the present immunohistochemical study and in earlier study by us on herniated disc tissue [26] bFGF could not be demonstrated in control discs. In a rat animal model on disc degeneration Nagano et al. [12] could not observe bFGF in control discs. When comparing the injured annulus fibrosus of merinos to intact ones, bFGF, TGFβ and osteonectin were strongly localized in blood vessels and cells in the vicinity of annular lesion [9] The immunohistochemical expression was maximal 12 month after the operation, and diminished by 26 months after the operation. In control discs, the expression of bFGF and TGFβ was localized to sparsely distributed cells in the annulus fibrosus
In an experimental animal model the degeneration process was delayed with the reinsertion of autogenous activated nucleus pulposus [15]. This activation was produced by coculturing nucleus pulposus cells with annulus fibrosus cells. During the coculture both cell types were proliferating. Furthermore, reinsertion of the nucleus pulposus delayed especially the formation of clusters of chondrocyte-like disc cells [15]. Such clusters were noted in the present study both in the nucleus pulposus and in annular areas of the degenerated discs. Furthermore, injection of intact nucleus pulposus has been demonstrated to be far more effective in delaying degeneration rather than injection of only nucleus pulposus cells [13], thus highlighting the importance of the extracellular matrix. In chondrocyte-like disc cell clusters marked matrix metalloproteinase activity has been demonstrated [19]. Interestingly, such enzyme activity was particularly intense in herniated discs [19]. The presence of growth factors, matrix metalloproteinases and oncoproteins [29] suggests that these particular disc cells have been activated in pathological conditions and regulate the turnover of extracellular matrix components. In rat degenerated intervertebral discs bFGF and its receptor were localized in chondrocyte-like rounded cells, and the proliferation capacity of these cells exceeded that of normal annular spindle-shaped cells [12].
Experimental animal models suggest important functions for the above growth factors in intervertebral disc physiology and pathophysiology. Minamide et al. [10] have shown that epidural application of bFGF in the rabbit facilitates the resorption of the sequestrated intervertebral disc fragment, and stimulates neovascularization and proliferation of inflammatory cells. This effect was dose-dependent. In cell cultures, TGFβ and FGF have been demonstrated to be potent cellular proliferation stimulators [25]. Cells from the nucleus pulposus and the transition zone reacted more than annulus fibrosus cells. Furthermore, TGFβ was far more potent than FGF as a stimulator of cellular proliferation. In addition, TGFβ-1 decreased the level of active matrix metalloproteinase-2 (MMP-2) in nucleus pulposus cells [17]. Furthermore, the cell surface levels of metalloproteinase inhibitors also decreased. In another cell culture study, the presence of TGFβ first enhanced cellular proliferation, later on the mitogenic response decreased [5]. In addition, TGFβ-1 is a potent stimulator for proteoglycan production by disc cells [1].
Since there was a time gap between discography and the operation, possible local irritation by the discography procedure was not present at the time of operation when the tissue samples were taken for analysis. Of note, the growth factor immunoreactivity was detected throughout the discs, not only in the disrupted posterior areas.
Earlier we noted marked PDGF immunoreactivity in herniated disc tissue [27]. This immunoreactivity was located in blood vessels and cells, both chondrocyte-like disc cells and fibroblast-like disc cells. In the present study such disc cell-associated immunoreactivity was not observed. Furthermore, normal control discs were totally PDGF immunonegative. This may suggest that PDGF is latent until the disc becomes herniated. Characterising the nature of the PDGF activator, whether another growth factor or some other substance in the nerve root area, will require further research. We expect that more information on disc cell remodelling, production of different proteins that affect the extracellular matrix and cell proliferation, may be obtained by focusing future research on signal transfer mechanisms in disc cells and the gene activation process in these cells.
Conclusions
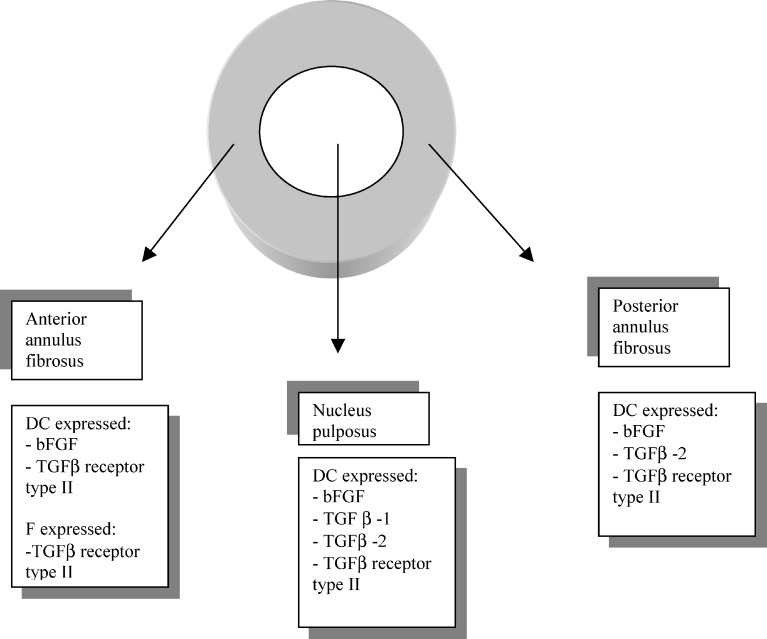
Our results show that growth factors are expressed in degenerated discs, in a different pattern than in control discs. Different types of expression were observed in the various disc areas (Fig. 2). In degenerated intervertebral disc tissue chondrocyte-like disc cells in the nucleus pulposus were immunopositive to all other growth factors except PDGF. In the anterior annulus fibrosus the most prevalent growth factor present in chondrocyte-like disc cells was bFGF. TGFβ receptor type II was expressed in both chondrocyte-like and fibroblast-like disc cells, whereas in the posterior annulus fibrosus the most prevalent growth factors expressed in chondrocyte-like disc cells were bFGF and TGFβ-2.
Fig. 2.
Expression of growth factors (bFGF, TGFβ-1, −2 and TGFβ receptor type II) in different disc regions. (DC chondrocyte-like disc cell, F fibroblast-like disc cell, bFGF basic fibroblast growth factor, TGFβ transforming growth factor β)
Acknowledgements
Financial support from the Paulo Foundation, the Yrjö Jahnsson Foundation and research funding from Helsinki University Central Hospital is gratefully acknowledged.
References
- 1.Alini M, Li W, Markowic P, et al. The potential and the limitations of a cell-seeded collagen/hyaluronan scaffold to engineer in intervertebral disc like matrix. Spine. 2003;28:446–454. doi: 10.1097/01.BRS.0000048672.34459.31. [DOI] [PubMed] [Google Scholar]
- 2.Burke JG, Watson RWG, Conhyea D, et al. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine. 2003;28:2685–2693. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 3.Fraser RD, Osti OL, Vernon-Roberts B. Intervertebral disc degeneration. Eur Spine J. 1993;1:205–213. doi: 10.1007/BF00298361. [DOI] [PubMed] [Google Scholar]
- 4.Gibson MJ, Buckley J, Mawhinney R, Mulholland RC, Worthington BS. Magnetic resonance imaging and discography in the diagnosis of disc degeneration. J Bone Joint Surg [Br] 1986;68:369. doi: 10.1302/0301-620X.68B3.3733797. [DOI] [PubMed] [Google Scholar]
- 5.Gruber HE, Fisher EC, Jr, Desai B, et al. Human intervertebral disc cells from the annulus: three-dimensional culture in agrose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 6.Gruber HE, Hanley EN. Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation. BMC Musculoskelet Disord. 2000;1:1–9. doi: 10.1186/1471-2474-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konttinen YT, Kemppinen P, Li P, et al. Transforming and epidermal growth factors in degenerated intervertebral discs. J Bone Joint Surg [Br] 1999;81:1058–1063. doi: 10.1302/0301-620X.81B6.9321. [DOI] [PubMed] [Google Scholar]
- 8.Kääpä E, Wang W, Takala TE, et al. Elevated protein content and prolyl 4-hydroxylase activity in severely degenerated human annulus fibrosus. Connect Tissue Res. 2000;2:93–99. doi: 10.3109/03008200009067661. [DOI] [PubMed] [Google Scholar]
- 9.Melrose J, Smith S, Little CB, et al. Spatial and temporal localization of transforming growth factor beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in injured anulus fibrosus: implication for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- 10.Minamide A, Hashizume H, Yoshida M, et al. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine. 1999;24:940–945. doi: 10.1097/00007632-199905150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nachemson A. In vivo discometry in lumbar discs with irregular nucleograms. Acta Orthop Scand. 1965;36:418–434. doi: 10.3109/17453676508988651. [DOI] [PubMed] [Google Scholar]
- 12.Nagano T, Yonenobu K, Miyamoto S, et al. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop. 2001;389:94–101. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Oegema TR, Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine. 2000;21:2742–2747. doi: 10.1097/00007632-200011010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988–997. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 16.Osti OL, Fraser RD. MRI and discography of annular tears and intervertebral disc degeneration. A prospective clinical comparison. J Bone Joint Surg [Br] 1992;74:431–435. doi: 10.1302/0301-620X.74B3.1587896. [DOI] [PubMed] [Google Scholar]
- 17.Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production of ovine intervertebral disc nucleus pulposus cells grown in alginate culture by transforming growth factor-β(1) and insulin growth factor-I. Cell Biol Int. 2001;25:679–689. doi: 10.1006/cbir.2000.0718. [DOI] [PubMed] [Google Scholar]
- 18.Pritzher KPH. Aging and degeneration in the lumbar intervertebral disc. Orthop Clin N Am. 1977;8:65–77. [PubMed] [Google Scholar]
- 19.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;23:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sachs BL, Vanharanta H, Spivey MA, et al. Dallas Discogram Description. A new classification of CT/discography in low-back disorders. Spine. 1987;12:287–294. doi: 10.1097/00007632-198704000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Schneiderman G, Flannigan B, Kingston S, Thomas J, Dillin WH, Watkins RG. Magnetic resonance imaging in the diagnosis of disc degeneration: correlation with discography. Spine. 1987;12:276–281. doi: 10.1097/00007632-198704000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Schollmeier G, Lahr-Eigen R, Lewandrowski KU. Observations on the fiber-forming collagens in the annulus fibrosus. Spine. 2000;21:2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Specchia N, Pagnotta A, Toesca A, et al. Cytokines and growth factor in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11:145–151. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IKY, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1117/12.45701. [DOI] [PubMed] [Google Scholar]
- 26.Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju E. Basic fibroblast growth factor immunoreactivity in blood vessels and cells of disc herniation. Spine. 1995;20:271–276. doi: 10.1097/00007632-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju EO. Platelet-derived growth factor and vascular endothelial growth factor expression in disc herniation tissue: an immunohistochemical study. Eur Spine J. 1997;6:63–69. doi: 10.1007/BF01676576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju E. Transforming growth factor beta receptor induction in herniated intervertebral disc tissue: an immunohistochemical study. Eur Spine J. 2001;10:172–176. doi: 10.1007/s005860000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju E. Oncoprotein c-Fos and c-Jun immunopositive cells and cell clusters in herniated intervertebral disc tissue. Eur Spine J. 2002;11:452–458. doi: 10.1007/s00586-001-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidenbaum M, Foster RJ, Best BA, et al. Correlation of magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res. 1990;10:552–561. doi: 10.1002/jor.1100100410. [DOI] [PubMed] [Google Scholar]