Abstract
Objectives. We evaluated the relationship between secondhand tobacco smoke (SHS) exposure and blood lead levels in US children and adolescents.
Methods. We analyzed data from 6830 participants aged 3–19 years in the National Health and Nutrition Examination Survey (1999–2004) who were not active smokers and for whom SHS exposure information and blood lead measurements were available.
Results. After multivariable adjustment, participants in the highest quartile of serum cotinine (≥ 0.44 μg/L) had 28% (95% confidence interval = 21%, 36%) higher blood lead levels than had those in the lowest quartile (< 0.03 μg/L). Similarly, blood lead levels were 14% and 24% higher in children who lived with 1 or with 2 or more smokers, respectively, than they were in children living with no smokers. Among participants for whom lead dust information was available, the associations between SHS and blood lead levels were similar before and after adjustment for lead dust concentrations.
Conclusions. SHS may contribute to increased blood lead levels in US children. Lead dust does not appear to mediate this association, suggesting inhalation as a major pathway of exposure. Eliminating SHS exposure could reduce lead exposure in children.
Secondhand tobacco smoke (SHS) remains a major source of indoor air pollution worldwide,1–3 causing major health effects in children, including sudden infant death syndrome, lower respiratory tract infections, reduced lung growth,1 and behavioral problems.4–6 In the United States, around 1 in 5 children aged 3 to 11 years live with at least 1 individual who smokes.1,7 Globally, the burden of SHS exposure during childhood is even higher.3,8 Lead, a major neurocognitive and kidney toxicant for children at relatively low levels,9 is a tobacco constituent that is measured in mainstream smoke (exhaled by the smoker) and sidestream smoke (from the burning cigarette), including the gas phase.10–13 During the period 1988 to 1994, US children exposed to SHS showed increased blood lead levels.14
National and local childhood lead poisoning prevention programs identify and follow children at risk for elevated blood lead levels (≥ 10 μg/dL) by collecting data on age of housing, occupancy status (rental or owner occupied), dwelling type, lead paint hazards (including lead in paint, dust, and soil), drinking water source, and industrial point sources near the home.15–17 Information on SHS, a potentially preventable source of lead exposure, is generally not considered.
Our goal was to evaluate the relationship between SHS exposure (identified by the number of smokers at home and by levels of serum cotinine, a biomarker of recent tobacco smoke exposure that integrates active and passive exposure, including exposure in the home, vehicles, and public places) and blood lead levels in US children aged 3 to 19 years who participated in the National Health and Nutrition Examination Survey (NHANES; 1999–2004). NHANES measured lead dust concentrations in the windows and floors of the homes of children aged 3 to 5 years. Because SHS may increase levels of home lead dust,18 we evaluated the potential mediation of lead dust concentrations in the association between SHS and blood lead levels in children aged 3 to 5.
METHODS
NHANES (1999–2004) conducted health and nutrition measurements in nationally representative samples of about 5000 people in each year of the survey.19 The participation rate for children aged 1 to 19 years completing the questionnaire and physical and laboratory examinations was 85.3%.
We restricted the sample to 10 553 children and adolescents aged 3 to 19 years for whom blood lead determinations were available. Children younger than 3 years were excluded because they were not eligible for serum cotinine testing. To ensure that the sample contained nonsmokers only, we excluded 389 participants with missing serum cotinine data, 873 participants reporting to be nonsmokers but with serum cotinine levels of 10 micrograms per liter or higher (indicative of active smoking),20 346 participants who reported smoking at least once per day in the last month, and 1295 participants who reported having ever smoked but with missing information on active smoking for the last month. We also excluded 820 participants missing other variables of interest, leaving 6830 participants for our study. Study participants were similar to overall NHANES (1999–2004) participants aged 3 to 19 years in terms of sociodemographic characteristics (age, gender, race/ethnicity, education of reference person in household; data not shown).
For the analysis based on lead dust samples, we further restricted the sample to 791 children aged 3 to 5 years because this is the only NHANES population for whom concentrations of window and floor lead dust are available.
Secondhand Smoke Exposure
We assessed SHS exposure using self-reported data from the home questionnaire (number of smokers at home) and serum cotinine levels. The National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) measured serum cotinine by means of an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry.21,22 The limit of detection for serum cotinine was 0.05 micrograms per liter for NHANES (1999–2000) and the first phase of NHANES (2001–2002), and 0.015 micrograms per liter for the second phase of NHANES (2001–2002) and for NHANES (2003–2004).21,22 In our study sample, serum cotinine levels were below the limit of detection for 40.1% of participants in NHANES (1999–2000), 23.4% of participants in NHANES (2001–2002), and 16.4% of participants in NHANES (2003–2004). Serum cotinine levels below the limit of detection were replaced by limit of detection divided by the square root of 2. The interassay coefficients of variation ranged from 3.3% to 9.0%.21,22
Blood Lead Measures
The National Center for Environmental Health measured blood lead levels following standardized protocols, including confirmation that collection and storage materials were not contaminated with lead. Lead in whole blood was measured by means of graphite furnace atomic absorption spectrometry in NHANES (1999–2002) and inductively coupled plasma mass spectrometry in NHANES (2003–2004).23–25 The limit of detection for both methods was 0.3 microgram per deciliter. For those participants (< 1%) whose blood lead levels were below the limit of detection, blood lead levels were replaced by limit of detection divided by the square root of 2.26 The interassay coefficients of variation ranged from 1.3% to 7.0%.23–25
Lead Dust Measures
NHANES (1999–2004) collected wipe samples from the floor and sill area of a window located in the room where the children spent the most time. After transfer of the sample into a beaker for partial digestion, NHANES determined the lead content of the digestate with a flame-atomic absorption spectrometer (Perkin-Elmer, Waltham, MA) for window samples and a graphite furnace atomic absorption spectrometer (Perkin-Elmer, Waltham, MA) for floor samples.27 NHANES reanalyzed floor samples with lead levels 5 micrograms per square foot or greater by flame-atomic absorption spectrometer.
Other Variables
Questionnaire information included age, gender, race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other), country of birth (United States, Mexico, other), education of the household reference person (< high school, high school or equivalent, > high school), family socioeconomic status, and the year the family house was built (by self-report). NHANES (1999–2004) established family socioeconomic status by categorizing the poverty-to-income ratio (PIR; the ratio of the family's income to its appropriate poverty threshold as defined by the US Census Bureau) as low (≤ 1.30), medium (1.31–3.50), or high (> 3.50) according to government food assistance programs.28 We categorized the year in which the family house was built by risk of lead paint as follows: before 1950 (highest risk), 1950 to 1978, after 1978 (year in which lead paint was banned in the United States), and unknown.
NHANES (1999–2004) measured height and weight during the physical examinations. We calculated percentiles of body mass index (BMI; defined as weight in kilograms divided by height in meters squared) on the basis of the CDC's BMI-for-age sex-specific growth charts.29 We categorized BMI percentiles of 85 to 94 and of 95 or higher as overweight and obese, respectively.
Statistical Analysis
We performed statistical analyses with Stata version 11.0 (StataCorp LP, College Station, TX) using the survey command to account for the complex sampling design and weights in NHANES. Serum cotinine and blood lead levels were right skewed and log transformed to achieve normality in the statistical analyses. To evaluate the potential contribution of SHS exposure to increased blood lead levels, we estimated the ratio (with 95% confidence intervals [CI]) of the geometric mean of blood lead levels (dependent variable) by SHS exposure (serum cotinine quartiles or number of smokers at home as separate independent variables) using linear regression models on log-transformed blood lead levels. We obtained the ratios of the geometric means (with 95% CIs) by exponentiating the coefficients and standard errors from the linear regression models on log-transformed blood lead levels.
We performed the models with progressive levels of adjustment for relevant determinants of blood lead. First, we adjusted for gender, age, race/ethnicity, country of birth, BMI percentile, and survey year. Second, we further adjusted for household education and PIR. Third, we further adjusted for housing age (according to the year the family house was built). We conducted plots of model residuals to confirm the adequacy of the models. We conducted a test for trend in the association between increasing cotinine and blood lead concentrations by including cotinine medians corresponding to each quartile as continuous variables. We conducted a test for trend in the association between increasing number of smokers at home by including 0, 1, and 2 as continuous variables in the regression model. We used stratified analysis by participant characteristics (gender, age, race/ethnicity, BMI, household education, household PIR, and age of housing) to evaluate the consistency of the findings across categories.
For children aged 3 to 5 years for whom house dust samples were available (n = 791), we evaluated the multivariable adjusted ratio of the geometric mean (with 95% CI) of blood lead levels by measures of SHS exposure before and after adjustment for window and floor lead dust concentrations in the participant's home.
RESULTS
Median blood lead levels were 1.1 micrograms per deciliter (interquartile range [IQR] = 0.8–1.7 μg/dL; Table 1). In univariate and multivariate analyses, lead levels decreased with increasing age, education, and PIR level and were higher in boys, in Black and Mexican American children, in children born outside of the United States, in children living in houses built before 1950 or with year unknown, and in homes with a higher number of smokers (Table 1; Appendix 1, available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 1.
Blood Lead and Serum Cotinine Concentrations in Children and Adolescents: National Health and Nutrition Examination Survey, United States, 1999–2004
| No. (Weighted %) | Blood Lead, μg/dL, Median (IQR) | Serum Cotinine, μg/L, Median (IQR) | |
| Total sample | 6830 (100) | 1.1 (0.8–1.7) | 0.07 (0.03–0.44) |
| Age, y | |||
| 3–5 | 1039 (16) | 1.6 (1.1–2.5) | 0.12 (0.04–0.63) |
| 6–11 | 2407 (45) | 1.3 (0.9–1.9) | 0.08 (0.04–0.53) |
| 12–14 | 1704 (20) | 1.0 (0.7–1.4) | 0.06 (0.03–0.32) |
| 15–19 | 1680 (19) | 0.9 (0.6–1.2) | 0.05 (0.02–0.29) |
| Gender | |||
| Male | 3353 (51) | 1.3 (0.9–1.9) | 0.08 (0.03–0.43) |
| Female | 3477 (49) | 1.0 (0.7–1.6) | 0.07 (0.03–0.47) |
| Race/ethnicity | |||
| White | 1795 (60) | 1.0 (0.7–1.5) | 0.07 (0.03–0.50) |
| Black | 2151 (15) | 1.6 (1.1–2.6) | 0.27 (0.07–0.84) |
| Mexican American | 2333 (13) | 1.3 (0.9–2.0) | 0.03 (0.02–0.11) |
| Other | 551 (12) | 1.2 (0.8–1.6) | 0.05 (0.02–0.25) |
| Birth country | |||
| United States | 6099 (94) | 1.1 (0.8–1.7) | 0.08 (0.03–0.47) |
| Mexico | 496 (2) | 1.8 (1.2–2.6) | 0.03 (0.02–0.10) |
| Other | 235 (4) | 1.3 (0.9–2.2) | 0.06 (0.03–0.21) |
| BMI percentile | |||
| < 85 | 4440 (68) | 1.2 (0.8–1.8) | 0.06 (0.03–0.39) |
| 85–94 | 1103 (16) | 1.1 (0.8–1.6) | 0.09 (0.03–0.51) |
| ≥ 95 | 1287 (16) | 1.1 (0.8–1.6) | 0.10 (0.03–0.71) |
| Educationa | |||
| < high school | 2532 (22) | 1.5 (1.0–2.3) | 0.17 (0.04–1.00) |
| High school or equivalent | 1672 (27) | 1.2 (0.8–1.8) | 0.15 (0.04–0.75) |
| > high school | 2626 (51) | 1.0 (0.7–1.5) | 0.04 (0.02–0.18) |
| PIR | |||
| Low (≤ 1.30) | 3063 (34) | 1.6 (1.0–2.4) | 0.25 (0.05–1.08) |
| Medium (1.31–3.50) | 2428 (37) | 1.1 (0.8–1.6) | 0.07 (0.03–0.40) |
| High (3.51–5.00) | 1339 (29) | 0.9 (0.7–1.3) | 0.04 (0.01–0.10) |
| Year house built | |||
| Before 1950 | 945 (15) | 1.3 (0.9–2.0) | 0.13 (0.04–0.63) |
| 1950–1978 | 1749 (26) | 1.1 (0.8–1.5) | 0.07 (0.03–0.40) |
| After 1978 | 2054 (40) | 1.0 (0.7–1.4) | 0.05 (0.02–0.27) |
| Unknown | 2082 (19) | 1.6 (1.0–2.6) | 0.17 (0.04–0.78) |
| Blood lead level, μg/dL | |||
| ≤ 0.8 | 1780 (30) | 0.7 (0.5–0.8) | 0.04 (0.02–0.19) |
| 0.9–1.1 | 1293 (21) | 1.0 (0.9–1.1) | 0.06 (0.02–0.33) |
| 1.2–1.7 | 1691 (25) | 1.4 (1.3–1.6) | 0.11 (0.04–0.53) |
| ≥ 1.8 | 2066 (24) | 2.4 (2.0–3.3) | 0.25 (0.05–1.05) |
| Serum cotinine level, μg/L | |||
| ≤ 0.03 | 1538 (25) | 0.9 (0.7–1.2) | 0.01 (0.01–0.02) |
| 0.031–0.074 | 1876 (25) | 1.1 (0.8–1.7) | 0.04 (0.04–0.05) |
| 0.075–0.44 | 1804 (25) | 1.3 (0.8–1.9) | 0.17 (0.11–0.27) |
| ≥ 0.441 | 1612 (25) | 1.4 (1.0–2.3) | 1.36 (0.77–2.37) |
| Smokers at home, no. | |||
| 0 | 5484 (78) | 1.1 (0.8–1.6) | 0.04 (0.02–0.14) |
| 1 | 929 (14) | 1.4 (1.0–2.2) | 0.34 (0.83–1.68) |
| ≥ 2 | 417 (8) | 1.5 (1.0–2.3) | 0.96 (1.79–3.23) |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); IQR = interquartile range; PIR = poverty-to-income ratio (the ratio of the family's income to its appropriate poverty threshold as defined by the US Census Bureau).
Education of reference person in household.
Median serum cotinine levels were 0.07 micrograms per deciliter (IQR = 0.03–0.44 μg/L; Table 1). Serum cotinine levels decreased with increasing age, education, and PIR level, and were higher in Black children, in children born in the United States, in children who were overweight or obese, and in children living in houses built before 1950 or with year unknown. Serum cotinine levels markedly increased with the number of smokers at home.
Secondhand Smoke and Blood Lead Levels
Blood lead levels increased with increasing serum cotinine levels and with an increasing number of smokers at home (Tables 1 and 2). After adjustment for personal and household characteristics, participants in the highest serum cotinine quartile had 28% higher blood lead levels than did participants in the lowest quartile (Table 2). After similar adjustment, blood lead levels were 14% and 24% higher in children who lived with 1 and with 2 or more smokers, respectively, than they were in children living with no smokers.
TABLE 2.
Blood Lead Levels in Children and Adolescents Aged 3–19 Years, by Secondhand Smoke Exposure Levels: National Health and Nutrition Examination Survey, United States, 1999–2004
| No.(Weighted %) | Blood Lead Level, μg/dL, Geometric Mean (95% CI) | Ratio of Geometric Mean (95% CI) | |||
| Model 1 | Model 2 | Model 3 | |||
| Cotinine, μg/L | |||||
| ≤ 0.03 (Ref) | 1538 (25) | 0.91 (0.86, 0.95) | 1.00 | 1.00 | 1.00 |
| 0.031–0.074 | 1876 (25) | 1.13 (1.08, 1.19) | 1.10 (1.04, 1.16) | 1.09 (1.03, 1.15) | 1.08 (1.02, 1.15) |
| 0.075–0.44 | 1804 (25) | 1.31 (1.22, 1.40) | 1.26 (1.21, 1.32) | 1.19 (1.14, 1.24) | 1.17 (1.12, 1.23) |
| ≥ 0.441 | 1612 (25) | 1.52 (1.41, 1.62) | 1.47 (1.40, 1.55) | 1.30 (1.23, 1.37) | 1.28 (1.21, 1.35) |
| P for trenda | < .001 | < .001 | < .001 | < .001 | |
| Smokers at home, no. | |||||
| 0 (Ref) | 5484 (78) | 1.12 (1.08, 1.17) | 1.00 | 1.00 | 1.00 |
| 1 | 929 (14) | 1.46 (1.33, 1.61) | 1.26 (1.18, 1.33) | 1.16 (1.08, 1.23) | 1.14 (1.07, 1.22) |
| ≥ 2 | 417 (8) | 1.56 (1.42, 1.72) | 1.39 (1.32, 1.47) | 1.25 (1.17, 1.33) | 1.24 (1.16, 1.33) |
| P for trenda | < .001 | < .001 | < .001 | < .001 | |
Note. CI = confidence interval. Model 1 was adjusted for gender; age (continuous); race (White, Black, Mexican American, other); birth country (United States, Mexico, other); body mass index (BMI; defined as weight in kilograms divided by the square of height in meters) percentile; and survey year (1999–2000, 2001–2002, 2003–2004). Model 2 was further adjusted for household education (< high school, high school or equivalent, > high school) and poverty-to-income ratio (PIR; the ratio of the family's income to its appropriate poverty threshold as defined by the US Census Bureau [≤ 1.30, 1.31–3.50, 3.51–5.00]). Model 3 was further adjusted for year in which house was built (before 1950, 1950–1978, after 1978, unknown).
P values for trend across cotinine categories were obtained by including cotinine medians corresponding to each quartile as continuous variables in the regression models. P values for trend across categories of no. of smokers at home were obtained by including 0, 1, and 2 as continuous variables in the regression models.
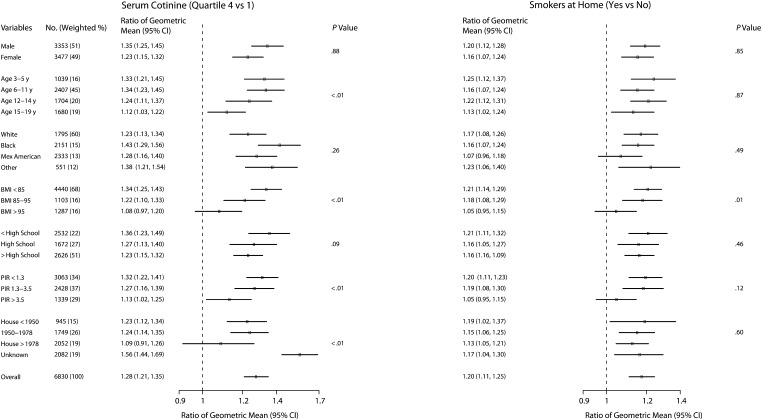
Across all participant characteristics studied, blood lead levels were higher for participants in the highest serum cotinine quartile (vs those in the lowest quartile) and for participants living with smokers at home (vs those without smokers at home; Figure 1). Effect modification was statistically significant (P < .05) for age, BMI, PIR, and age of the house; younger age, lower BMI, lower PIR, and unknown year of house construction were related to stronger associations between blood lead and serum cotinine. Those characteristics were also associated with stronger associations between living with a smoker and blood lead levels, although effect modification was statistically significant only for BMI (P = .01).
FIGURE 1.
Ratio of geometric mean of blood lead level, by children's and adolescents' secondhand smoke exposure and other characteristics: National Health and Nutrition Examination Survey, United States, 1999–2004.
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); CI = confidence interval; Mex = Mexican; PIR = poverty-to-income ratio. Ratios are adjusted for gender, age, race, country of birth, BMI percentile, survey year, household education, poverty level (PIR; the ratio of the family's income to its appropriate poverty threshold as defined by the US Census Bureau), and year in which housing was built. Dots represent the ratios and horizontal lines represent the CIs. The P value is for the difference in the association between secondhand smoke exposure (cotinine or smokers at home) and blood lead levels by participant subgroups (interaction).
Among 24 children with blood lead levels of 10 micrograms per deciliter or higher, 33% lived with at least 1 smoker, and 8% lived with 3 or more smokers; among children with blood lead levels below 10 micrograms per deciliter, the respective figures were 20% and 1% (data not shown). Among 195 children with blood lead levels of 5 micrograms per deciliter or higher, 37% lived with at least 1 smoker, and 5% lived with 3 or more smokers; among children with blood lead levels below 5 micrograms per deciliter, the respective figures were 19% and 1% (data not shown).
Adjustment for Lead Dust
Median lead dust in the homes of children aged 3 to 5 years was 4.9 micrograms per square foot (IQR = 2.2–18.7 μg/sq ft) in windows and 0.46 micrograms per square foot (IQR = 0.24–0.93 μg/sq ft) in floors (Table 3). Lead dust levels in windows and floors were higher in the homes of children of Black race/ethnicity, higher BMI, lower household education, lower family PIR, older houses, higher blood lead, higher serum cotinine, or living with a smoker at home (Table 3).
TABLE 3.
Lead Dust in the Homes of Children Aged 3–5 Years: National Health and Nutrition Examination Survey, United States, 1999–2004
| No. (Weighted %) | Lead Dust on Window, μg/sq ft, Median (IQR) | Lead Dust on Floor, μg/sq ft, Median (IQR) | |
| Total sample | 791 (100) | 4.9 (2.2–18.7) | 0.46 (0.24–0.93) |
| Gender | |||
| Male | 421 (54) | 4.6 (2.2–15.1) | 0.45 (0.23–0.88) |
| Female | 370 (46) | 5.2 (2.2–20.8) | 0.24 (0.46–0.95) |
| Race/ethnicity | |||
| White | 225 (57) | 4.3 (2.0–14.0) | 0.41 (0.23–0.73) |
| Black | 256 (16) | 10.9 (3.1–41.3) | 0.82 (0.38–1.74) |
| Mexican American | 236 (15) | 5.3 (2.4–26.1) | 0.51 (0.27–1.05) |
| Other | 74 (12) | 3.8 (1.5–7.4) | 0.38 (0.18–0.85) |
| Birth country | |||
| United States | 763 (97) | 5.0 (2.1–18.7) | 0.46 (0.24–0.90) |
| Mexico | 14 (1) | 4.2 (2.2–23.8) | 0.87 (0.29–3.40) |
| Other | 14 (2) | 3.6 (2.3–6.9) | 0.38 (0.29–0.92) |
| BMI percentile | |||
| < 85 | 590 (78) | 4.8 (2.1–16.5) | 0.46 (0.24–0.94) |
| 85–94 | 103 (12) | 4.6 (2.0–21.0) | 0.41 (0.22–0.90) |
| ≥ 95 | 98 (10) | 5.9 (2.4–22.2) | 0.50 (0.21–0.99) |
| Educationa | |||
| < high school | 281 (24) | 7.5 (2.2–25.3) | 0.67 (0.27–1.30) |
| High school or equivalent | 203 (25) | 6.1 (2.4–16.6) | 0.49 (0.23–0.90) |
| > high school | 307 (51) | 4.1 (2.0–14.1) | 0.39 (0.22–0.67) |
| PIR | |||
| Low (≤ 1.30) | 431 (41) | 6.9 (2.6–22.4) | 0.60 (0.29–1.30) |
| Medium (1.31–3.50) | 257 (37) | 4.3 (2.0–16.5) | 0.42 (0.23–0.81) |
| High (3.51–5.00) | 103 (22) | 3.8 (1.8–7.7) | 0.35 (0.18–0.54) |
| Year house built | |||
| Before 1950 | 110 (16) | 24.7 (4.3–100.7) | 0.78 (0.43–1.70) |
| 1950–1978 | 189 (22) | 5.4 (2.1–16.9) | 0.52 (0.27–0.99) |
| After 1978 | 220 (39) | 2.9 (1.8–7.2) | 0.28 (0.17–0.50) |
| Unknown | 272 (23) | 7.7 (2.5–28.5) | 0.75 (0.36–1.41) |
| Child's blood lead level, μg/dL | |||
| ≤ 0.8 | 72 (13) | 2.8 (1.6–4.8) | 0.28 (0.17–0.46) |
| 0.9–1.1 | 98 (15) | 2.4 (1.6–5.9) | 0.35 (0.19–0.53) |
| 1.2–1.7 | 184 (27) | 4.6 (2.4–12.2) | 0.39 (0.23–0.67) |
| ≥ 1.8 | 437 (45) | 8.9 (2.8–37.7) | 0.70 (0.36–1.56) |
| Child's serum cotinine level, μg/L | |||
| ≤ 0.03 | 133 (20) | 2.9 (1.8–6.3) | 0.33 (0.16–0.52) |
| 0.031–0.074 | 189 (21) | 3.3 (2.0–15.8) | 0.50 (0.26–0.82) |
| 0.075–0.44 | 236 (31) | 5.4 (2.1–22.3) | 0.45 (0.24–0.90) |
| ≥ 0.441 | 233 (28) | 7.9 (3.0–29.2) | 0.66 (0.27–1.23) |
| Smoking at home | |||
| No | 623 (79) | 4.3 (2.0–13.1) | 0.41 (0.23–0.80) |
| Yes | 168 (21) | 12.3 (3.0–34.9) | 0.67 (0.28–1.24) |
Note. BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); IQR = interquartile range; PIR = poverty-to-income ratio (the ratio of the family's income to its appropriate poverty threshold as defined by the US Census Bureau).
Education of reference person in household.
Compared with a multivariate model adjusted for personal and household characteristics (Table 4, model 1), models incorporating further adjustment for lead dust concentrations in windows and floors, added separately (models 2 and 3) and jointly (model 4), did not modify the association between measures of SHS and blood lead levels. The ratios of blood lead levels comparing the highest with the lowest window and floor lead dust quartiles were 1.38 (95% CI = 1.27, 1.48) and 1.34 (95% CI = 1.20, 1.48), respectively, before adjustment for cotinine level and number of smokers at home and 1.33 (95% CI = 1.23, 1.42) and 1.27 (95% CI = 1.12, 1.43), respectively, after adjustment (data not shown).
TABLE 4.
Blood Lead in Children Aged 3–5 Years for Whom House Dust Data Were Available (n = 791), by Secondhand Smoke Exposure Levels: National Health and Nutrition Examination Survey, United States, 1999–2004
| No. (Weighted %) | Ratio of Geometric Mean (95% CI) | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Cotinine, μg/L | |||||
| ≤ 0.03 (Ref) | 133 (17) | 1.00 | 1.00 | 1.00 | 1.00 |
| 0.031–0.074 | 189 (24) | 1.01 (0.88, 1.14) | 1.00 (0.87, 1.13) | 1.00 (0.87, 1.14) | 1.00 (0.87, 1.13) |
| 0.075–0.44 | 236 (30) | 1.14 (1.02, 1.25) | 1.13 (1.02, 1.25) | 1.13 (1.01, 1.24) | 1.12 (1.01, 1.24) |
| ≥ 0.441 | 233 (29) | 1.31 (1.21, 1.42) | 1.31 (1.19, 1.42) | 1.31 (1.20, 1.42) | 1.30 (1.19, 1.41) |
| P for trenda | < .001 | < .001 | < .001 | < .001 | |
| Smokers at home, no. | |||||
| 0 (Ref) | 623 (78) | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥ 1 | 168 (22) | 1.17 (1.04, 1.30) | 1.17 (1.04, 1.30) | 1.17 (1.04, 1.30) | 1.17 (1.04, 1.30) |
Note. CI = confidence interval. Model 1 was adjusted for gender, age, race, country of birth, body mass index (BMI; defined as weight in kilograms divided by the square of height in meters) percentile, survey y (1999–2000, 2001–2002, 2003–2004), household education (< high school, high school or equivalent, > high school), poverty-to-income ratio (PIR; the ratio of the family's income to its appropriate poverty threshold as defined by the US Census Bureau [≤ 1.30, 1.31–3.50, 3.51–5.00]), and year in which housing was built (before 1950, 1950–1978, after 1978, unknown). Model 2 was the same as model 1 but further adjusted for window lead dust as a continuous variable. Model 3 was the same as model 1 but further adjusted for floor lead dust as a continuous variable. Model 4 was the same as model 1 but further adjusted for window lead dust and floor lead dust.
P values for trend across cotinine categories were obtained by including cotinine medians corresponding to each quartile as continuous variables in the regression models.
DISCUSSION
Living with 1 or more smokers and increasing serum cotinine levels were associated with elevated blood lead levels in a nationally representative sample of nonsmoking US children and adolescents from NHANES (1999–2004). Blood lead levels increased steadily with SHS measures after adjustment for sociodemographic and household characteristics. The association between SHS measures and blood lead was somewhat stronger among younger participants, maybe because they spent more time in the home and were in closer contact with their parents or other adults compared with older children. Importantly, among children aged 3 to 5 years, adjustment for lead dust did not modify the magnitude of the association between SHS and blood lead levels, suggesting that the measures of SHS exposure were not simply markers of living in homes with high lead and that direct inhalation could be a major exposure pathway. These findings extend previous evidence that SHS is an important source of lead in the general population10,14,30 and suggest that current SHS exposure levels affect lead levels in children.
Lead is an important environmental hazard for children, particularly recognized for its neurocognitive effects.31,32 Despite a dramatic overall decline in lead levels in children and young adults over the last few decades, inner-city children and young adults of low socioeconomic status continue to experience high lead exposure. Established sources include lead paint, dust and soil, industrial sources, and recently, urban drinking water.33 SHS, on the other hand, remains a relatively unrecognized source of lead in the population. It is well-established, however, that tobacco smoke contains lead.10,13 Controlled experiments have shown that the more puffs of smoke a smoker produces, the higher the lead content in mainstream and sidestream smoke, as measured in both particulate matter and gas.13 Lead in the gas phase is due to the formation of volatile compounds such as tetramethyl lead and plumbane in the burning zone of the cigarette13 and can be readily inhaled. Tobacco smoke particles, moreover, are relatively small1,10,34,35 and can be more easily absorbed in the bronchiole-alveolar region than can larger particles (e.g., dust particles or paint chips).36 Airway diameter, respiratory rates, time spent at home, and housing characteristics such as size, ventilation, and presence or absence of a home smoking ban are possible contributors to the differences in the magnitude of the association between SHS and blood lead by age, BMI, and socioeconomic status. Although information on home smoking policy, ventilation, or size was not available, the consistent findings regarding serum cotinine levels and self-reported number of smokers in the home support the conclusion that household members who smoke represent a major source of SHS exposure in US children.
In the context of the Healthy People 2010 initiative, the Environmental Protection Agency, the CDC, and the US Department of Housing and Urban Development have developed federal initiatives to eliminate the risk of lead poisoning in US children.37 These efforts focused mainly on controlling lead paint hazards. Our findings, together with previous evidence,10,14,30 indicate that lead prevention programs need to incorporate strategies to prevent potential lead exposure from SHS, particularly in children who live with smokers. Lead poisoning prevention programs should collect data on the number of smokers living in the home, their relationship with the child, and the number of cigarettes smoked per day in the home, as well as the presence and level of enforcement of smoking bans at home (established by the family or property owner), in motor vehicles, and in other environments where children spend time with adults who smoke. In homes with at least 1 smoker, lead prevention programs can borrow strategies from SHS prevention programs, such as explaining the health impacts of SHS38 and the benefits of smoke-free homes and motor vehicles39–42 and providing or referring to smoking cessation counseling.43–45 In addition, family physician and pediatrician visits should be considered as opportunities for SHS prevention,6,45–47 especially for children with clinical symptoms related to SHS exposure or elevated blood lead levels.
Incorporating lead prevention and control as an additional benefit of smoke-free programs can also be a useful argument for implementing tobacco control initiatives in the home environment, particularly in disadvantaged communities that are at increased risk of both lead and SHS exposure. Recently, the Department of Housing and Urban Development encouraged public authorities to implement smoke-free policies in public housing units.48,49 As of July 2010, at least 171 local housing authorities in 25 states have adopted smoke-free policies for some or all of their housing,50 and there are several community initiatives for smoke-free multiunit housing across the country.51 Additional efforts, however, are urgently needed because fewer than 40% of smokers' homes in the United States have smoke-free policies in place.40 The stronger association between serum cotinine and blood lead in children living in housing of unknown age and in families with lower education and income could be associated with living in smaller rental units with poorer ventilation than in other types of housing. Although regulating smoking in private homes can be challenging from a public policy perspective,49,52 banning smoking in environments where young children spend time is consistent with protecting their health and has been evaluated as ethically acceptable.52,53
Strengths and Limitations
Strengths of our study include the large sample size, the national representativeness of the study sample, the high quality of the study protocol and laboratory methods, and the inclusion of children from different ethnicities and socioeconomic statuses. To ensure that only nonsmokers were evaluated, we applied strict criteria that excluded participants who acknowledged ever smoking or whose cotinine levels were indicative of tobacco use, or who had missing responses to the smoking questions.
Several limitations, however, must also be considered. First, this is a cross-sectional study. Although reverse causality is unlikely, potential confounding by common sources of exposure or unmeasured socioeconomic factors remains possible. The association between SHS measures and blood lead, however, persisted after adjustment for measures of education and income, age of housing, and lead dust levels. Second, cotinine and lead were measured only once, and the half-lives of both biomarkers differ markedly (16 hours for serum cotinine54 and 30 days for blood lead55). The adequacy of serum cotinine, however, as a marker of ongoing SHS exposure is supported by the consistent findings with both cotinine and self-reported measures of exposure. Third, the lack of specific information on smoking at home or number of cigarettes smoked could have led to an underestimation of the association between SHS and lead. Also, other sources and routes of lead, such as oral intake from toys, were not included. Finally, we could not evaluate the role of SHS deposition in children younger than 3 years because lead dust information was not available for those children. The contribution of SHS exposure to blood lead levels in younger children is particularly important because they spend more time in closer proximity to adults and at home than do older children.
Conclusions
Our study provides support for SHS as a modifiable source of lead exposure in US children and adolescents and suggests that direct inhalation could be a major exposure pathway. These findings have important public health implications for lead and SHS prevention programs. First, lead prevention programs should systematically evaluate smoking at home and promote smoke-free environments to reduce lead exposure. Second, smoke-free legislation initiatives in public places, motor vehicles, and other environments where children are present could further prevent lead exposure. Third, health care professionals should evaluate potential SHS exposure and provide recommendations to minimize exposure as part of children's medical care. Lead-related health effects occur at levels well below the CDC current action level for children (10 μg/dL).56 Eliminating exposure to SHS in children could thus result in lower lead exposure and fewer adverse lead-related health effects, including neurocognitive effects.4,57,58
Acknowledgments
This study was supported by the National Cancer Institute (grant R03CA153959).
Human Participant Protection
The NHANES protocol was reviewed and approved by the National Center for Health Statistics institutional review board, and informed consent was provided by the participants and their guardians for those younger than 18 years of age.
References
- 1.The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Dept of Health and Human Services, Centers for Disease Control and Prevention; 2006 [PubMed] [Google Scholar]
- 2.European Commission Council recommendation on smoke-free environments. Available at: http://ec.europa.eu/health/ph_determinants/life_style/Tobacco/Documents/tobacco_prec2009_en.pdf. Accessed August 15, 2010
- 3.GTSS Collaborative Group A cross country comparison of exposure to secondhand smoke among youth. Tob Control. 2006;15(suppl 2):4–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froehlich TE, Lanphear BP, Auinger P, et al. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(6):e1054–e1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsen KH, Carlsen KC. Respiratory effects of tobacco smoking on infants and young children. Paediatr Respir Rev. 2008;9(1):11–19 [DOI] [PubMed] [Google Scholar]
- 6.Best D. From the American Academy of Pediatrics: technical report—secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009;124(5):E1017–E1044 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Vital signs: nonsmokers' exposure to secondhand smoke—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59(35):1141–1146 [PubMed] [Google Scholar]
- 8.Wipfli H, Avila-Tang E, Navas-Acien A, et al. Secondhand smoke exposure among women and children: evidence from 31 countries. Am J Public Health. 2008;98(4):672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL. Blood lead level and kidney function in US adolescents: The Third National Health and Nutrition Examination Survey. Arch Intern Med. 2010;170(1):75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galazyn-Sidorczuk M, Brzoska MM, Moniuszko-Jakoniuk J. Estimation of Polish cigarettes contamination with cadmium and lead, and exposure to these metals via smoking. Environ Monit Assess. 2008;137(1–3):481–493 [DOI] [PubMed] [Google Scholar]
- 11.Pappas RS, Polzin GM, Zhang L, Watson CH, Paschal DC, Ashley DL. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem Toxicol. 2006;44(5):714–723 [DOI] [PubMed] [Google Scholar]
- 12.Chang MJ, Walker K, McDaniel RL, Connell CT. Impaction collection and slurry sampling for the determination of arsenic, cadmium, and lead in sidestream cigarette smoke by inductively coupled plasma-mass spectrometry. J Environ Monit. 2005;7(12):1349–1354 [DOI] [PubMed] [Google Scholar]
- 13.Kalcher K, Kern W, Pietsch R. Cadmium and lead in the smoke of a filter cigarette. Sci Total Environ. 1993;128(1):21–35 [DOI] [PubMed] [Google Scholar]
- 14.Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in US children. Epidemiology. 2003;14(6):719–727 [DOI] [PubMed] [Google Scholar]
- 15.Advisory Committee on Childhood Lead Poisoning Prevention Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials. Atlanta, GA: Centers for Disease Control and Prevention; 2002. Available at: http://www.cdc.gov/nceh/lead/publications/screening.htm. Accessed August 16, 2010 [Google Scholar]
- 16.Centers for Disease Control and Prevention Preventing lead poisoning in young children. 2005. Available at: http://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf. Accessed August 16, 2010
- 17.US Environmental Protection Agency Lead in paint, dust, and soil. 2010. Available at: http://www.epa.gov/opptintr/lead. Accessed August 16, 2010
- 18.Roberts JW, Wallace LA, Camann DE, et al. Monitoring and reducing exposure of infants to pollutants in house dust. Rev Environ Contam Toxicol. 2009;201:1–39 [DOI] [PubMed] [Google Scholar]
- 19.National Health and Nutrition Examination Survey Analytic and reporting guidelines. 2006. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Accessed August 16, 2010
- 20.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2001;153(8):807–814 [DOI] [PubMed] [Google Scholar]
- 21.National Health and Nutrition Examination Survey Questionnaires, data sets, and related documentation. Laboratory procedure manual. Cotinine. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab06_met_cotinine.pdf. Accessed August 16, 2010
- 22.National Health and Nutrition Examination Survey Questionnaires, data sets, and related documentation. Laboratory procedure manual. Cotinine. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l06_b_met_cotinine.pdf. Accessed August 16, 2010
- 23.National Health and Nutrition Examination Survey Questionnaires, data sets, and related documentation. Laboratory procedure manual. Lead, cadmium, and mercury. Whole blood. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06_c_met_pb_cd_hg.pdf. Accessed August 2, 2010
- 24.National Health and Nutrition Examination Survey Questionnaires, data sets, and related documentation. Laboratory procedure manual. Cadmium and lead. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab06_met_lead_and_cadmium.pdf. Accessed August 16, 2010
- 25.National Health and Nutrition Examination Survey Questionnaires, data sets, and related documentation. Laboratory procedure manual. Cadmium and lead. Blood. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l06_b_met_lead_and_cadmium.pdf. Accessed August 16, 2010
- 26.Hornung W, Reed D. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51 [Google Scholar]
- 27.National Health and Nutrition Examination Survey Questionnaires, data sets, and related documentation. Analytic guidelines. Lead dust. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l20_c.pdf. Accessed August 16, 2010
- 28.US Census Bureau How the Census Bureau measures poverty. Available at: http://www.census.gov/hhes/www/poverty/methods/measure.html. Accessed August 16, 2010
- 29.Centers for Disease Control and Prevention About BMI for children and teens. Available at: http://www.cdc.gov/healthyweight/assessing/bmi/childrens_BMI/about_childrens_BMI.html. Accessed August 16, 2010
- 30.Willers S, Schutz A, Attewell R, Skerfving S. Relation between lead and cadmium in blood and the involuntary smoking of children. Scand J Work Environ Health. 1988;14(6):385–389 [DOI] [PubMed] [Google Scholar]
- 31.Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations < 10 microg/dL in US children and adolescents. Public Health Rep. 2000;115(6):521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin R, Brown MJ, Kashtock ME, et al. Lead exposures in US children, 2008: implications for prevention. Environ Health Perspect. 2008;116(10):1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klepeis NE, Apte MG, Gundel LA, Sextro RG, Nazaroff WW. Determining size-specific emission factors for environmental tobacco smoke particles. Aerosol Sci Technol. 2003;37(10):780–790 [Google Scholar]
- 35.National Research Council, Committee on Passive Smoking Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington, DC: National Academy Press; 1986 [PubMed] [Google Scholar]
- 36.Agency for Toxic Substances and Disease Registry Toxicological profile for lead. 2007. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. Accessed August 16, 2010 [PubMed]
- 37.Centers for Disease Control and Prevention Healthy people. Available at: http://www.cdc.gov/nchs/healthy_people.htm. Accessed August 16, 2010 [PubMed]
- 38.Jimenez-Gonzalez C, Santini V, Figueroa Cosme WI, Parilla IC. Do parents know about the adverse effects of passive smoking and the relationship with respiratory illness on their children? Bol Asoc Med P R. 2008;100(2):39–46 [PubMed] [Google Scholar]
- 39.Gilpin EA, White MM, Farkas AJ, Pierce JP. Home smoking restrictions: which smokers have them and how they are associated with smoking behavior. Nicotine Tob Res. 1999;1(2):153–162 [DOI] [PubMed] [Google Scholar]
- 40.Messer K, Mills AL, White MM, Pierce JP. The effect of smoke-free homes on smoking behavior in the US. Am J Prev Med. 2008;35(3):210–216 [DOI] [PubMed] [Google Scholar]
- 41.Mills AL, Messer K, Gilpin EA, Pierce JP. The effect of smoke-free homes on adult smoking behavior: a review. Nicotine Tob Res. 2009;11(10):1131–1141 [DOI] [PubMed] [Google Scholar]
- 42.Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007;32(6):542–543 [DOI] [PubMed] [Google Scholar]
- 43.Caponnetto P, Polosa R, Best D. Tobacco use cessation counseling of parents. Curr Opin Pediatr. 2008;20(6):729–733 [DOI] [PubMed] [Google Scholar]
- 44.Winickoff JP, Tanski SE, McMillen RC, Klein JD, Rigotti NA, Weitzman M. Child health care clinicians' use of medications to help parents quit smoking: a national parent survey. Pediatrics. 2005;115(4):1013–1017 [DOI] [PubMed] [Google Scholar]
- 45.US Dept of Health and Human Services Treating tobacco use and dependence: 2008 update. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. Accessed June 10, 2011
- 46.Mullins S, Fagan HB, Reed JF, III, Bercaw D. Ask and act: Delaware physicians demonstrate the effectiveness of the American Academy of Family Physicians' initiative to promote tobacco cessation counseling. Del Med J. 2009;81(4):155–160 [PubMed] [Google Scholar]
- 47.Perez-Stable EJ, Juarez-Reyes M, Kaplan C, Fuentes-Afflick E, Gildengorin V, Millstein S. Counseling smoking parents of young children: comparison of pediatricians and family physicians. Arch Pediatr Adolesc Med. 2001;155(1):25–31 [DOI] [PubMed] [Google Scholar]
- 48.US Dept of Housing and Urban Development, Office of Public and Indian Housing, Office of Healthy Homes and Lead Hazard Control. Non-smoking policies in public housing. Available at: site. http://www.hud.gov/offices/pih/publications/notices/09/pih2009-21.pdf. Accessed August 16, 2010
- 49.Winickoff JP, Gottlieb M, Mello MM. Regulation of smoking in public housing. N Engl J Med. 2010;362(24):2319–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smoke-Free Environments Law Project Housing authorities/commissions which have adopted smoke-free policies. Available at: http://www.tcsg.org/sfelp/SFHousingAuthorities.pdf. Accessed August 16, 2010
- 51.American Academy of Pediatrics Smoke-free multiunit housing. Available at: http://www.aap.org/richmondcenter/pdfs/Tobacco_Policy_Appendix3.pdf. Accessed August 16, 2010
- 52.Jarvie JA, Malone RE. Children's secondhand smoke exposure in private homes and cars: an ethical analysis. Am J Public Health. 2008;98(12):2140–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization Tobacco and the rights of the child. 2001. Available at: http://www.who.int/tobacco/media/en/CRCreport.pdf. Accessed August 16, 2010
- 54.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;(192):29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbosa F, Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113(12):1669–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) Advisory Committee on Childhood Lead Poisoning Prevention Interpreting and managing blood lead levels < 10 microg/dL in children and reducing childhood exposures to lead: recommendations of CDC's Advisory Committee on Childhood Lead Poisoning Prevention. MMWR Recomm Rep. 2007;56(RR-8):1–16 [PubMed] [Google Scholar]
- 57.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among US children and adolescents. Environ Health Perspect. 2005;113(1):98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braun JM, Froehlich TE, Daniels JL, Dietrich KN, Hornung R. Association of environmental toxicants and conduct disorder in US children: NHANES 2001–2004. Environ Health Perspect. 2008;116(7):956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]