Abstract
Background: As the immature spinal cord was nerve growth permissive, we examined glial reactions that influence regeneration of the spinal cord in a fetal rat spinal cord injury model. Methods: Three, 7, 21, and 35 days after intrauterine surgery, offsprings were killed and the thoracic and lumbar spinal cords were carefully removed from the spinal column and then cut into 10 μm longitudinal sections. These sections were stained with hematoxylin-eosin, anti-glial fibrillary acidic protein antibody (GFAP) as a marker of astrocytes, and anti-complement CR3 antibody (OX-42) as a marker of microglia. A cordotomy model in a young adult rat was utilized as a control. Results: In the present study, collagen fibers and scar formation were seen in the severed spinal cords of mature rats, but scar formation was not seen in the fetal rat cordotomy group, regardless of spinal continuity. In the control group, biological activity of GFAP-positive cells increased over time. In the fetal rat cordotomy model, activity elevated slightly immediately after cordotomy, and disappeared shortly thereafter. In the control group, OX-42-positive macrophage-like cells proliferated over time. However, in the fetal rat cordotomy model, OX-42- positive macrophage-like cells were recognized on postoperative days 3 and 7, and then disappeared. At 5 mm from the cordotomy site, reactive microglia were recognized in the white matter of control group spinal cords, but these microglia were not recognized in the fetal rat cordotomy model. Conclusions: In the present study, collagen fibers and scar formation were seen in the severed spinal cords of adult rats, but scar formation was not seen in the fetal rat cordotomy group. Lack of inflammation and scar formation thus appear advantageous for regeneration of the fetal spinal cord. Between fetal and mature rats, chronological changes in the immunohistochemical reactions of astrocytes and microglia following cordotomy were compared, and the results confirmed many differences. The results of the present study suggest that the presence of activated glial cells around damaged central nervous tissue and the quick disappearance of these cells after injury are important for the repair of damaged central nervous system tissue, and that the role of glial cells in nerve regeneration can change depending on the level of maturity of glial cells or surrounding cells, site of injury, or the state of tissue around the injury.
Keywords: Fetal surgery, Immunohistochemistry, Astrocytes, Microglia, Spinal cord injury
Introduction
The spinal cords of various kinds of vertebrates, such as lampreys and newts, display regenerative properties. However, spinal cords in mammals do not demonstrate marked regeneration following injury. Recently, research aimed at facilitating regeneration of the spinal cord has been undertaken using peripheral nerves [6], central neural tissue [16], neural stem cells [4], and neurotrophic factors [22]. Furthermore, functional recovery of the spinal cord and axonal regeneration has been reported. This kind of research is paving the way toward the ability to reconstruct neuronal circuits in the spinal cord.
In the fetus, during development of the neuronal circuitry, various guidance cues induce growth of nerve fiber. In addition, guidance cues are involved in the repair of the central nervous system following damage. Guidance cues [13] may affect neurons directly or indirectly through glial cells. Previous research has made actual axonal regeneration of the principal objective, but the cellular environment including glial cells has barely been investigated.
The present study aimed to immunohistochemically observe changes to glial cells using a spinal cord injury model in fetal rat, and to elucidate the cellular environment required to allow spinal cord regeneration.
Materials and methods
Study design
The current study was approved by the Animal Care Committee of Hiroshima University School of Medicine and conformed to NIH Guidelines for use of animals in research (NIH publication No. 86-23). Pair of male and female Wistar rat (15-week-old, 280–320 g) was housed in the same cage for one night. The next morning, female rats with a vaginal plug were considered to be in the first day of pregnancy (n= 75). Spinal cords of one fetus from each mother were transected using intrauterine surgical techniques on day 19 of pregnancy. Fetuses were delivered 3 days after intrauterine surgery (Fetal Group).
The following items were examined: (1) pathohistology of the spinal cord on the 3rd, 7th, 21st, and 35th postoperative days (postnatal days 0, 4, 18, and 32); (2) immunohistochemistry of astrocytes and microglia on the 0, 4th, 18th, and 32nd postnatal days. As controls, spinal cords of young adult rats were transected and investigated pathohistologically and immunohistologically (Control Group).
Surgical procedure for intrauterine surgery and animal care
Fetal group
Following intraperitoneal administration of 0.5 ml/kg pentobarbital sodium (Abbott Laboratories Co., North Chicago, III) for anesthesia, 75 pregnant Wistar rats underwent intrauterine surgery. The rat uterus displays a bicornuate structure, with each uterine horn containing about six fetuses. One of the fetuses convenient for the intrauterine procedure was chosen for the operation. The operation was performed under an operating microscope (Olympus OME, Olympus Optical Co., Tokyo, Japan), while the fetus was wrapped with gauze soaked with 40°C saline. After laminectomy of T8-10, the spinal cord was transected using a sharpened, 0.1-mm-thick Feather blade (Feather S Blade, Feather Safety Razor Co., Osaka, Japan). Complete transection of the spinal cord was confirmed under operating microscopy.
Fetal skin was closed using 10-0 nylon sutures, and the fetus was returned to the uterus. The amnion and uterus were sutured and decreased amniotic fluid was supplemented with 37°C saline. Fetuses were delivered on day 22 of pregnancy, and the maternal rat fostered neonates in the same cage.
Control group
At 7-week-old, 20 males Wistar rats underwent laminectomy of T8-10 and transection of the spinal cord following an induction of anesthesia using intraperitoneal administration of 0.5 ml/kg pentobarbital sodium.
Tissue processing for histology
A 18- and 32-day-old rats in the fetus group were killed by pentobarbital overdose (50 mg/100 g bodyweight) and perfused transcardially with Ringer solution containing 1 × 105 IU/l heparin and 0.25% NaNO2, followed by fixative (4% paraformaldehyde in 0.1 M phosphate buffer with 5% sucrose). Spinal cords were removed and postfixed overnight. After immersion for 2–3 days in 30% sucrose solution, spinal cords were embedded in OCT compound (Miles No. 4583, Elkhart, IN, USA.) and frozen in isopentane at −40°C. Sections of 50 μm in thickness were cut in the sagittal plane. Zero- and 4-day-old rats were put on ice for a few minutes to enter suspended animation, then tissue processing was performed as described.
Immunohistochemistry
Tissue sections were air-dried, then rinsed, blocked, and incubated overnight at 4°C with primary antibodies. Astrocytes were identified with both rabbit anti-bovine and monoclonal mouse ascites glial fibrillary acidic protein (GFAP; Biomeda Co., Hayward, CA, USA). Microglial (MG) cells were identified using monoclonal mouse anti-rat OX-42 (CD11b) (Biosys Co., Compiegne, France). Diaminobenzamine (DAB; DAKO Corp., Carpinteria, CA, USA) staining was performed by incubating sections for 90 min with either biotinylated goat anti-rabbit or goat anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA). Sections were rinsed, incubated with 0.3% hydrogen peroxide/methanol to quench endogenous tissue peroxidases, and incubated with avidin and biotinylated horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Immunostaining was visualized with DAB and sections counterstained with hematoxylin (Sigma, St. Louis, MO, USA). Slides were dehydrated through alcohols and xylene and mounted in permount (Sigma). In negative controls, normal goat and rabbit serum were added instead of primary antibodies.
Quantitative image analysis
For quantitative analysis of MG activation, several parameters were studied using image analysis [23]. Signal intensity of each marker was evaluated using densitometry in material derived from animals in fetus and control groups. In addition, OX-42-immunostained cells were counted. Researchers blinded to the experimental group assignments of animals conducted these analyses.
For densitometric evaluations, a zero value of optical density was assigned in each section to the background, defined as intercellular spaces within the spinal cord. Integrated density gives the sum of ratiometric gray values for each pixel in the selected area (100 × 100 μm). Higher numbers indicate more intense staining and more stained MG cell branches in the area. Single numbers represent whole sections with means of pooled data from the 50 areas taken, using the ×20 objective lens.
Numbers of OX-42-immunostained cells in the spinal cord were counted using a ×16 objective lens and a rectangular field within a 300 × 220-μm frame. Immunopositive cells were counted in a total of 40 fields sampled randomly from the spinal cord of rats, and an equal number of fields from three control animals. Morphological signs of MG activation include changes of phenotype and staining intensity of individual MG cells. Immunological score [23] was calculated based on population and morphological signs of OX-42-positive cells. Immunological scores were based on cell counts as follows: scores of 0.5, 1, 1.5, 2, 3, 4 or 5 were given to spinal cord where average numbers of immunoreactive cells/section were 4–7, 8–12, 13–20, 21–40, 41–100, 101–200, and >201, respectively. When, in addition to cells, immunoreactive fibrous processes were also noted, the scores were incremented by 0.5, 1 or 2 for small, moderated or large amounts of such fibrous material.
An image analyzer comprising a Nikon FXA microscope, XC-003 CCD high- sensitivity monochrome video camera (Sony, Tokyo, Japan) with control unit, and a Macintosh Quadra 700 (Apple Computer, CA, USA) computer was used. Pictures were captured using a Scion LG-3 frame grabber card. Data were evaluated using Mac ASPECT software (Mitani Co., Fukui, Japan).
For statistical analysis, differences between fetus and control groups in mean optical density value of OX-42 immunoreactivity and number of OX-42-immunostained cells were evaluated using Kruskal–Wallis test and Mann–Whitney test. Values of P< 0.05 were considered statistically significant.
Control group
On days 3, 7, 21, and 35 after cordotomy, pathohistological, and immunohistochemical investigations of the spinal cord were performed in adult cordotomy models using the same methods described above for the fetal model.
Results
Survival rates of fetal cordotomy model
Only 35 of the 75 fetal cordotomy rats were alive at parturition, with 29 prenatal deaths and 11 stillbirths. Natality was 46.7%. Ten of the 35 rats that were alive at parturition had disappeared from the cage within a week after birth. Twenty-five rats remained alive and hence was available for postbirth study. Fifteen rats were sacrificed at the 3rd, 7th, and 21st postoperative days (five each), and 10 rats were sacrificed at 35st postoperative days. Predation by the dam was suspected.
Pathohistological findings of the spinal cord
Control group
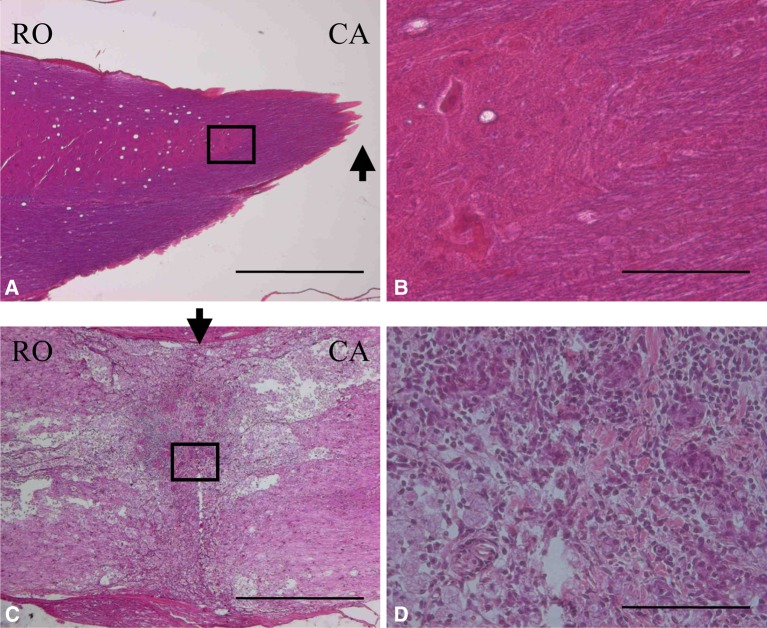
On day 3 after cordotomy, necrotic tissue, infiltration of leukocytes, and obvious edema were recognized. On day 7 after cordotomy, several intramedullary cavities were formed as scar tissue disappeared with macrophage infiltration. A rich network of capillary vessels was observed. On day 21 after cordotomy, the boundary between normal and degenerative tissue became clear as glial cells grew. On postoperative day 35, substantial amounts of scar tissue comprising collagen fibers were formed at the cordotomy site (Fig. 1).
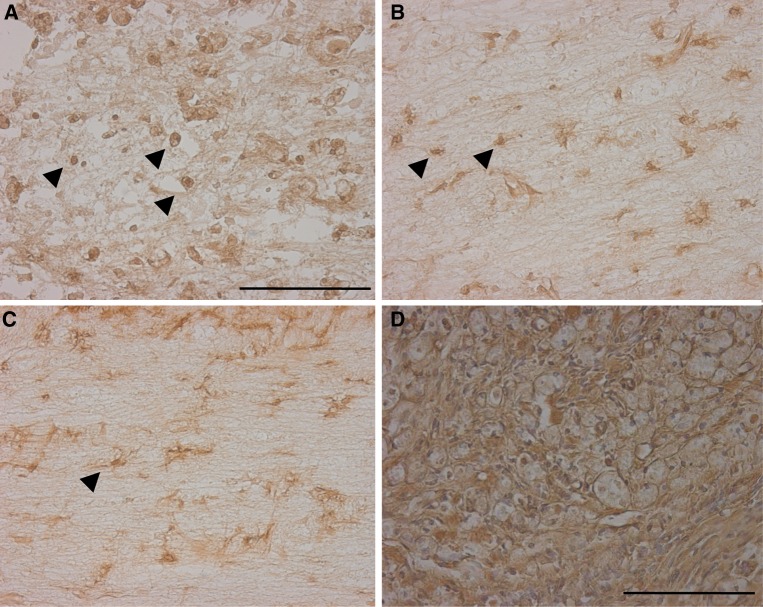
Fig. 1.
Spinal cord at 5 weeks after fetal surgery (a and b) and adult surgery (c and d). (Hematoxilin-Eosin (HE) stain). The cut end of the spinal cord. White matter was wrapped around the spinal cord. Higher magnification of the box shown in a. Connective tissue scarring at the site of transection was absent. The lesion sites of the spinal cord. Cysts occurred more frequently in the adults than in the fetal rats. Higher magnification of the box shown c. In the adult rat, there was significant connective tissue scar formation. Arrows show the lesion sites of the spinal cord. (RO rostral, CA caudal) (Scale bar = 1 mm, applicable to a and c) (Scale bar = 100 μm, applicable to b and d)
Fetal rat cordotomy group
On day 3 after cordotomy, edematous changes and mild infiltration of leukocytes were seen in the severed spinal cord. On day 7 after cordotomy, neither infiltration of macrophages nor intramedullary cavitation were observed in the injured spinal cord. Level of capillary vessel formation was mild. On day 21 after cordotomy, neither proliferation of glial cells nor cavitation was seen. Even on day 35 after cordotomy, scar tissue was not found in the severed spinal cord (Fig. 1).
Immunohistochemical findings
GFAP staining
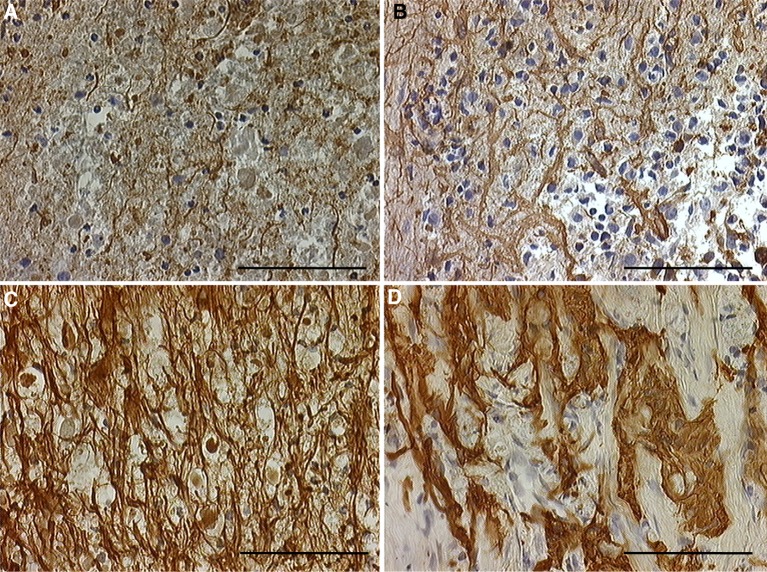
Control group On day 3 after cordotomy, no GFAP-positive cells were seen in necrotic tissue, but some GFAP-positive cells were detected in tissue around the severed cord. These cells were slightly thickened, and cell processes were extended. On day 7 after cordotomy, GFAP-positive cells were further thickened, and cell processes were also thickened, extended, and arranged densely at the boundary with the injured cord. On day 21 after cordotomy, a boundary wall was beginning to form between the injured cord and surrounding tissue. In this area, numerous GFAP-positive cells were present and cell processes were densely arranged. On day 35 after cordotomy, the number of GFAP-positive cells was even greater, density of cell processes was higher, and collagen fibers (i.e., scar tissue) were present in the severed spinal cord. The number of activated GFAP-positive cells increased over time (Fig. 2).
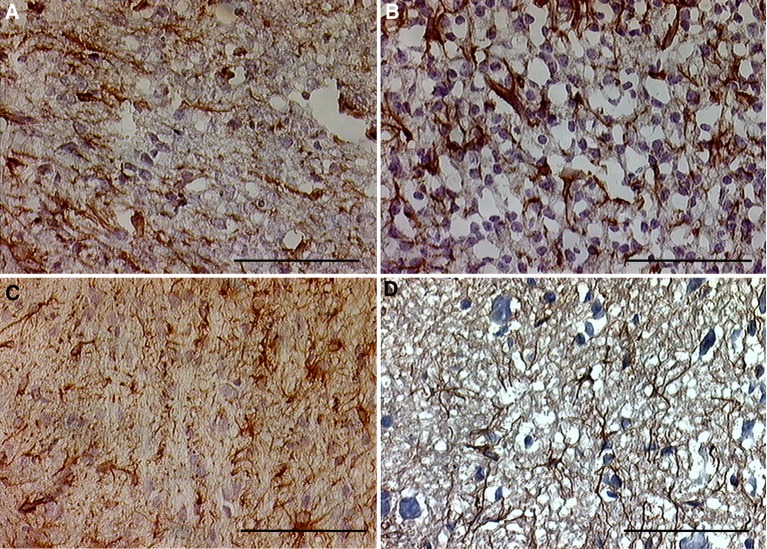
Fetal rat cordotomy group On day 3 after cordotomy GFAP-positive cells were seen around the severed cord, but no hypertrophy was observed and cell processes were not extended. On day 7 after cordotomy GFAP-positive cells were slightly thickened, and cell processes were mildly thickened and extended. On days 21 and 35 after cordotomy GFAP-positive cells around the severed spinal cord were not thickened, cell processes were not thickened or extended, and no cellular multiplication was detected. In addition, no scar tissue had developed in the severed spinal cord. Although some activated GFAP-positive cells were seen soon after cordotomy, they quickly disappeared (Fig. 3).
Fig. 2.
(Control group) GFAP immunostaining after spinal cord transection in the severed spinal cord. a Three days after surgery. b Seven days after surgery. c Three weeks after surgery. d Five weeks after surgery. Glial reaction was increased from 3 days after surgery to 5 weeks after surgery. Five weeks after surgery there was significant glial or connective tissue scar formation in the adult rats (scale bar = 100 μm, applicable to all sections)
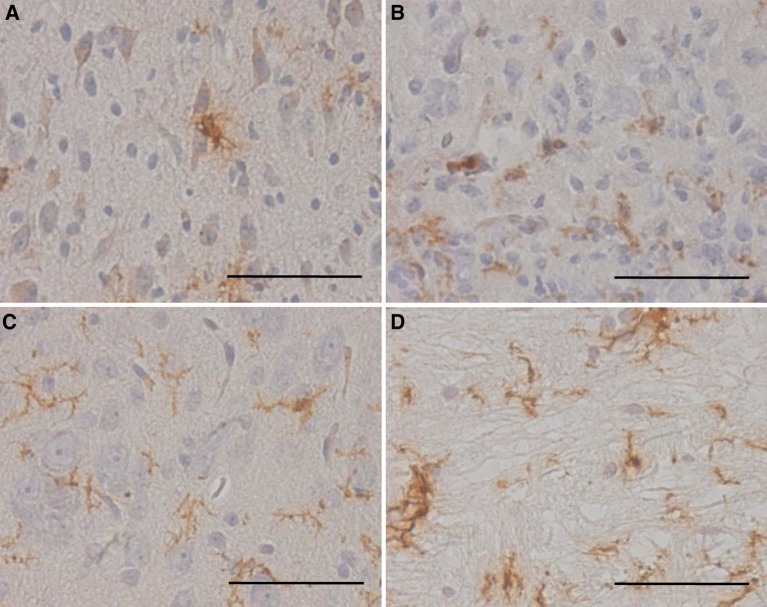
Fig. 3.
(Fetal rat cordotomy group) GFAP immunostaining after spinal cord transection in the severed spinal cord. a Three days after surgery. b Seven days after surgery. The glial reaction was slightly increased. c Three weeks after surgery. d Five weeks after surgery. The glial reaction was restored. There was no significant glial or connective tissue scar formation in the offspring, whereas there was the dense glial or connective tissue scar in adult rats (scale bar = 100 μm, applicable to all sections)
OX-42 staining
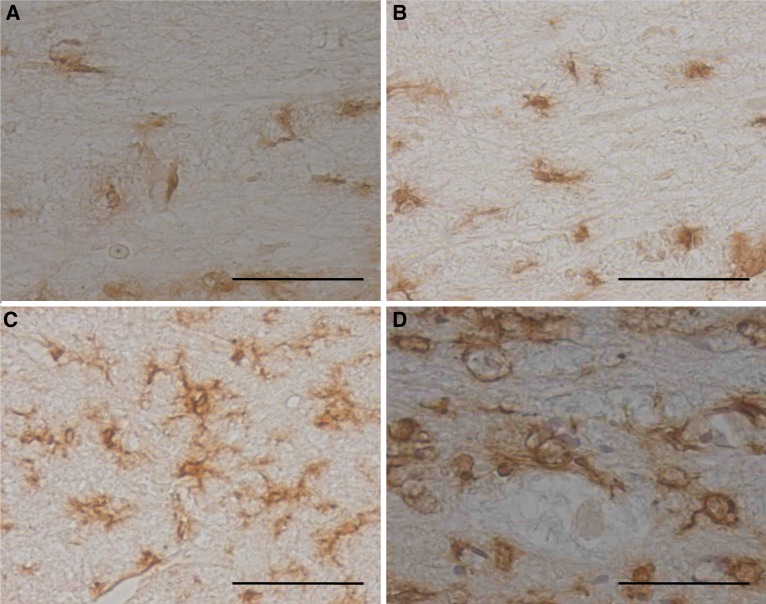
Control group In areas 5 mm cranial and caudal to the severed cord, chronological changes were seen in the morphology of OX-42-positive cells in white matter. While no clear changes were seen on days 3 and 7 after cordotomy, cell processes were extended and enclosed surrounding damaged cells on days 21 and 35 after cordotomy (Fig. 4). Conversely, in the severed spinal cord, semicircular OX-42-positive cells were seen on day 3 after cordotomy, and similar semicircular OX-42-positive cells were detected on days 7 and 21 after cordotomy, but no semicircular OX-42-positive cells were seen around the severed spinal cord on day 35 after cordotomy (Fig. 5).
Fetal rat cordotomy group In areas 5 mm cranial and caudal to the severed cord, OX-42-positive cells rich in cell processes were seen, but the extension and hypertrophy of cell processes in white matter seen with the control group were not observed (Fig. 6). In the severed spinal cord, semicircular OX-42-positive cells with less cell processes were seen on days 3 and 7 after cordotomy, but no semicircular OX-42-positive cells were seen on days 21 or 35 after cordotomy (postnatal days 18 and 32, respectively (Fig. 7). On day 3 after cordotomy, around the severed cord, three types of microglia were observed: small cells with irregular cell processes that exist; semicircular cells without cell processes; and cells that fall somewhere between the above two types (Fig. 8).
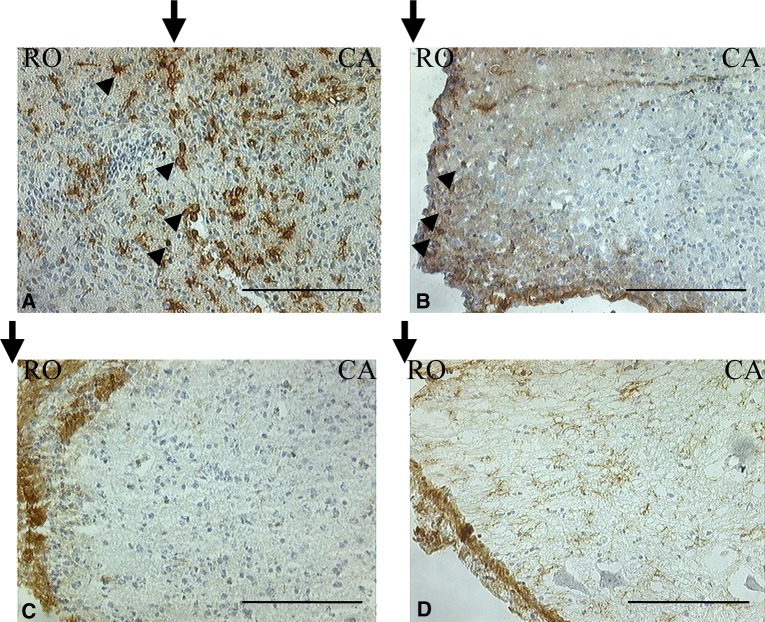
Fig. 4.
(Control group) OX-42 immunostaining after spinal cord transection in areas 5 mm cranial and caudal to the severed cord. a Three days after surgery. b Seven days after surgery. In the white matter OX-42-positive cells were not activated. c Three weeks after surgery. d Five weeks after surgery. In the white matter OX-42-positive cells were activated. Fine antler-like cytoplasmic processes are identifiable. These microglia were characterized by plump terminal knob formations containing myelin debris at the periphery of their antlers (scale bar = 50 μm, applicable to all sections)
Fig. 5.
(Control group) OX-42 immunostaining after spinal cord transection in the severed spinal cord. Small arrowheads show OX-42-positive round cells. a Three days after surgery. b Seven days after surgery. c Three weeks after surgery. OX-42- positive round cells were observed. d Five weeks after surgery. OX-42-positive round cell was not observed (scale bar = 100μm, applicable to all sections)
Fig. 6.
(Fetal rat cordotomy group) OX-42 immunostaining after spinal cord transection in areas 5 mm cranial and caudal to the severed cord. a Three days after surgery. b Seven days after surgery. c Three weeks after surgery. d Five weeks after surgery. In the white matter OX-42-positive cells were not activated (scale bar = 50 μm, applicable to all sections)
Fig. 7.
(Fetal rat cordotomy group) OX-42 immunostaining after spinal cord transection in the severed spinal cord. Arrows show the lesion sites of the spinal cord. Small arrowheads show OX-42- positive round cells. a Three days after surgery. b Seven days after surgery. c Three weeks after surgery. OX-42-positive round cells were observed. d Five weeks after surgery. OX-42- positive round cell was not observed. (CR cranial, CA caudal) (scale bar = 200 μm, applicable to all sections)
Fig. 8.
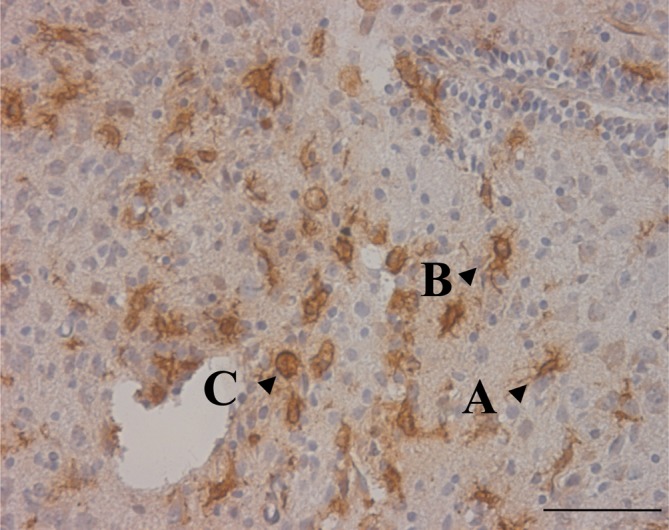
(Fetal rat cordotomy group) OX-42 immunostaining after spinal cord transection in the severed spinal cord. (Three days after surgery.) Ramified-type, intermediate-type, and macrophage-like cell. On day 3 after cordotomy, around the severed cord, three types of microglia were observed: small cells with irregular cell 0processes that exist; semicircular cells without cell processes; and cells that fall somewhere between the above two types (scale bar = 50 μm, applicable to all sections)
Quantitative assessment of OX-42 staining
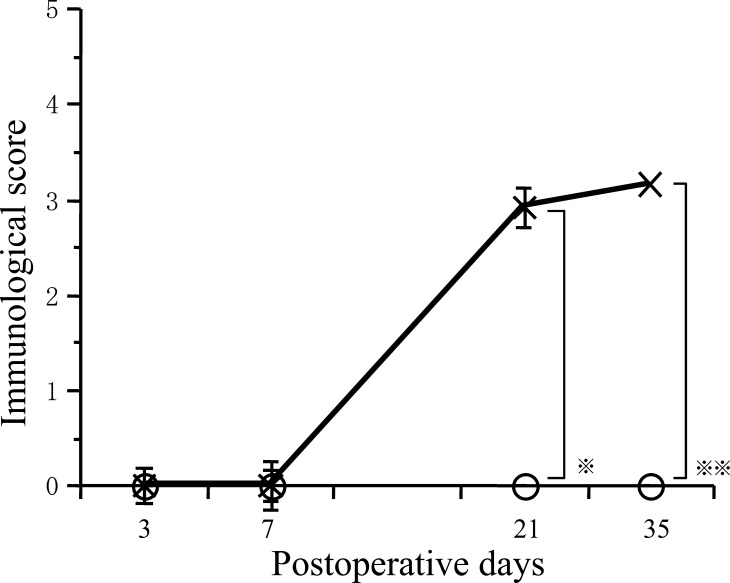
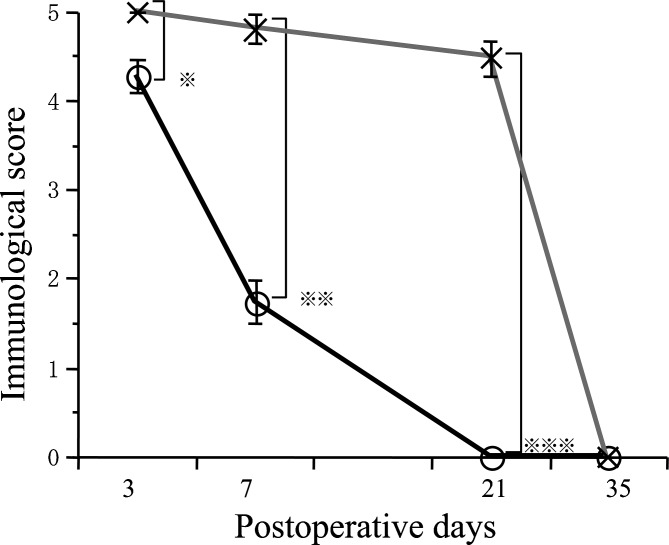
OX-42-positive cells in areas 5 mm away from severed cord Immunological score for the control group increased significantly over time (P< 0.01) (Fig. 9). Immunological score for the control group was significantly higher than that for the fetal rat cordotomy group on days 21 and 35 after cordotomy (P< 0.01). Compared to the fetal rat cordotomy group, immunological scores increased significantly more over the time in the control group.
OX-42-positive cells in the severed cord For both control and fetal rat cordotomy groups, immunological scores decreased significantly over the time (P< 0.01) (Fig. 10). Immunological scores for the fetal rat cordotomy group on days 3, 7, and 21 after cordotomy were significantly lower than those for the control group (P< 0.01). Semicircular OX-42-positive cells (macrophage-like cells) disappeared much more quickly from the fetal rat cordotomy group when compared to the control group.
Fig. 9.
Postoperative course of immunological score in areas 5 mm cranial and caudal to the severed cord. Immunological score of control group were significantly higher than fetal surgery group on day 21 (* P< 0.01) and on day 35 (** P< 0.01) (O: Fetal rat cordotomy group, ×: Control group)
Fig. 10.
Postoperative course of immunological score in the severed spinal cord. Immunological scores of control group were significantly higher than fetal surgery group on day 3 (* P< 0.01), on day 7 (** P< 0.01) and on day 21 (*** P< 0.01) (O: Fetal rat cordotomy group, ×: Control group)
Discussion
Scar tissue formation following cordotomy
Wound healing during the fetal period lacks inflammation and scar formation. Dixon [9] investigated burn-induced inflammation in fetal rats, and documented that when the skin was burned after day 18 of gestation, monocytes and polymorphonuclear leukocytes were seen. However, when the skin was burned prior to day 17 of gestation, these inflammatory cells were not observed. Longaker et al. [19] compared healing of skin cuts between adult sheep and fetuses on days 75, 100, and 120 of gestation. They reported that expression of collagen in fetuses on days 75 and 100 of gestation was faster than that in adult sheep. While normal dermis was formed within 2 weeks for fetuses in days 75 and 100 of gestation, scar tissue was formed within 2 weeks for both adult sheep and fetuses on day 120 of gestation. This suggests maturation of the pattern of wound healing during gestation. As to fetal tissue reactions attributable to central nerve damage, Ikuta and co-workers [15, 20] reported no scar formation following brain injury in fetal rats on day 16 of gestation, and hypothesized that, in fetal rats, scar formation does not form because the cellular structure of the central nervous system is rough, water content is high, and macrophages in the extracellular fluid can rapidly eliminate dead cells. Berry et al. [3] stated that differences in the maturity of fibroblasts play a role in scar formation. In the present study, collagen fibers and scar formation were seen in the severed spinal cords of control group, but scar formation was not seen in the fetal rat cordotomy group. Lack of inflammation and scar formation thus appear advantageous for regeneration of the fetal spinal cord.
Astrocytes
Astrocytes are capable of synthesizing various cytokines, such as NGF and IL-6, neurotrophic factors, and growth factors and they exist between nervous tissue and vessels to transport select compounds required by nervous tissues. In general, when nervous tissue is damaged, astrocytes are activated and proliferated to become reactive astrocytes with large cell processes and rich soma. These cells synthesize neurotrophic and growth factors to play a large role in nerve repair. However, in mature central nervous tissue, reactive astrocytes inhibit repair by forming a glial scar at the injury site, and this functional diversity has formed a cause of confusion [5]. Das [7] hypothesized that glial scar formation associated with spinal injury does not reflect the ability of astrocytes to hinder regeneration, but rather indicates repair failure.
Barrett et al. [1] severed the spinal cords of rat pups, and reported that the spinal cord was not regenerated, and that since hypertrophy and growth of the cell processes and soma of astrocytes was mild, the lack of reactive astrocytes had led to failed spinal cord regeneration. In addition, Oyanagi et al. [20] induced brain injury using fetal rats, and documented that compared to brain injury in adult rats, reactions of astrocytes and level of glial scar formation were much milder.
Compared to the control group, hypertrophy and growth of the cell processes and soma of astrocytes were much milder in the present fetal rat cordotomy group, and while some activated astrocytes were seen soon after cordotomy, they quickly disappeared. Results of the present and past studies [1, 2] seem to suggest that when the spinal cord of fetal rats or rat pups is damaged, astrocytes do not proliferate or become thickened, and reactions are mild. Differences exist in the repair of damaged spinal cord, and several factors could be involved. Although astrocytes play an important role in the repair of damaged central nervous tissue, the repair is not solely dependent on astrocytes, and differences between fetal rats and adult rats in other factors besides astrocytes may contribute to differences in the repairs of damaged central nervous tissue. Furthermore, several subtypes of astrocyte exist, and while some subtypes facilitate nerve regeneration, others hinder this process [21]. Second, different subtypes of astrocytes may be found in adult and fetal rats. Moreover, the degree of involvement of astrocytes in nerve regeneration may change depending on maturity or cellular environment [24]. Third, between adult and fetal rats, the role of astrocytes in nerve regeneration may change over time from facilitation to inhibition. Establishment of a method that can identify astrocyte subtypes and ascertain the function of each subtype is therefore necessitated.
Microglia
Microglia are mildly stained using silver carbonate and can be classified into three types: small cells with irregular cell processes that exist in normal central nervous tissue (ramified microglia); semicircular cells without cell processes that engulf damaged central nervous tissue (ameboid microglia); and cells that fall somewhere between the above two types (intermediate microglia) [8]. In general, ramified microglia are considered static-type microglia, while intermediate and ameboid microglia are considered reactive microglia. In the past, the primary role of microglia was generally accepted to comprise engulfing damaged central nerve tissue, but major histocompatibility complex (MHC) antigens have recently been reported to be expressed on the cell surface, suggesting that microglia are involved in antigen presentation to the central nervous tissue [14]. Furthermore, cultured microglia have been shown to synthesize cytokines such as IL-1 and IL-3, and various functions of microglia have been identified [12]. The OX-42 antibody recognizes a portion of complement iC3b receptor (CR3) and has been utilized widely as a specific surface marker of microglia in the CNS. The cell surface antigens on microglia in the CNS include the Fc and complement type 3 receptors (CR3) that express on microglia of class II antigen.
In the present study, marked differences in microglia reactions were observed between control and fetal rat cordotomy groups. In the control group, reactive microglia were seen in white matter in areas 5 mm cranial and caudal to the severed cord on days 21 and 35 after cordotomy, but no reactive microglia were detected in the fetal rat cordotomy group. Proliferation of reactive microglia in the white matter of mature rats is related to Wallerian degeneration, which occurs after the spinal tract is severed, and microglia are activated to engulf or repair damaged tissue [10, 17]. In the fetal rat cordotomy group, the spinal cord was severed while the spinal neural circuit was still immature. Wallerian degeneration therefore did not occur, and as a result, reactive microglia were not detected. In the fetal cordotomy group, semicircular OX-42-positive cells (macrophage-like cells) were seen around the severed cord soon after cordotomy, but these disappeared within a short period of time. The reason for this might be that these macrophage-like cells quickly engulfed and eliminated damaged tissue. We believe that these macrophage-like cells represent a mixture of macrophages originating from monocytes associated with tissue injury, and microglia that transformed into macrophage-like cells due to nerve damage. Around the severed cord, not only were ameboid microglia observed, but also intermediate microglia with thickened cell processes and rich soma. This suggests that microglia change from ramified microglia to intermediate type, and then from intermediate type to ameboid microglia [17, 25].
Like astrocytes, several subtypes of microglia exist, and while subtypes facilitate nerve regeneration, others hinder this process. In addition, Kreutzberg [18] studied a severed facial nerve model and reported that when nerve cell damage was extensive and many cells died, microglia transform into phagocyte-like cells to eliminate nerve cells. In other cases, microglia protect nerve cells by producing cytokines. Like astrocytes, microglia are involved in repair of damaged central nerve, and various factors can alter their cellular functions.
Between fetal and mature rats, chronological changes in the immunohistochemical reactions of astrocytes and microglia following cordotomy were compared, and the results confirmed many differences. The results of the present study suggest that the presence of activated glial cells around damaged central nervous tissue and the quick disappearance of these cells after injury are important for the repair of damaged central nervous system tissue, and that the role of glial cells in nerve regeneration can change depending on the level of maturity of glial cells or surrounding cells, site of injury, or the state of tissue around the injury. In future, reconstruction of central nervous system tissue might be possible if the diverse functions of glial cells can be elucidated and the effects of various factors on these cells can be determined.
References
- 1.Barrett CP, Donati EJ, Guth L. Differences between adult and neonatal rats in their astroglial response to spinal injury. Exp Neurol. 1984;84:374–385. doi: 10.1016/0014-4886(84)90234-6. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein DR, Bechard DE, Stelzner DJ. Neuritic growth maintained near lesion site long after spinal cord transection in the newborn rat. Neurosci Lett. 1981;26:55–60. doi: 10.1016/0304-3940(81)90425-0. [DOI] [PubMed] [Google Scholar]
- 3.Berry M, Maxwell WL, Logan A, Mathewson A, McConnell P, Ashurst DE, Thomas GH. Deposition of scar tissue in the central nervous system. Acta Neurochir Suppl. 1983;32:31–53. doi: 10.1007/978-3-7091-4147-2_3. [DOI] [PubMed] [Google Scholar]
- 4.Bregman BS, Kunkel-Bagden E, Shnell L, Dai HN, Gao D, Schwab ME.Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors Nature 199532498–501. 10.1038/378498a01995Natur.378..498B [DOI] [PubMed] [Google Scholar]
- 5.Brown BS, McCouch GP. Abortive regeneration of the transected spinal cord. J Comp Neurol. 1947;87:131–137. doi: 10.1002/cne.900870204. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Cao R, Olson L.Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function Science 1996273510–513.1996Sci...273..510C 10.1126/science.273.5274.510 [DOI] [PubMed] [Google Scholar]
- 7.Das GD. Neural transplantation in spinal cord under different condition of lesions and their functional significance. In: Das GD, Wallace RB, editors. Neural transplantation and regeneration. New York: Springer; 1986. pp. 1–62. [Google Scholar]
- 8.Del Rio-Hortega P, Penfield W. Cerebral cicatrix: the reaction of neuroglia and microglia to brain wounds. Johns Hopkins Hops Bull. 1927;41:278–303. [Google Scholar]
- 9.Dixon JB. Inflammation in the fetal and neonatal rat; the local reactions to skin burns. J Path Bact. 1960;80:73–82. doi: 10.1002/path.1700800109. [DOI] [PubMed] [Google Scholar]
- 10.George R, Griffin JW. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol. 1994;129:225–236. doi: 10.1006/exnr.1994.1164. [DOI] [PubMed] [Google Scholar]
- 11.Giovanini MA, Reier PJ, Eskin YA. Characteristics of human fetal spinal cord grafts in the adult rat spinal cord: influence of lesion and grafting conditions. Exp Neurol. 1997;164:523–534. doi: 10.1006/exnr.1997.6703. [DOI] [PubMed] [Google Scholar]
- 12.Giulian D, Baker TJ, Shin L-C, Lachman LB. Interleukin 1 of the central nervous system is produced by amebiod microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman CS. Mechanisms and molecules that control growth cone guidance. Ann Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 14.Hayes GM, Woodroofe MN, Cuzner ML. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci Bull. 1987;80:25–37. doi: 10.1016/0022-510X(87)90218-8. [DOI] [PubMed] [Google Scholar]
- 15.Ikuta F. Neuropathology in brain research. Amsterdam: Elsevier; 1991. The process of brain lesion repair and activity of astrocytes; pp. 211–231. [Google Scholar]
- 16.Iwashita Y, Kawaguchi S, Murata M.Restoration of function by replacement of spinal cord segments in the rat Nature 1994367167–170. 10.1038/367167a01994Natur.367..167I [DOI] [PubMed] [Google Scholar]
- 17.Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995;12:209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- 18.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 19.Longaker MT, Whitby DJ, Adzick NS. Studies in fetal wound healing VII Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–69. doi: 10.1016/S0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 20.Oyanagi K, Yoshida Y, Ikuta F. The chronology of lesion repair in the developing rat brain: biological significance of the pre-existing extracellular space. Virchows Arch (A) 1986;408:347–359. doi: 10.1007/BF00707693. [DOI] [PubMed] [Google Scholar]
- 21.Rugde JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinoda M, Hudson J, Strömberg IL, Hoffer BJ, Moorhead JW, Olson L. Allogeneic grafts of fetal dopamine neurons: immunological reactive and adoptive immunizations. Brain Res. 1995;680:180–195. doi: 10.1016/0006-8993(95)00260-W. [DOI] [PubMed] [Google Scholar]
- 24.Smith GM, Miller RH, Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J Comp Neurol. 1990;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Yamamoto T, Saito N, Kumagai T, Kayama H. Differential activation of microglia after experimental spinal cord injury. J Neurotrauma. 1999;16:255–265. doi: 10.1089/neu.1999.16.255. [DOI] [PubMed] [Google Scholar]