Abstract
The use of polymer-based bioresorbable materials is now expanding to the realm of spinal interbody fusion. Bioresorbable polymers have important advantages over metals, because they are temporary, much less stiff, and radiolucent. Most promising is a group of α-polyesters, in particular polylactide acids (PLAs). Their biocompatibility is excellent, and they have sufficient stiffness and strength to provide initial and intermediate-term stability required for bone healing. However, polylactides have characteristics that make them vulnerable to complications if not properly controlled. Degradation rate strongly depends on polymer type, impurities, manufacturing process, sterilization, device size, and the local environment. The fact that larger implants degrade faster is contra-intuitive, and should be considered in the design process. Also optimal surgical techniques, such as careful bone bed preparation, are required for a successful application of these materials. The purpose of this paper is to highlight the specific properties of these bioresorbable polymers and to discuss their potential and limitations. This is illustrated with early preclinical and clinical data.
Bioresorbable cage technology is just emerging: their time-engineered degradation characteristics allow controlled dynamization in interbody applications, facilitating spinal fusion. Their radiolucency improves image assessment of fusion healing. Acceptance and use of bioresorbable implants may increase as further research and clinical studies report on their safety, efficacy, and proper usage.
Keywords: Bioresorbable, Polymer, Polylactic acid (PLA), Spinal fusion, Interbody cage
Introduction
Spinal fusion surgery in general and the use of interbody cages in particular is rapidly increasing [30, 31]. The increased popularity of interbody devices for spinal arthrodesis is based on (1) a better understanding of biomechanics and spinal pathologies; (2) the widespread availability of CT and MRI, offering accurate, non-invasive assessment of the spine; (3) failure of autogenous grafts as intervertebral spacers and the morbidity associated with harvesting these; (4) technical developments (materials and techniques) in spine surgery. Also secondary factors, like promotion by manufacturers and surgeons involved in the development of such products, contributed to a widespread application of interbody fusion techniques today.
Early reports on interbody cages are mainly positive, but with longer follow-up, the number of complications reported is increasing [99]. Most of these complications are inherent to the fact that the cages are non-degradable, especially when made of metal. First, there is a remarkable mismatch between the mechanical properties of metals and bone. High stiffness decreases graft loading within the cage, a condition calling stress shielding. A metal implant thus slows down the fusion process, eventually resulting in pseudarthrosis, corrosion, wear, and ultimately migration [78, 79, 85, 105]. A second disadvantage of metal implants is that they are radiopaque, and thus eclipse the fusion zone during radiological evaluations [77]. Finally, metals remain as permanent foreign bodies; after their function (stabilization) is completed, a second surgical intervention is sometimes required to remove the implant. When non-resorbable cages are placed in locations difficult to access, repair or dislodgement can be quite problematic [36].
These inherent limitations of current cages gave some impetus for the development of bioresorbable cages: their stiffness is comparable to that of bone, they are radiolucent, and they resorb over time. As such, they also have great potential as drug release systems [115]. Furthermore, biodegradable cage devices eliminate the risks of permanent implants and thus the need for a second intervention to remove the implant. Bioresorbables, however, have their own drawbacks and pitfalls. First, their strength is usually considerably lower than that of metals or non-degradable polymers. Also the brittleness of some frequently used polymers is worrisome. The main concern, though, is the concentration of degradation products like acids and crystals, because very high concentrations may lead to serious tissues responses like inflammation and osteolysis. The local concentration of degradation products depends on their rate of production and their rate of drainage. Therefore, good vascularization of the tissues around bioresorbable implants is of utmost importance. Degradation depends on many factors such as material properties (chemical species, molecular weight distribution, and permeability), implant design (bulkiness and porosity), handling (sterilization and thermal history), and environment (pH and mechanical loading). Slow degradation is desirable not only to maintain the mechanical function of the implant until fusion is obtained, but also to reduce the risk of tissue reactions. Such reactions may occur many years after implantation of the device [9, 13, 16, 95].
Although bioresorbable polymers have been used in orthopedic surgery for more than 30 years [61, 62], bioresorbable polymer implants have only recently been applied in spinal surgery [29, 52, 102]. Despite encouraging initial results in animal and clinical studies, there is little and confusing documentation about this family of materials in the orthopedic and spinal literature. The purpose of this paper is to discuss these issues in order to better understand the potentials and limitations of bioresorbable polymers for spinal cage devices.
Polylactic acids
Polymers are long-chain molecules derived from repeating units, typically with a carbon backbone [103]. When the backbone is hydrolytically unstable, these chains will degrade when placed in an aqueous environment. This material property to degrade over time has led to a variety of medical applications [83]. The most commonly used biodegradable materials are polyesters that are derived from so-called poly(α-hydroxy acids), like poly(lactic acid) (polylactide, PLA), and poly(glycolic acid) (polyglycolide, PGA) [27, 55, 108]. Importantly, lactic and glycolic acid are present in the biochemical pathways of cells and organisms; PLA and PGA thus degrade to natural metabolic compounds. Both polymers have US Food and Drug Administration (FDA) clearance for some applications, and thus are attractive for commercialization and clinical usage. PGA, however, is very unstable and loses its strength within a month. Therefore, it is not a suitable material for a cage device, unless as a minor component in a copolymer. Its main application is in sutures, which only need to be strong for a few weeks [27].
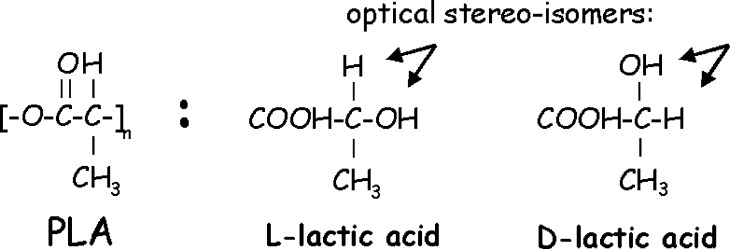
The most useful and applied base material for spinal interbody cages is poly(lactic acid) or polylactide (PLA). PLA can be engineered to possess appropriate mechanical properties and is more resistant to hydrolytic degradation than PGA [62, 108]. Lactic acid exists as two optical isomers, the naturally occurring L (Levorotary) isomer, and the D (Dexorotary) isomer; their polymers are usually referred to as PLLA and PDLA, respectively (Fig. 1). L-homopolymers and D-homopolymers (i.e. polymers consisting of only L-isomers or D-isomers) have the same chemical and physical properties; PLLA is more commonly used, however, because the basic molecule is naturally available (Table 1).
Fig. 1.
Fischer projections of the molecular structure of PLA and its L-isomers and D-isomers
Table 1.
Chemical and physical characteristics of PLLA and PLDLLA from literature and used in own preclinical experiments
| PLLA (literature) | PLLA (own use) | 70/30 PLDLLA (literature) | 70/30 PLDLLA (own use) | |
|---|---|---|---|---|
| Mn (g/mol) | 240500 | 140250 | ||
| Mw (g/mol) | 395500 | 253300 | ||
| D (Mw/Mn) | 1.64 | 1.81 | ||
| Intr. visc. (dl/g) | 2.68 | 1.57 | ||
| Inh. visc. (dl/g) | 2.42 | 1.45 | ||
| Crystallinity (%) | 13.0 | Amorphous | Amorphous | |
| Tg (°C) | 60–65 | 64–67 | 55–60 | 60–62 |
| Tm (°C) | 173–178 | 180 | Amorphous | Amorphous |
| Degr. time (min) | >24 | 36–48 | 12–18 | >12 |
Being isotaetic molecular chains (having side groups on the same side of the backbone), both homopolymers meet the basic requirement to form crystals, and therefore are considered semi-crystalline. Polymers are never fully crystalline [108]; the naturally occurring form PLLA, for example, is about 37% crystalline [115]. When L-isomers and D-isomers are co-polymerized in equal proportions (i.e. chains are formed consisting of equal numbers of L-isomers and D-isomers), a racemic polylactide is formed. Its molecular chains cannot easily pack together to crystallize, because the side groups are located on both sides of the polymer backbone; consequently, racemic polylactide (P-L-DLA) is entirely amorphous. Non-racemic copolymers are usually mixed from L-lactide and a racemic (50:50) mixture of D-lactide and L-lactide:PLDLLA. 70:30 PLDLLA, for example, also referred to as 70/30 PLDLA—indicates the molar ratio of L-lactide (70%) and the racemic DL–lactide mixture (30%). This polymer thus contains 85% L-isomers and 15% D-isomers of lactic acid. Less common mixtures in the orthopeadic literature are 80:20, 85:15, and 96:4.
Characteristics of polylactides
One of the greatest advantages of polymers is that they can be produced with a broad range of physical and chemical properties. This allows engineering products with particular properties tailored to specific needs. On the other hand, it makes it difficult to compare polymers, because many parameters, including thermal history, implant design, and application environment influence their behavior. Polymers with the same composition and molecular weight can still have different properties. There are several parameters, though, that help to characterize polymers like PLA, the most important ones being crystallinity and average molecular weight (or, alternatively, inherent viscosity). Other relevant parameters are molecular weight distribution (polydispersity), impurities (such as residual monomers, water, and free radicals), and glass transition temperature.
With all other parameters constant, a polymer with higher crystallinity will be stronger and stiffer, and typically degrade at a slower rate. Also the glass transition temperature is higher with increasing crystallinity. These are important factors for spinal cages, which need to sustain high dynamic loads and need to function at 37°C for at least 6 months. Polymers like PLLA crystallize easily, due to their symmetric repeat unit and the flexibility of the long chain. Crystallization begins at multiple points along the chain, or at impurities within the polymer. Amorphous or disordered chains are entrapped between the crystallites, thus creating amorphous regions; consequently, the whole polymer is semi-crystalline. Crystalline regions in the polymer have stronger secondary bonds between the chains of the polymer, and make it difficult for water to penetrate, which makes these regions degrade more slowly. Racemic polylactide, which is entirely amorphous, degrades within months, whereas high-crystalline PLLA has been reported to require more than 4 years to degrade [16].
The second factor that has major impact on the properties and degradation kinetics of polymers is molecular weight. Polymer strength increases with molecular weight by the formation of secondary bonds between the chains and by the entanglements in the structure. Degradation occurs more slowly, because more secondary bonds have to be broken per chain. Molecular weight can be described in several ways, most commonly as number average molecular weight (Mn), and weight average molecular weight (Mw). Mn simply describes the average molecular weight of all chains in the polymer. Mw is more indicative, however, because it is related to the viscosity of the polymer in melted condition, and thus relevant for its processability in, for example, injection molding. In Mw, polymer chains are weighed by their weight fraction in a linear fashion. Clearly, not all chains in a polymer sample have the same length, but a range of molecular weights exists, a property called polydispersity. A simple measure for polydispersity is the ratio between Mw and Mn; for polylactides produced under well-defined conditions, polydispersity is generally lower than two. Large polydispersity indicates a low number average molecular weight (Mn), and thus a large number of small molecules. This reduces the resorption time of the polymer, because small molecules are more easily degraded than large ones. Low polydispersity thus is an important quality for bioresorbable polymers, because it indicates a smaller spectrum of molecular masses and allows a better prediction of the degradation kinetics. Residual monomer content is related to this, because it gives the number of monomer molecules that have not been polymerized; it is a key quality for polymers and well controlled by the manufacturers. A general method to determine the entire molecular weight distribution is gel permeation chromatography (GPC, also known as size exclusion chromatography (SEC)), which gives the whole spectrum of weight distribution, from which Mn, Mw, polydispersity, and monomer residue can be derived [103]. Mw is also related to intrinsic viscosity, and thus also can be derived from experiments with a viscometer.
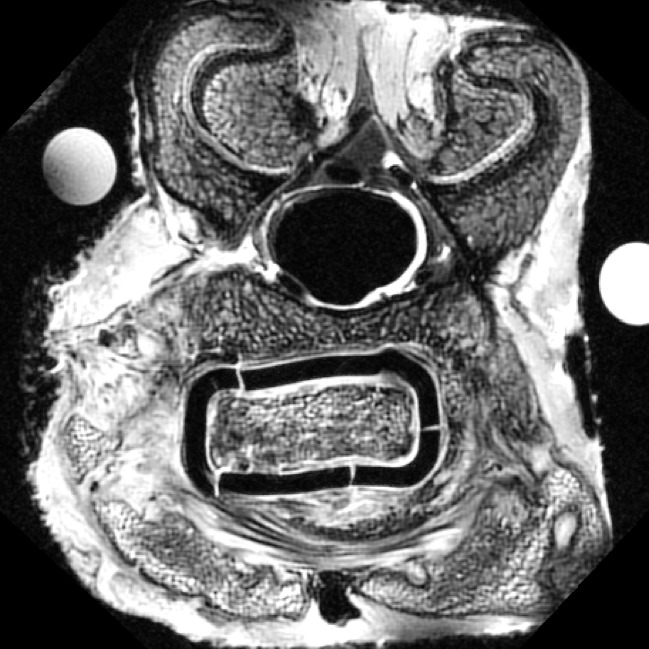
The glass transition temperature is another important—but not independent—property of polymers. Glass is defined as a material with the mechanical properties of a solid, but the molecular properties of a liquid, that is, with a disordered, non-crystalline structure. It has a glass transition temperature (Tg), above which the amorphous part of the polymer can flow, albeit not as freely as in a molten state. Polymers above Tg have a Young’s modulus orders of magnitudes lower than in their glass condition. This is important for bioresorbable interbody cages, as these devices must maintain their mechanical integrity for a long period of time, and require a Tg well above body temperature. Tg’s of polylactides are typically in the range of 55–60°C [65], but water can act as a plasticizer, which might result in a decrease of Tg even below body temperature (37°C) [108]. Tg also depends on the time-scale of mechanical loading [103]: deformation of a glass strongly depends on the motion of single chains, and in the case of an impact load, the time is simply too short for conformational deformations to occur. However, under long-lasting loads such as in the spine, the material effectively starts to creep, resulting in considerable deformations of the implant as well as micro-fractures (Fig. 2) [59]. This effect is stronger for materials with lower Tg, which thus should be well above body temperature.
Fig. 2.
Transverse micro-MRI section of a spinal goat segment at 3 months follow-up, showing plastic deformation and micro-fracturing of an interbody cage made of 70:30 PLDLLA [62]. Plastic deformation indicates that the polymer has experienced continuous loading at temperatures above the glass transition temperature (Tg). The micro-fractures indicate that the strength of the cage is now below the spinal loads in the goat in vivo
The very existence of a rubber phase in polymers also has implications for the production and handling of degradable polymers. Slowly cooling down a molten polymer below the melt temperature (Tm) can lead to crystallization. The amorphous part of the substance, however, maintains their liquid behavior until Tg is reached, below which molecular motion effectively ceases. If cooling is rapid, and/or molecular weight (i.e. viscosity) is high, crystallization is limited with small crystals and the resulting polymer becomes more glassy (amorphous) and—consequently—more brittle. Cooling rate—for example, after injection molding—thus strongly determines crystallinity and consequently affects mechanical strength, stiffness, and degradation rate. The same applies to periods of increased temperatures during handling or sterilization.
All peculiarities discussed above are of great importance to control the properties of polylactides and their behavior in vivo. Literature is sometimes incomplete or even misleading because authors do not exactly define the polymers they worked with. In the biomedical field, any scientific contribution dealing with polylactide composites should mention the identity of the investigated compounds as accurately as possible, starting at the level of the gross chain composition (raw material), and describing the relevant biomechanical, biochemical, processing, and sterilization characteristics, a requirement underlined over 20 years ago and still not respected [109].
Production and processing
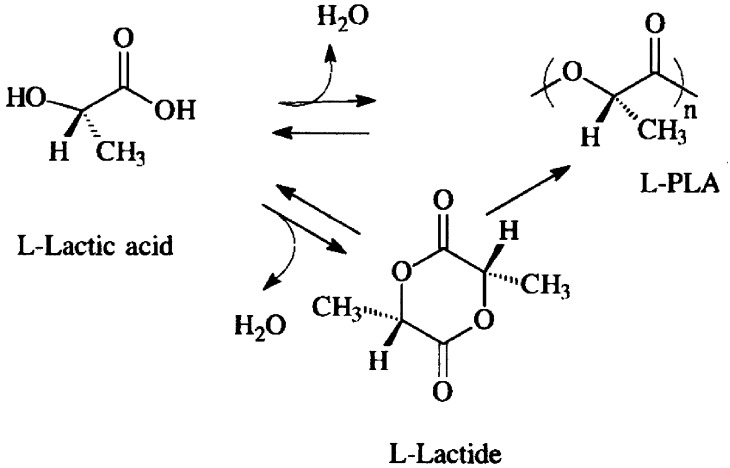
Nearly all polylactides for medical device application are obtained from only two suppliers: Purac (Gorinchem, The Netherlands) and Boehringer (Ingelheim, Germany). The basic compound for the production of polylactides is lactic acid, a molecule manufactured through fermentation of sugars. Many microorganisms produce lactides, but genetically engineered strains of, for example, Lactobacillus and Escherichia Coli are particularly useful due to their ability to selectively produce D-isomers or L-isomers [21, 118]. As the physical properties of polylactides critically depend on their isomeric composition [110], production by fermentation is preferred over the production by chemical processes, which only yield racemic mixtures [21]. Polymers can be produced either by direct condensation of lactic acid, or by ring-opening polymerization of the cyclic lactide dimer (Fig. 3) [40, 68, 83]. Lactic acids in cyclic dimers are bound by two ester bonds; one is broken with a catalyst, which stabilizes the other. The resulting molecules are dimeric chains (chains of two monomers) that subsequently join to form polylactides. This process strongly depends on the environmental conditions and is difficult to control, resulting in some variety of polymer properties from batch to batch.
Fig. 3.
Polymerization routes to polylactic acid. Note that water (H2O) is released during this process. This should be removed in order to prevent the reverse process of hydrolysis
Fabrication of raw PLA material into devices can be accomplished in many ways, for example, by direct machining, compression molding, or melt spinning, and hot drawing. Devices directly machined from a block of raw PLLA exhibit better mechanical properties than a specimen prepared by a process in which the raw polylactide is molten again. This is due to the degradation of the high-molecular weight polymers under high temperatures. Melt spinning and hot drawing, for example, may reduce the molecular weight of PLLA by as much as 85% [51]. The main complication during processing, however, is molecular weight decrease due to the hydrolytic sensitivity of the polymer bonds. The presence of moisture during processing reduces molecular weight and thus the final properties. PLA processing also proves to be very sensitive for high temperatures and shear stresses; this makes high molecular weight polymers, which have high-melt viscosities and melt at higher temperatures, more difficult to handle.
Sterilization
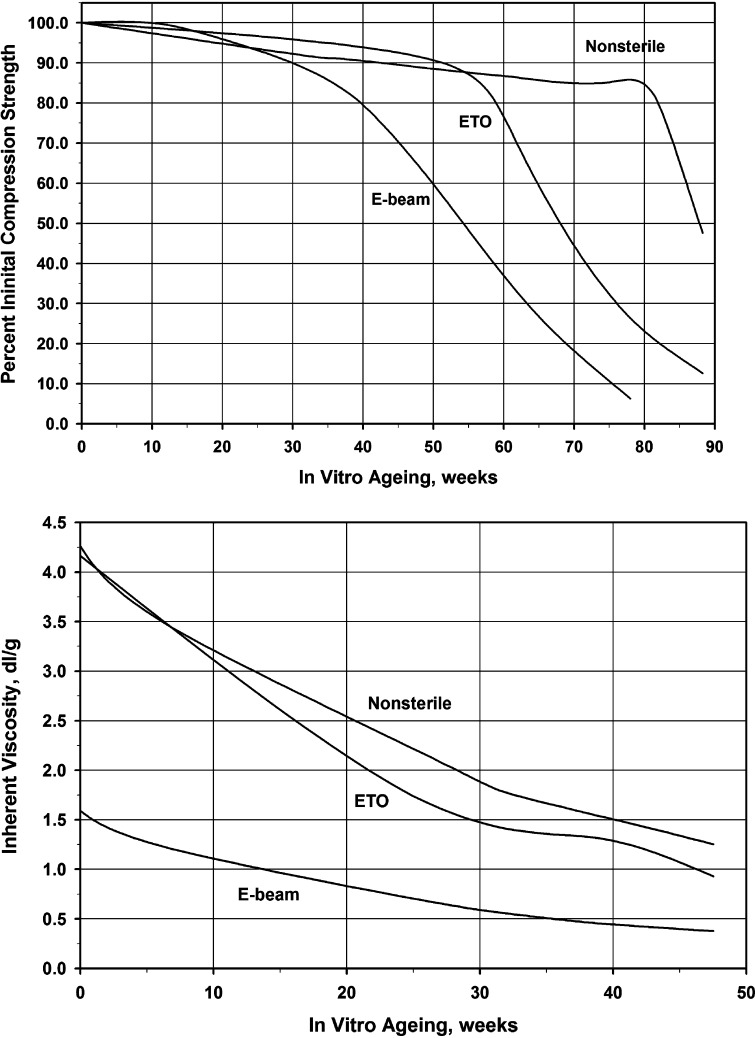
Sterilization can have dramatic effects on the physical and mechanical properties of polymers (Fig. 4), and the most common methods are most harmful. Hospital steam sterilization techniques, for example, use high moisture and temperatures in excess of 100°C. This exceeds the thermal transition temperatures of most medical polymers, thereby altering their physical and mechanical properties. For this reason they should not be not used for PLA and PGA.
Fig. 4.
Increased degradation of 70/30 PLDLLA as a result of sterilization: a compression strength; b inherent viscosity
Ionizing radiation, either gamma-radiation from 60Co or 137Cs or beta-radiation from accelerated electrons (e-beam radiation), is another way of sterilization. The high-energy particles exert their sterilizing effect by inducing ionizing events in the materials; the released energetic electrons collide with neighboring atoms and create a shower of secondary electrons. These bombard DNA molecules in the harmful microorganisms and induce irreversible damage to inactivate them. However, they also induce severe damage in polymers causing changes in biocompatibility and the biomechanical characteristics. Both, γ-radiation and e-beam sterilization, are known to cause chain scission and cross-linking in PLA, leading to a decrease of intrinsic viscosity and molecular weight (Fig. 4) [6, 67, 83, 84].
Chemical sterilization by gases such as ethylene oxide (EtO) is often used for PLA-based polymers that are sensitive to heat and moisture [83]. However, chemical sterilization can leave residues in harmful quantities on the surface and within the polymer. It is crucial that the polymeric implants are subjected to adequate degassing or aeration following sterilization, so that the concentration of residuals is reduced to acceptable levels [76]. So far, detrimental effects of chemical sterilization using EtO on the mechanical properties of PLA material in vitro and in vivo have rarely been reported [84] (see also Fig. 4).
Plasma sterilization offers several advantages over the other sterilization processes, such as a shorter processing time, minimal effect on material properties, no toxic or carcinogenic by-products, and no disposal problems [38, 84]. Low temperature radio-frequency glow discharge (RGFD) plasma sterilization is reported to have only a limited effect on molecular weight and tensile strength of PLA-based materials [5, 47]. In fact, it is surprising that plasma is not routinely used for the sterilization of polymers; the relative unfamiliarity of plasma sterilization is probably the main reason for that.
The data provided show that information about the sterilization process used is important for appropriate interpretation of the physical and mechanical properties of PLA. Therefore, it is strongly recommended that identification of the investigated compounds includes processing and sterilization protocols and these data should be included in any paper in the field.
Packaging
Because PLA (and related polymers) are naturally hygroscopic and hydrolytically unstable, the presence of moisture can degrade them during processing, after fabrication, and in storage. Therefore, the polymers are quickly packaged and generally double bagged under inert atmosphere or vacuum. The bag material should be very resistant to water permeability. The polymers are typically stored in freezers to minimize the effect of moisture present, but when packaged in desiccated moisture-proof bags they can be stored at room temperature [114]. The polymer package should always be opened at room temperature to minimize condensation and should be handled as little as possible at ambient atmospheric conditions.
Degradation
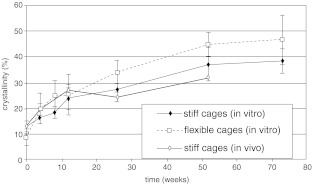
Virtually all polymers are susceptible to degradation by heat, oxidation, mechanical pertubation, hydrolysis, enzymatic action, and radiation. Degradation in vivo can result either from cell-mediated or from chemistry-mediated cleavages of labile bonds (hydrolysis), or both [115]. Principally, there are two mechanisms of hydrolytic degradation [96]: surface erosion and bulk erosion. Surface erosion occurs when water is unable to penetrate the device in large concentrations. The degradation products at the surface of the implant are rapidly dissolved in the surrounding fluid and removed from the bulk polymer. Consequently, the device will shrink in size, which happens to some extent in PLA. However, bulk erosion is much more common in PLA, because these polymers have relatively high porosity and permeability [57, 86]. Hydrolysis first occurs in the amorphous phase, resulting in a reduction in molecular weight within several months (Fig. 4b). Initially there is no loss in physical properties, because the matrix is held together by the crystalline regions; only when water fragments these regions, the physical properties reduce, usually after several months of mechanical integrity (Fig. 4a). When PLA degrades, crystallinity increases, because the amorphous part of the polymer is absorbed first. Also, molecules released from the device are free to move again and may crystallize; this way, crystallinity may increase from an initial 10% to some 40% after several months (Fig. 5). As the polymer degrades further, lactic acid is formed that becomes eliminated through the tricarboxylic acid cycle, primarily as carbon dioxide and water [16]; this results in the final loss of polymer mass, usually after several years.
Fig. 5.
Increase of crystallinity of PLLA as a function of degradation time in vivo
Bulk erosion is a complex phenomenon, depending on multiple factors of different nature. First, it requires penetration of water into the matrix. This is a diffusion process, and as such depends on porosity and permeability of the matrix, as well as the mechanical loading regime: dynamic loading pumps water into the matrix, and simultaneously drains the degradation waste products. This is important, as degradation can be enhanced by the degradation products themselves (autocatalysis); the hydrolysis of PLA (and other α-polyesters) is catalyzed by the presence of carboxylic end-groups [64, 96]. When there is insufficient flow to drain the acidic degradation products from the matrix, the rate of degradation is thus enhanced [1, 3, 45]. Likewise, PLA devices degrade faster when they are less porous [2], less permeable [81], have a bulkier design [39], and function under static loading conditions [1, 97]. Due to these many factors, it is difficult to predict degradation kinetics of a device in a particular application, even if an identical, well characterized, material is used.
The role of enzymatic involvement in the biodegradation of PLA remains controversial [37, 93]. Most literature conclude that erosion of these materials occurs strictly by hydrolysis, with no enzymatic involvement [88]. However, other investigators suggest that enzymes do play a role in the breakdown of polylactides and related polymers [33, 100, 112, 113]. Much of these speculations are based upon the differences observed between in vitro and in vivo degradation rates: phenomena like adsorption of proteins, absorption of lipids, and greater solubility of lactic acid-based oligomers in blood have been suggested of sources of difference [44, 90]. Some evidence for degradation by biologic activity was observed with tiny crystalline particles, which were phagocytosed by macrophages to undergo intracellular degradation [35, 93].
Biocompatibility and toxicity
In order to be biocompatible, bioresorbable polymers should be free from potentially toxic or carcinogenic residual monomers, stabilizers, polymerization initiators, solvents, and emulsifiers. The same applies to the leachables, degradation products, and subsequent metabolites. In general, PLA (and related polymers like PGA) have demonstrated good biocompatibility and absence of significant toxicity, although some reduction in cell proliferation has been reported on PLA in vitro [106, 107]. Also, it has been reported that PLA produces toxic solutions in vitro, probably as a result of the acidic degradation products [94].
When a foreign material is implanted in the body, the surrounding tissues are compromised, and the inflammatory response is activated to heal the damage. The first step in this inflammatory response is opsonization, a process of coating with plasma proteins like immunoglobin G (IgG), which makes the device a target for neutrophils and enhance the adhesion for phagocytic cells [48]. Neutrophils are forerunners of macrophages and multinucleated giant cells, and in fact trigger them when they die. Macrophages, like neutrophils, release free radicals and degradative enzymes, such as acid phosphatase and lactic dehydrogenase (LDH), which can affect both the implant and the surrounding tissues. Local reduction in pH may be responsible for adverse tissue reactions such as osteolysis: osteoclasts—cells that are responsible for bone resorption—become more active at lower pH [92], and thus may resorb the bone around the implant, leading to clinical failure.
In early literature, between 0% and 22% of patients treated with degradable PLA or related degradable polymer implants develop irritation at the implant site, a sterile sinus, or osteolysis [14, 20, 23]. However, bacterial cultures of the drainage routinely tested negative, indicating that the biological response was a consequence of the chemical irritation accompanying acidic polymer degradation products [15].
PLA and PGA have found to exhibit sufficient biocompatibility with bone [75], but studies involving sensitive tissues such as the dura mater and nerve roots show differences. PGA-based polymers become inseparable from the dura only 2 weeks after implantation [34]; this effectively disqualifies PGA for use in intervertebral cages. PLA materials, however, did not adhere to the dura [28, 34], and no adverse events of the dura were noted histologically [63, 72]. PLA-based material has also been shown to be biocompatible with neural cells: proliferation of spinal cord Schwann cells is not affected by the material itself or its by-products during degradation and has no effect on neuronal cells, non-neuronal cells, and axon growth [34]. In addition, PLA is biocompatible with nerve roots and peripheral nerves; polymer tubes are actually used for reattachment of nerve stumps [28, 89]. PLA thus seems to be a more appropriate material for intervertebral cages than (copolymers of) PGA, not only in terms of degradation time, but also in terms of biocompatibility.
Cage design
It has been shown that polymer degradations kinetics is influenced by the implant design itself [3, 39, 41]. Specifically, greater (wall) thickness of a polymer cage device may lead to a faster degradation rate. To illustrate this, inappropriate wall thickness may well explain the disappointing results of an in vivo animal study using vertical PLDLLA ring cages with a wall thickness of about 5 mm to induce a cervical spinal fusion [54]. The authors saw rapid disintegration, collapse, and tissue reaction after a follow-up of only 12 weeks, a phenomenon not seen in a long-term in vivo animal study (up to 24 months) using PLDLLA cages with thinner walls [101]. However, factors not described in their study, such as the processing and fabricating technique, type of sterilization, and material-related parameters may have played a role in the premature degradation of their cage and the concomitant tissue reactions [4, 49, 108]. To further emphasize the importance of using appropriately sized PLA implants, Eitenmuller et al. [32] showed a significant difference in the percentage of aseptic tissue problems between patients treated for ankle fractures with a bigger PLA plate and screw system (52%) as compared to a similar but volume reduced bioresorbable fixation system (0% aseptic tissue reaction). Bioresorbable cages thus should be made with the least amount of material and be as small as the mechanical specifications allow. A high content of (semi-crystalline) PLLA or smart processing methods to enhance the mechanical properties of the amorphous PLAs is helpful in meeting these requirements.
Surgical technique
Providing a satisfactory environment for fusion is vital for clinical success. To enable fusion, a sufficient number of potentially osteogenic cells is necessary [11]; therefore, bleeding bone should be present next to the graft. MacNab [82] suggested that unless a massive graft is used to replace the entire excised disc, a fusion is likely to fail, because it is easier for fibrous tissue from the remnants of the disc to invade the graft than it is for bone to grow from one vertebra to another. McAffee et al. [80] illustrated this in a clinical study: 100% fusion rate with a BAK cage had only been achieved when the patients underwent complete discectomy and thorough endplate cleaning, whereas 16% of the patients who had partial reamed channel discectomy with the BAK implantation required revision surgery because of pseudarthrosis or cage displacement.
Complete disc excision and thorough removal of the cartilage endplate down to healthy bleeding bone are even more important when using a polymer cage device. Both, disc material and endplate are avascular tissues; incomplete removal precludes sufficient vascularity, thereby creating an unfavorable environment for the degradation of the polymer. However, a dilemma arises: to maximize graft and polymer contact with high-quality bony bed, greater amounts of the endplate need to be excised and larger cages need to be used. This bears a risk for destabilization of the bony endplate with potential secondary subsidence. Hollowel [46] reported that the endplate did not increase the resistance significantly when tested in compression until failure; Closkey [24] agreed but showed that a minimum contact area was needed to avoid subsidence. However, Steffen [94] and Polikeit [87, 91] found that placement of cages in the central area of the vertebral body, whether the endplate has been removed or not, could cause early failure. More recently Lowe et al. [70] showed that the central region of the endplate provided the least resistance. There was a significant reduction in the vertebral strength with the complete removal of the endplate. However, partial anterior third removal of the endplate showed marginal decrease in compressive strength, while still a highly vascularized environment for fusion could be created. This scenario could be ideal when using PLIF or TLIF technique with small bioresorbable interbody spacers.
PLA cages: clinical experiences
At present there is limited clinical experience with bioresorbable interbody spacers; publications are based on the Hydrosorb copolymer of polylactide, the 70:30 poly(L-D,L-lactide) polymer (distributed by Medtronic Sofamor Danek, Memphis, TN). In 2002, the first clinical results of the use of vertical cylindric bioresorbable cages have been reported [69, 71]. A series of 60 patients underwent the TLIF procedure using Hydrosorb vertical mesh cages. There were no cage-related complications, but the mean follow-up was very short (4.7 months). Kuklo et al. [60] reported a 87% fusion rate observed on lateral radiographs and a 97% rate on CT-scans at a mean follow-up evaluation of 12.4 months using a Hydrosorb vertical cylinder cage packed with recombinant bone morphogenetic protein rhBMP2 (Infuse; Medtronic Sofamor Danek) for single and multiple-level TLIF procedures over a period of 18 months. In patients who underwent repeated CT scanning, the fusion mass appeared to increase in time, whereas the disc space height remained stable. They noted no infections or complications related to the cages. In another single institutional study using the same approach and the same bioresorbable cage but packed with iliac crest autocraft, an equivalent percentage of solid fusion (96.8%, 30 out of 31 patients) after a mean follow-up 18.4 months was achieved [25].
Limitations of the above-mentioned studies are that (1) they include a relative small number of patients; (2) single and multilevel surgeries are performed in a patient group having heterogeneous spinal pathologies; (3) there is no control group with non-resorbable implants; (4) there were relative short follow-up periods. In addition, none of the authors mentioned the degradation of the cage in their studies, although the mean follow-up duration in the study performed by Coe [25] equalized or exceeded the predicted biological life expectancy of this material (12–18 months). Nevertheless, early research into this bioresorbable cage technology is promising, and longer follow-up periods with appropriate selected patients are needed.
Future directions of biobasorbable PLA-based cages
Although implants made of PLA generally perform well, there are technical developments addressing certain risks associated with the material. Most importantly, PLA degradation can result in low local pH, with subsequent risks for inflammation and osteolysis. The incorporation of a long-acting buffer such as hydroxylapatite (HA) or tricalcium–phosphate composites effectively controls the rates of acids generated from polymer hydrolysis and at the same time acts as an osteoconductive scaffold [43]. Studies with PLA and calcium phosphate indicate that the addition of the inorganic phase decreases the mechanical characteristics of the composite, but does not change degradation time [50]. The reduction in strength may be due to difficulties in forming a true composite of polymer and ceramic, especially once hydrolysis begins. When using HA, neutralization of the acids increases the projected resorption time of the composite device [43] and exhibits a more controlled decrease in mechanics.
A second general problem of PLA is its intrinsic brittleness, with the risk of fracturing the device during surgery. Materials can be made stronger by using larger molecular weights or by self-reinforcing fibers, but more promising is the addition of a small volume percentage of trimethylene carbonate (TMC), an amorphous polymer with a low glass-transition temperature (approximately −20°C) [117]. TMC makes PLA more ductile, thus increasing the resistance to impact forces, for example, from hammering at insertion of the implant. Other resorbable polymers with low glass-transition temperatures may show the same effect, however, risk faster degradation.
Another reported development of PLAs in spinal surgery are the use of bioresorbable cages in combination with bioresorbable rods [10, 58]. Both studies clearly demonstrate the feasibility of such a combination; further studies should also be initiated in larger animal models.
At present there are few preclinical and clinical investigations on the effect of bone growth stimulation on fusion involving cages: such studies are underway using bioresorbable cage devices in combination with bone graft substitutes such as synthetic carriers, bone morphologic proteins (BMP) or a combination of both [66, 101]. Preclinical in vivo experiments demonstrated good fusion results using rhBMP-2 sponge in combination with a PLDLLA threaded cage in a small animal study [101] and clinically in a study using a Hydrosorb vertical cylinder cage [60]. Other potential bone graft substitutes that may generate interest in combination with bioresorbable cages include other growth factors such as recombinant human osteogenic protein-1 (rhOP-1) [26], local gene therapy [12], and stem cells [42]. The introduction of graft substitutes may help curb many of the complications associated with iliac crest bone harvesting, while offering the ability to assess fusion success radiographically. Although promising, much more preclinical in vitro and in vivo research with larger groups, prospective randomized studies and longer follow-up periods are needed to come to valid conclusions.
Discussion
Since Bagby’s [7] initial experiences with a stainless steel basket in cervical spinal fusions, there has been a rapid evolution in the technology of interbody fusion cages, using various designs and materials [73, 111]. Metal and titanium cages devices provide adequate mechanical stability and withstand loading with ease; however, a major disadvantage of these cages is that their modulus of elasticity is much higher as compared to vertebral bone tissue, leading to stress shielding within the cage [53, 105]. In addition, micro-motion through the motion segment before interbody fusion is unavoidable and may lead to particle debris [98, 99]. Even though these materials have superior mechanical strength and stiffness, they may fail if interbody fusion is not obtained and stresses on the implant and graft are high enough. Finally, metallic cage devices interfere with visualization of interbody fusion on radiographs, CT and MRI. With the introduction of carbon fiber reinforced cages and more recently polyetheretherketone (PEEK) cages, a major step in cage technology was taken with good clinical success [17–19, 22]. Both materials have a modulus of elasticity approximating that of cortical bone and are radiolucent. However, in a retrieval of failed cages, it could be shown that carbon fiber cages had significantly more debris in the surrounding tissues than failed metal cages, although no histological evidence of bone resorption or inflammation was found [99].
With the use of newer generations of PLA and related polymers, early concerns about sterile abscess or sinus tract formation, osteolysis, allergic reactions, or hypertrophic fibrous encapsulation have almost been eliminated [8, 9, 116]. The most important improvement in this respect has been the increase of molecular weight in the final sterilized device, which slows down the degradation rate and thus the temporal concentration of degradation products (crystals and lactic acids). Also the increased purity of the polymers reduced the risk for adverse reactions described in earlier studies. Another advantage of these new bioresorbable materials as interbody spacers is that they confer initial and intermediate-term stability that is adequate for spinal interbody healing. This is followed by gradual resorption after biologic fixation has occurred [104]. As they slowly degrade, the load is gradually transferred to the healing bone and the void filled with bone. These potential advantages, however, require the right type of polymer in terms of biological and physical properties by primary molecular weight, crystallinity, residual monomers, purity, size, geometry and thickness of a device and by considering the appropriate fabrication and sterilization method [74]. Nevertheless, further research into this bioresorbable cage technology is needed with longer follow-up periods and appropriately selected patients. At present, the best bioresorbable material or a combination of bioresorbable materials, the optimal cage stiffness, and the desired period over which the cage should biodegrade are unknown. In addition, it is unclear what kind of tissue reaction will occur when a cage fails and there are no clear guidelines with respect to the type of treatment in such events.
What has become clear from current preclinical and human clinical studies using bioresorbable polymer cage devices is that technical expertise is crucial for the outcome. Preparation of the intervertebral implant site is important: it may be anticipated that complete discectomy rather than partial reamed channel discectomy should be performed. In addition, packing bone in between the cages and anteriorly is recommended to enhance fusion. In all patients posterior instrumentation to supplement the interbody devices is mandatory. Supplement pedicle screw fixation not only greatly enhances the stability of the intervertebral device, but also enables restoration of lordosis [56]. The duration of follow-up required to rule out cage subsidence, the fate of the void left by the cage after bioabsorption, and late complications due to the resorption of the cage has not been determined, but likely must be more than 4 years. Future follow-up studies will help to delineate the truly successful cage design.
Conclusions
The use of bioresorbable cage devices in the field of spinal surgery is a novel, but well-anticipated approach that is only beginning to develop. The particular situation in which a specific polymer material may be applied might be very limited; for example, polymers used for stand alone interbody devices should have different physical and chemical properties than those used in combination with a supplement internal fixation device. Tailoring of specific functional properties of the biodegradable polymers can be contrived in the laboratory to render a particular, unique task. However, it is essential that uniform standards for quality estimation are defined to enable comparison of the different polymer materials. Essential is to know specific details of the chemical composition (i.e. copolymer ratios), fabrication process, crystallinity, inherent viscosity and/or molecular weight, glass-transition temperature, sterilization method, monomer residue, yield strength, ultimate tensile strength, tensile or flexural modulus, and the degradation time. Reporting this information will lead to a more effective further development and prudent application of these materials as an impotant component of interbody fusion cages.
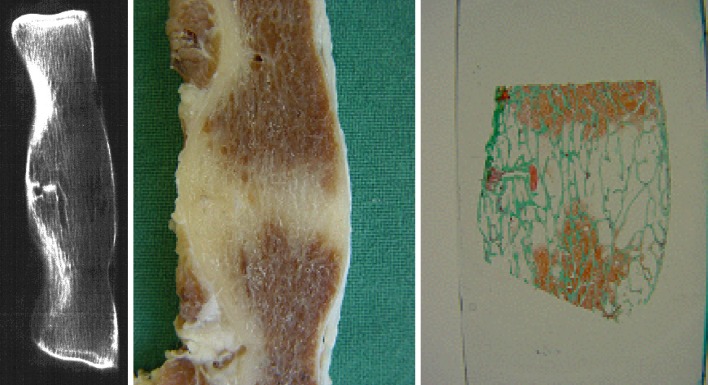
Last but not least, surgeons wishing to perform interbody procedures using bioresorbable cage devices should understand the fundamental differences between the non-resorbable and bioresorbable cages and should be properly trained in patient selection, surgical technique and correct device handling and placement. Spinal fusion with bioresorbable cages can be reached within months, but safe degradation of the implant is a process of years (Fig. 6). Once that has been achieved, however, the risk for late complications is eliminated.
Fig. 6.
Radiographic, macroscopic specimen and histology of a completely fused spinal segment, 48 months after inserting a PLLA cage in a goat model. Pictures show complete resorption of the cage, its void replaced by bone, and a well-aligned trabecular bone architecture
Glossary
- Biodegradation
Strictly: enzymatically promoted degradation. More loosely also referring to degradation to smaller fragments due to chemicals inside the body
- Bioresorbable
Degradable in the presence of chemicals in the body other than enzymes, generally referring to hydrolytically unstable polymers like polylactide
- Biocompatible
Having no toxic or injurious effects on biological systems and having an acceptable amount of tissue reactions to the material (qualitative term)
- Biomaterial
A material intended to interface with biological systems to evaluate, treat, augment, or replace any tissue, organ, or function of the body; includes both temporary and permanent devices, and natural tissues like autograft
- Biostability
Resistance of an implant to chemical or structural degradation
- Polymer
Long-chain molecules derived from repeating units
- Monomer
Single molecule with the ability to polymerize
- Isomer
A chemical species with the same number and types of atoms as another species, but with different configuration. There are structural isomers, geometric isomers, optical isomers, and stereoisomers
- Glass
Material with the structural properties of a liquid, namely disorder, and the mechanical properties of a solid
- PLA
Poly(lactic acid), also referred to as polylactide
- PGA
Poly(glycolic acid), also referred to as polyglycolide
- PLLA
Poly(L-lactic acid), polymer of L-lactic acid units only
- PDLA
Poly(D-lactic acid), polymer of D-lactic acid units only
- PLDLA
Poly(L,D-lactic acid), racemic (50/50) mixture of L-lactic and D-lactic acid
- x/y
PLDLLAMixture of x% L-lactic acid and y% 50/50 (racemic) D,L-lactic acid
- Mn
Number average molecular weight
- Mw
Weight average molecular weight
- Monodisperse
Property of polymers where all the polymer chains have the same chain length
- Polydisperse
Property of polymers with more than one (typically a spectrum of) chain lengths
- Polydispersity
Ratio of Mw over Mn, providing a measure for polydispersity
- Crystallinity
Degree to which polymer molecules arrange themselves into repeating structural patterns
- Intrinsic viscosity
A measure of the capability of a polymer in solution to enhance its viscosity. More technically: the ratio of specific viscosity to concentration at infinite dilution. Intrinsic viscosity is related to molecular weight
- Inherent viscosity
Or “logarithmic viscosity number”: ratio of the natural logarithm of the relative viscosity to the mass concentration of the polymer. Traditional name in polymer literature, not to be confused with intrinsic viscosity
- Tg
Glass transition temperature: temperature below which amorphous polymers are “frozen” into a glassy state. Above Tg, amorphous polymers behave like a rubber, allowing molecules to rearrange or move within the polymer (visco-elastic)
- Tm
(Crystalline) melting temperature: temperature above which polymers (both crystalline and amorphous) become a fluid
References
- 1.Agrawal CM, McKinney JS, Lanctot D, Athanasiou KA. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials. 2000;21:2443–2452. doi: 10.1016/S0142-9612(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 2.Athanasiou KA, Schmitz JP, Agrawal CM. The effects of porosity on in vitro degradation of polylactic acid–polyglycolic acid implants used in repair of articular cartilage. Tissue Eng. 1998;4:53–63. doi: 10.1089/ten.1998.4.53. [DOI] [Google Scholar]
- 3.Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14:726–737. doi: 10.1016/S0749-8063(98)70099-4. [DOI] [PubMed] [Google Scholar]
- 4.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 5.Ayhan F, Ayhan H, Piskin E. Sterilization of sutures by low temperature argon plasma. J Bioactive Biocompat Polym. 1998;13:65–72. [Google Scholar]
- 6.Babanalbandi A, Hill DJT, O’Donnell JH, Pomery PJ. An electron spin resonance analysis on -irradiated poly(glycolic acid) and its copolymers with lactic acid. Polym Degrad Stab. 1996;52:59–66. doi: 10.1016/0141-3910(95)00230-8. [DOI] [Google Scholar]
- 7.Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931–934. doi: 10.3928/0147-7447-19880601-13. [DOI] [PubMed] [Google Scholar]
- 8.Bergsma EJ, Rozema FR, Bos RR, Bruijn WC. Foreign body reactions to resorbable poly(L-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J Oral Maxillofac Surg. 1993;51:666–670. doi: 10.1016/s0278-2391(10)80267-8. [DOI] [PubMed] [Google Scholar]
- 9.Bergsma JE, Bruijn WC, Rozema FR, Bos RR, Boering G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials. 1995;16:25–31. doi: 10.1016/0142-9612(95)91092-D. [DOI] [PubMed] [Google Scholar]
- 10.Bezer M, Yildirim Y, Erol B, Guven O. Absorbable self-reinforced polylactide (SR-PLLA) rods vs rigid rods (K-wire) in spinal fusion: an experimental study in rabbits. Eur Spine J. 2005;14:227–233. doi: 10.1007/s00586-004-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden SD, Sumner DR. Biologic factors affecting spinal fusion and bone regeneration. Spine. 1995;20:102S–112S. doi: 10.1097/00007632-199512151-00006. [DOI] [PubMed] [Google Scholar]
- 12.Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1) Spine. 1998;23:2486–2492. doi: 10.1097/00007632-199812010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bostman O, Hirvensalo E, Makinen J, Rokkanen P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Joint Surg Br. 1990;72:592–596. doi: 10.1302/0301-620X.72B4.2199452. [DOI] [PubMed] [Google Scholar]
- 14.Bostman O, Hirvensalo E, Partio E, Tormala P, Rokkanen P. Resorbable rods and screws of polyglycolide in stabilizing malleolar fractures. A clinical study of 600 patients. Unfallchirurg. 1992;95:109–112. [PubMed] [Google Scholar]
- 15.Bostman OM. Absorbable implants for the fixation of fractures. J Bone Joint Surg Am. 1991;73:148–153. [PubMed] [Google Scholar]
- 16.Bostman OM, Pihlajamaki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop. 2000;371:216–227. doi: 10.1097/00003086-200002000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Brantigan JW, McAfee PC, Cunningham BW, Wang H, Orbegoso CM. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone. An investigational study in the Spanish goat. Spine. 1994;19:1436–1444. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 19.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine. 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 20.Bucholz RW, Henry S, Henley MB. Fixation with bioabsorbable screws for the treatment of fractures of the ankle. J Bone Joint Surg Am. 1994;76:319–324. doi: 10.2106/00004623-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Chang DE, Jung HC, Rhee JS, Pan JG. Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microbiol. 1999;65:1384–1389. doi: 10.1128/aem.65.4.1384-1389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC. Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery. 2002;51:1343–1349. doi: 10.1097/00006123-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Claes L, Ignatius A. Development of new, biodegradable implants. Chirurg. 2002;73:990–996. doi: 10.1007/s00104-002-0543-0. [DOI] [PubMed] [Google Scholar]
- 24.Closkey RF, Parsons JR, Lee CK, Blacksin MF, Zimmerman MC. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine. 1993;18:1011–1015. doi: 10.1097/00007632-199306150-00010. [DOI] [PubMed] [Google Scholar]
- 25.Coe JD. Instrumented transforaminal lumbar interbody fusion with bioabsorbable polymer implants and iliac crest autograft. Neurosurg Focus. 2004;16:E11. doi: 10.3171/foc.2004.16.3.12. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham BW, Kanayama M, Parker LM, Weis JC, Sefter JC, Fedder IL, et al. Osteogenic protein versus autologous interbody arthrodesis in the sheep thoracic spine. A comparative endoscopic study using the Bagby and Kuslich interbody fusion device. Spine. 1999;24:509–518. doi: 10.1097/00007632-199903150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Daniels AU, Chang MK, Andriano KP. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J Appl Biomater. 1990;1:57–78. doi: 10.1002/jab.770010109. [DOI] [PubMed] [Google Scholar]
- 28.Medinaceli L, al Khoury R, Merle M. Large amounts of polylactic acid in contact with divided nerve sheaths have no adverse effects on regeneration. J Reconstr Microsurg. 1995;11:43–49. doi: 10.1055/s-2007-1006510. [DOI] [PubMed] [Google Scholar]
- 29.Deguchi M, Cheng BC, Sato K, Matsuyama Y, Zdeblick TA. Biomechanical evaluation of translaminar facet joint fixation. A comparative study of poly-L-lactide pins, screws, and pedicle fixation. Spine. 1998;23:1307–1312. doi: 10.1097/00007632-199806150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350:722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 31.DiAngelo DJ, Kitchel S, McVay BJ, Scifert JL, Cornwall GB. Bioabsorbable anterior lumbar plate fixation in conjunction with anterior interbody fusion cages. Orthopedics. 2002;25:S1157–S1165. doi: 10.3928/0147-7447-20021002-06. [DOI] [PubMed] [Google Scholar]
- 32.Eitenmuller J, David A, Pommer A, Muhr G. Surgical treatment of ankle joint fractures with biodegradable screws and plates of poly-l-lactide. Chirurg. 1996;67:413–418. [PubMed] [Google Scholar]
- 33.Fukuzaki H, Yoshida M, Asano M, Kumakura M, Mashimo T, Yuasa H, et al. In vivo characteristics of low molecular weight copolymers composed of L-lactic acid and various DL-hydroxy acids as biodegradable carriers for drug delivery systems. Biomaterials. 1990;11:441–446. doi: 10.1016/0142-9612(90)90102-V. [DOI] [PubMed] [Google Scholar]
- 34.Gautier SE, Oudega M, Fragoso M, Chapon P, Plant GW, Bunge MB, et al. Poly(alpha-hydroxyacids) for application in the spinal cord: resorbability and biocompatibility with adult rat Schwann cells and spinal cord. J Biomed Mater Res. 1998;42:642–654. doi: 10.1002/(SICI)1097-4636(19981215)42:4<642::AID-JBM22>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Gibbon DF. Tissue response to resorbable synthetic polymers. In: Plank H, Daumer M, Renardy M, editors. Degradation phenomena on polymeric biomaterials. New York: Springer; 1992. pp. 97–104. [Google Scholar]
- 36.Glassman SD, Johnson JR, Raque G, Puno RM, Dimar JR. Management of iatrogenic spinal stenosis complicating placement of a fusion cage. A case report. Spine. 1996;21:2383–2386. doi: 10.1097/00007632-199610150-00018. [DOI] [PubMed] [Google Scholar]
- 37.Gogolewski S, Jovanovic M, Perren SM, Dillon JG, Hughes MK. Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA) J Biomed Mater Res. 1993;27:1135–1148. doi: 10.1002/jbm.820270904. [DOI] [PubMed] [Google Scholar]
- 38.Gogolewski S, Mainil-Varlet P, Dillon JG. Sterility, mechanical properties, and molecular stability of polylactide internal-fixation devices treated with low-temperature plasmas. J Biomed Mater Res. 1996;32:227–235. doi: 10.1002/(SICI)1097-4636(199610)32:2<227::AID-JBM12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Grizzi I, Garreau H, Li S, Vert M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials. 1995;16:305–311. doi: 10.1016/0142-9612(95)93258-F. [DOI] [PubMed] [Google Scholar]
- 40.Guilding DK, Reed AM. Biodegradable polymers for use in surgery-Polyglycolic/poly(lactic acid) homo- and copolymers: 1. Polymer. 1979;20:1459–1464. doi: 10.1016/0032-3861(79)90009-0. [DOI] [Google Scholar]
- 41.Hasirci V, Lewandrowski K, Gresser JD, Wise DL, Trantolo DJ. Versatility of biodegradable biopolymers: degradability and an in vivo application. J Biotechnol. 2001;86:135–150. doi: 10.1016/S0168-1656(00)00409-0. [DOI] [PubMed] [Google Scholar]
- 42.Heng BC, Cao T, Stanton LW, Robson P, Olsen B. Strategies for directing the differentiation of stem cells into the osteogenic lineage in vitro. J Bone Miner Res. 2004;19:1379–1394. doi: 10.1359/JBMR.040714. [DOI] [PubMed] [Google Scholar]
- 43.Hile DD, Doherty SA, Trantolo DJ. Prediction of resorption rates for composite polylactide/hydroxylapatite internal fixation devices based on initial degradation profiles. J Biomed Mater Res. 2004;71B:201–205. doi: 10.1002/jbm.b.30091. [DOI] [PubMed] [Google Scholar]
- 44.Holland SJ, Tighe BJ, Gould PL. Polymers for biodegradable medical devices. 1. The potential of polyesters as controlled macromolecular release systems. J Controlled Rel. 1986;4:155. doi: 10.1016/0168-3659(86)90001-5. [DOI] [Google Scholar]
- 45.Hollinger JO, Battistone GC. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin Orthop. 1986;207:290–305. [PubMed] [Google Scholar]
- 46.Hollowell JP, Vollmer DG, Wilson CR, Pintar FA, Yoganandan N. Biomechanical analysis of thoracolumbar interbody constructs. How important is the endplate? Spine. 1996;21:1032–1036. doi: 10.1097/00007632-199605010-00007. [DOI] [PubMed] [Google Scholar]
- 47.Holy CE, Cheng C, Davies JE, Shoichet MS. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials. 2001;22:25–31. doi: 10.1016/S0142-9612(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 48.Hunt J. Foreign body response. Encyclopedia of biomaterials and biomechanical engineering. New York: Marcel Dekker; 2004. pp. 641–648. [Google Scholar]
- 49.Hyon SH, Jamshidi K, Ikada Y. Synthesis of polylactides with different molecular weights. Biomaterials. 1997;18:1503–1508. doi: 10.1016/S0142-9612(97)00076-8. [DOI] [PubMed] [Google Scholar]
- 50.Ignatius AA, Augat P, Claes LE. Degradation behavior of composite pins made of tricalcium phosphate and poly(L,DL-lactide) J Biomater Sci Polym Ed. 2001;12:185–194. doi: 10.1163/156856201750180915. [DOI] [PubMed] [Google Scholar]
- 51.Incardona SD, Fambri L, Migliaresi C. Poly-L-lactic acid braided fibres produced by melt spinning: characterization and in vitro degradation. J Mater Sci Mater Med. 1996;7:387–391. doi: 10.1007/BF00122005. [DOI] [Google Scholar]
- 52.Johnsson R, Axelsson P, Stromqvist B. Posterolateral lumbar fusion using facet joint fixation with biodegradable rods: a pilot study. Eur Spine J. 1997;6:144–148. doi: 10.1007/BF01358748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanayama M, Cunningham BW, Haggerty CJ, Abumi K, Kaneda K, McAfee PC. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg Spine. 2000;93:259–265. doi: 10.3171/spi.2000.93.2.0259. [DOI] [PubMed] [Google Scholar]
- 54.Kandziora F, Pflugmacher R, Scholz M, Eindorf T, Schnake KJ, Haas NP. Bioabsorbable interbody cages in a sheep cervical spine fusion model. Spine. 2004;29:1845–1855. doi: 10.1097/01.brs.0000137060.79732.78. [DOI] [PubMed] [Google Scholar]
- 55.Katz JL, Ambrose CG, McMillin C, Spencer P. Orthopedic Biomaterials. In: Wnek GE, Bowlin GL, editors. Encyclopedia of biomaterials and biomedical engineering. New York: Marcel Dekker; 2004. pp. 1160–1171. [Google Scholar]
- 56.Klemme WR, Owens BD, Dhawan A, Zeidman S, Polly DW., Jr Lumbar sagittal contour after posterior interbody fusion: threaded devices alone versus vertical cages plus posterior instrumentation. Spine. 2001;26:534–537. doi: 10.1097/00007632-200103010-00017. [DOI] [PubMed] [Google Scholar]
- 57.Kohn J, Langer R. Bioresorbable and bioerodable materials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterial science. New York: Academic Press; 1996. pp. 64–72. [Google Scholar]
- 58.Kotani Y, Abumi K, Shikinami Y, Takahata M, Kadoya K, Kadosawa T, et al. Two-year observation of artificial intervertebral disc replacement: results after supplemental ultra-high strength bioresorbable spinal stabilization. J Neurosurg Spine. 2004;100:337–342. doi: 10.3171/spi.2004.100.4.0337. [DOI] [PubMed] [Google Scholar]
- 59.Krijnen MR, Smit TH, Strijkers GJ, Nicolay K, Pouwels PJ, Wuisman PI. The use of high-resolution magnetic resonance imaging for monitoring interbody fusion and bioabsorbable cages: an ex vivo pilot study. Neurosurg Focus. 2004;16:E3. doi: 10.3171/foc.2004.16.3.4. [DOI] [PubMed] [Google Scholar]
- 60.Kuklo TR, Rosner MK, Polly DW., Jr Computerized tomography evaluation of a resorbable implant after transforaminal lumbar interbody fusion. Neurosurg Focus. 2004;16:E10. doi: 10.3171/foc.2004.16.3.11. [DOI] [PubMed] [Google Scholar]
- 61.Kulkarni RK, Moore EG, Hegyeli AF, Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- 62.Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93:839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 63.Levy FE, Hollinger JO, Szachowicz EH. Effect of a bioresorbable film on regeneration of cranial bone. Plast Reconstr Surg. 1994;93:307–311. doi: 10.1097/00006534-199402000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Li SM, Garreau H, Vert M. Structure–property relationships in the case of the degradation of massive poly(a-hydroxy acids) in aqueous media. Part 3: influence of the morphology of poly(L-lactic acid) J Mater Sci: Mater Med. 1990;1:198–206. doi: 10.1007/BF00701077. [DOI] [Google Scholar]
- 65.Li SM, Vert M. Biodegradation of aliphaticolyesters. Degradableolymers. Principles and applications. Dordrecht: Scott G. Kluwer Academic Publ; 2002. pp. 71–132. [Google Scholar]
- 66.Lippman CR, Hajjar M, Abshire B, Martin G, Engelman RW, Cahill DW. Cervical spine fusion with bioabsorbable cages. Neurosurg Focus. 2004;16:E4. doi: 10.3171/foc.2004.16.3.5. [DOI] [PubMed] [Google Scholar]
- 67.Loo JS, Ooi CP, Boey FY. Degradation of poly(lactide-co-glycolide) (PLGA) and poly(l-lactide) (PLLA) by electron beam radiation. Biomaterials. 2005;26:1359–1367. doi: 10.1016/j.biomaterials.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Lowe CH (1952) Preparation of high molecular weight polyhydroxyacetic ester. US patent 2,668,162
- 69.Lowe TG, Coe JD. Bioresorbable polymer implants in the unilateral transforaminal lumbar interbody fusion procedure. Orthopedics. 2002;25:S1179–S1183. doi: 10.3928/0147-7447-20021002-09. [DOI] [PubMed] [Google Scholar]
- 70.Lowe TG, Hashim S, Wilson LA, O’Brien MF, Smith DA, Diekmann MJ, et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine. 2004;29:2389–2394. doi: 10.1097/01.brs.0000143623.18098.e5. [DOI] [PubMed] [Google Scholar]
- 71.Lowe TG, Tahernia AD. Unilateral transforaminal posterior lumbar interbody fusion. Clin Orthop. 2002;394:64–72. doi: 10.1097/00003086-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Lundgren D, Nyman S, Mathisen T, Isaksson S, Klinge B. Guided bone regeneration of cranial defects, using biodegradable barriers: an experimental pilot study in the rabbit. J Craniomaxillofac Surg. 1992;20:257–260. doi: 10.1016/s1010-5182(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 73.Martz EO, Goel VK, Pope MH, Park JB. Materials and design of spinal implants—a review. J Biomed Mater Res. 1997;38:267–288. doi: 10.1002/(SICI)1097-4636(199723)38:3<267::AID-JBM12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto M, Chosa E, Nabeshima K, Shikinami Y, Tajima N. Influence of bioresorbable, unsintered hydroxyapatite/poly-L-lactide composite films on spinal cord, nerve roots, and epidural space. J Biomed Mater Res. 2002;60:101–109. doi: 10.1002/jbm.1283. [DOI] [PubMed] [Google Scholar]
- 75.Matsusue Y, Yamamuro T, Oka M, Shikinami Y, Hyon SH, Ikada Y. In vitro and in vivo studies on bioabsorbable ultra-high-strength poly(L-lactide) rods. J Biomed Mater Res. 1992;26:1553–1567. doi: 10.1002/jbm.820261203. [DOI] [PubMed] [Google Scholar]
- 76.Matthews IP, Gibson C, Samuel AH. Enhancement of the kinetics of the aeration of ethylene oxide sterilized polymers using microwave radiation. J Biomed Mater Res. 1989;23:143–156. doi: 10.1002/jbm.820230202. [DOI] [PubMed] [Google Scholar]
- 77.McAfee PC, Boden SD, Brantigan JW, Fraser RD, Kuslich SD, Oxland TR, et al. Symposium: a critical discrepancy-a criteria of successful arthrodesis following interbody spinal fusions. Spine. 2001;26:320–334. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 78.McAfee PC, Cunningham BW, Lee GA, Orbegoso CM, Haggerty CJ, Fedder IL, et al. Revision strategies for salvaging or improving failed cylindrical cages. Spine. 1999;24:2147–2153. doi: 10.1097/00007632-199910150-00015. [DOI] [PubMed] [Google Scholar]
- 79.McAfee PC, Farey ID, Sutterlin CE, Gurr KR, Warden KE, Cunningham BW. 1989 Volvo Award in basic science. Device-related osteoporosis with spinal instrumentation. Spine. 1989;14:919–926. doi: 10.1097/00007632-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 80.McAfee PC, Lee GA, Fedder IL, Cunningham BW. Anterior BAK instrumentation and fusion: complete versus partial discectomy. Clin Orthop. 2002;394:55–63. doi: 10.1097/00003086-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 81.McKinney JS, Huang D, Athanasiou KA, Agrawal CM (1999) Degradation kinetics of highly permeable biodegradable scaffolds, 99 May 2; transactions 25th annual meeting of the society for biomaterials. Providence, RI
- 82.McNab I. Backache. Baltimore: Lippincott, Williams & Wilkins Company; 1977. [Google Scholar]
- 83.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/S0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 84.Nuutinen JP, Clerc C, Virta T, Tormala P. Effect of gamma, ethylene oxide, electron beam, and plasma sterilization on the behaviour of SR-PLLA fibres in vitro. J Biomater Sci Polym Ed. 2002;13:1325–1336. doi: 10.1163/15685620260449723. [DOI] [PubMed] [Google Scholar]
- 85.Ohlin A, Karlsson M, Duppe H, Hasserius R, Redlund-Johnell I. Complications after transpedicular stabilization of the spine. A survivorship analysis of 163 cases. Spine. 1994;19:2774–2779. doi: 10.1097/00007632-199412150-00007. [DOI] [PubMed] [Google Scholar]
- 86.Pitt CG, Gratzl MM, Kimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2:215–220. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- 87.Polikeit A, Ferguson SJ, Nolte LP, Orr TE. The importance of the endplate for interbody cages in the lumbar spine. Eur Spine J. 2003;12:556–561. doi: 10.1007/s00586-003-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puleo DA, Huh WW, Duggirala SS, DeLuca PP. In vitro cellular responses to bioerodible particles loaded with recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 1998;41:104–110. doi: 10.1002/(SICI)1097-4636(199807)41:1<104::AID-JBM13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 89.Seckel BR, Chiu TH, Nyilas E, Sidman RL. Nerve regeneration through synthetic biodegradable nerve guides: regulation by the target organ. Plast Reconstr Surg. 1984;74:173–181. doi: 10.1097/00006534-198408000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Sharma CP, Williams DF. The effects of lipids on the mechanical properties of polyglycolic acid structures. Eng Med. 1981;10:8–10. doi: 10.1243/EMED_JOUR_1981_010_005_02. [DOI] [Google Scholar]
- 91.Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine. 2000;25:1077–1084. doi: 10.1097/00007632-200005010-00007. [DOI] [PubMed] [Google Scholar]
- 92.Suganuma J, Alexander H. Biological response of intramedullary bone to poly-lactic acid. J Appl Biomater. 1993;4:13–27. doi: 10.1002/jab.770040103. [DOI] [Google Scholar]
- 93.Tabata Y, Ikada Y. Macrophage phagocytosis of biodegradable microspheres composed of L-lactic acid/glycolic acid homo- and copolymers. J Biomed Mater Res. 1988;22:837–858. doi: 10.1002/jbm.820221002. [DOI] [PubMed] [Google Scholar]
- 94.Taylor MS, Daniels AU, Andriano KP, Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994;5:151–157. doi: 10.1002/jab.770050208. [DOI] [PubMed] [Google Scholar]
- 95.Tegnander A, Engebretsen L, Bergh K, Eide E, Holen KJ, Iversen OJ. Activation of the complement system and adverse effects of biodegradable pins of polylactic acid (Biofix) in osteochondritis dissecans. Acta Orthop Scand. 1994;65:472–475. doi: 10.3109/17453679408995495. [DOI] [PubMed] [Google Scholar]
- 96.Therin M, Christel P, Li S, Garreau H, Vert M. In vivo degradation of massive poly(alpha-hydroxy acids): validation of in vitro findings. Biomaterials. 1992;13:594–600. doi: 10.1016/0142-9612(92)90027-L. [DOI] [PubMed] [Google Scholar]
- 97.Thompson DE, Agrawal CM, Athanasiou KA. The effects of dynamic compressive loading on biodegradable implants of 50–50% polylactic acid–polyglycolic acid. Tissue Eng. 1996;2:61–74. doi: 10.1089/ten.1996.2.61. [DOI] [PubMed] [Google Scholar]
- 98.Togawa D, Bauer TW, Brantigan JW, Lowery GL. Bone graft incorporation in radiographically successful human intervertebral body fusion cages. Spine. 2001;26:2744–2750. doi: 10.1097/00007632-200112150-00025. [DOI] [PubMed] [Google Scholar]
- 99.Togawa D, Bauer TW, Lieberman IH, Sakai H. Lumbar intervertebral body fusion cages: histological evaluation of clinically failed cages retrieved from humans. J Bone Joint Surg Am. 2004;86A:70–79. [PubMed] [Google Scholar]
- 100.Tomihata K, Suzuki M, Ikada Y. The pH dependence of monofilament sutures on hydrolytic degradation. J Biomed Mater Res. 2001;58:511–518. doi: 10.1002/jbm.1048. [DOI] [PubMed] [Google Scholar]
- 101.Toth JM, Wang M, Scifert JL, Cornwall GB, Estes BT, Seim HB, III, et al. Evaluation of 70/30 D,L-PLa for use as a resorbable interbody fusion cage. Orthopedics. 2002;25:S1131–S1140. doi: 10.3928/0147-7447-20021002-03. [DOI] [PubMed] [Google Scholar]
- 102.Vaccaro AR, Singh K, Haid R, Kitchel S, Wuisman P, Taylor W, et al. The use of bioabsorbable implants in the spine. Spine. 2003;3:227–237. doi: 10.1016/S1529-9430(02)00412-6. [DOI] [PubMed] [Google Scholar]
- 103.Van der Vegt AK, Govaert LE (2003) Polymeren, van keten tot kunststof. DUP Blue Print, Delft
- 104.Dijk M, Smit TH, Burger EH, Wuisman PI. Bioabsorbable poly-L-lactic acid cages for lumbar interbody fusion: three-year follow-up radiographic, histologic, and histomorphometric analysis in goats. Spine. 2002;27:2706–2714. doi: 10.1097/00007632-200212010-00010. [DOI] [PubMed] [Google Scholar]
- 105.Dijk M, Smit TH, Sugihara S, Burger EH, Wuisman PI. The effect of cage stiffness on the rate of lumbar interbody fusion: an in vivo model using poly(l-lactic Acid) and titanium cages. Spine. 2002;27:682–688. doi: 10.1097/00007632-200204010-00003. [DOI] [PubMed] [Google Scholar]
- 106.Sliedregt A, Blitterswijk CA, Hesseling SC, Grote JJ, Groot K. The effect of the molecular weight of polylactic acid on in vitro biocompatibility. Adv Biomater. 1990;9:207–211. [Google Scholar]
- 107.Sliedregt A, Radder AM, Groot K, Blitterswijk CA. In vitro biocompatibility testing of polylactides. Part I: proliferation of different cell types. J Mater Sci: Mater Med. 1992;3:365–370. doi: 10.1007/BF00705369. [DOI] [Google Scholar]
- 108.Vert M. Poly(lactic acid)s. In: Wnek GE, Bowlin GL, editors. Encyclopedia of biomaterials and biomedical engineering. New York: Marcel Dekker; 2004. pp. 1254–1264. [Google Scholar]
- 109.Vert M, Chabot F, Leray J, Chrsitel P. Bioresorbable polyesters for bone surgery. Makromol Chem. 1981;5(Suppl):30–41. doi: 10.1002/macp.1981.020051981103. [DOI] [Google Scholar]
- 110.Wehrenberg RH. II. Lactic acid polymers: strong, degradable thermoplastics. Mater Eng. 1981;94:63–66. [Google Scholar]
- 111.Weiner BK, Fraser RD. Spine update lumbar interbody cages. Spine. 1998;23:634–640. doi: 10.1097/00007632-199803010-00020. [DOI] [PubMed] [Google Scholar]
- 112.Williams DF. Enzyme hydrolysis of polylactic acid. Eng Med. 1981;10:5. doi: 10.1243/EMED_JOUR_1981_010_004_02. [DOI] [Google Scholar]
- 113.Williams DF, Most E. Enzyme-accelerated hydrolysis of poly(glycolic acid) J Bioeng. 1977;1:231. [PubMed] [Google Scholar]
- 114.Williams C, Middleton JC, Sims KR, Swaim RP, Whitfield DR, Yarbrough JC (1998) Long-term stability of biodegradable polymers, 98 February 6; San Antonio, TX. Publication Date: 6–8 Feb 1998: Birmingham Polymers; ISSN: 1086–4105, 1998
- 115.Wright DD. Degradable polymer composites. In: Wnek GE, Bowlin GL, editors. Encyclopedia of biomaterials and biomedical engineering. New York: Marcel Dekker; 2004. pp. 423–432. [Google Scholar]
- 116.Wuisman PI, Dijk M, Smit TH. Resorbable cages for spinal fusion: an experimental goat model. Orthopedics. 2002;25:S1141–S1148. doi: 10.3928/0147-7447-20021002-04. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Z, Grijpma DW, Feijen J. Creep-resistant porous structures based on stereo-complex forming triblock copolymers of 1,3-trimethylene carbonate and lactides. J Mater Sci Mater Med. 2004;15:381–385. doi: 10.1023/B:JMSM.0000021105.02301.ff. [DOI] [PubMed] [Google Scholar]
- 118.Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO. Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol. 2003;69:399–407. doi: 10.1128/AEM.69.1.399-407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]