Abstract
Object: Spinal cord compression from spinal metastasis represents a substantial clinical problem. Complete resection of spinal metastases is difficult in many cases, and conventional surgical decompression of the spinal cord with or without instrumentation often results in unsatisfactory neurological recovery and local recurrence, even if combined with external radiotherapy. To increase rates of local control and improve neurological recovery in such cases, we introduced decompressive surgery combined with intraoperative radiotherapy (IORT) for the treatment of spinal metastasis in 1992. We report the results of neurological recovery and local control in cases that received surgery with IORT. Methods: Between November 1992 and December 2001, 133 cases (117 patients) were treated using IORT at Tokyo Metropolitan Komagome Hospital. The 79 cases (74 patients) that received posterior spine surgery only for spinal paresis due to spinal metastasis were reviewed. Results: Improvement of at least one level according to Frankel’s classification was attained in 68 cases (86%). Of the 58 patients unable to walk preoperatively, 45 patients (78%) regained walking ability postoperatively. Rate of local recurrence was 2.5%. Conclusions: IORT, combined with posterior surgery and FERT, might be one of the effective methods for local control of spinal metastasis and neurological improvement, especially in cases with progressive and multi-level lesions.
Keywords: Spinal metastasis, Intraoperative radiotherapy, Decompressive surgery, Local control
Introduction
Incidence of spinal metastasis is about 30% in patients with cancer at time of death [12, 19], and about 5% of cancer patients are estimated to develop neurological deficit as a result of spinal metastases [2]. Recent advances in oncological therapy are increasing the duration that patients with metastases can expect to live, and effective treatment is required to control both spinal metastases and associated neurological symptoms. However, results of surgical intervention are often unsatisfactory. Conventional surgical decompression of the spinal cord with or without instrumentation often results in local recurrence. Surgery combined with external radiotherapy often does not completely control the disease, particularly if the tumor is radioresistant. In addition, treatment of progressive neurological symptoms during or after external radiotherapy is difficult, as conventional decompressive surgery does not achieve satisfactory neurological recovery.
To increase rates of local control and improve neurological recovery of such patients, we introduced decompressive surgery combined with intraoperative radiotherapy (IORT) for the treatment of spinal metastasis in 1992. We report the results for neurological recovery and local control in patients who received surgery with IORT.
Materials and methods
Between November 1992 and December 2001, a total of 133 cases (117 patients) were treated using IORT at Tokyo Metropolitan Komagome Hospital. To determine rates of neurological improvement in patients with spinal metastasis, we reviewed 79 cases (74 patients) that received:
posterior spine surgery (excluding anterior spine surgery);
for spinal paresis (excluding patients who received surgery for intractable pain or cauda equina paresis);
due to spinal metastasis (excluding primary spinal tumors).
Patients comprised 48 men and 26 women, with a mean age of 61.4 years (range, 42–85 years).
Primary tumor sites comprised: breast (n=13); lung (n=12); colon (n=9); thyroid (n=7); prostate (n=7); kidney (n=6); liver (n=5); multiple myeloma (n=4); malignant lymphoma (n=3); pharynx (n=3); stomach (n=1); esophagus (n=1); bladder (n=1); uterus (n=1); parotid gland (n=1); pancreas (n=1); malignant melanoma (n=1); leiomyosarcoma of the thigh (n=1); squamous cell carcinoma of the external genitalia (n=1); and unknown (n=1).
Surgery was performed in the following vertebrae: cervical (n=3); cervicothoracic (n=7); thoracic (n=60); and thoracolumbar (n=9).
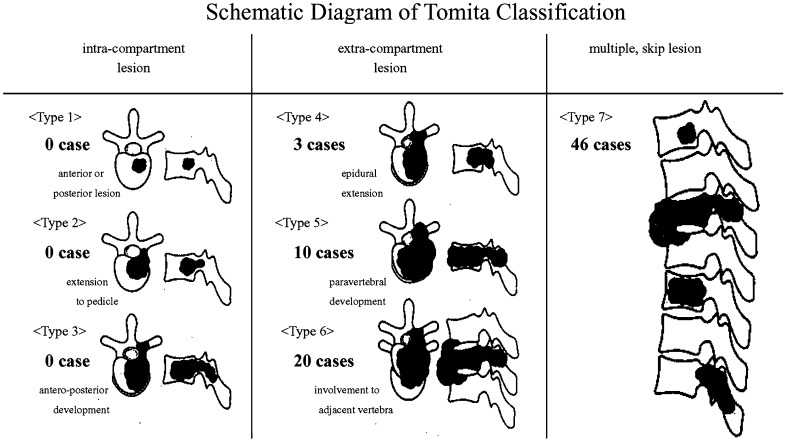
Metastases were assessed using the surgical classification system for spinal tumors proposed by Tomita et al. [17] (Fig. 1). In the current study, types 6 and 7 progressive and multi-level lesions were common.
Fig. 1.
Schematic diagram of surgical classification for vertebral tumors proposed by Tomita et al. [17] In the current study, metastatic lesions were extensive in many cases
Simulation study and in vivo measurement
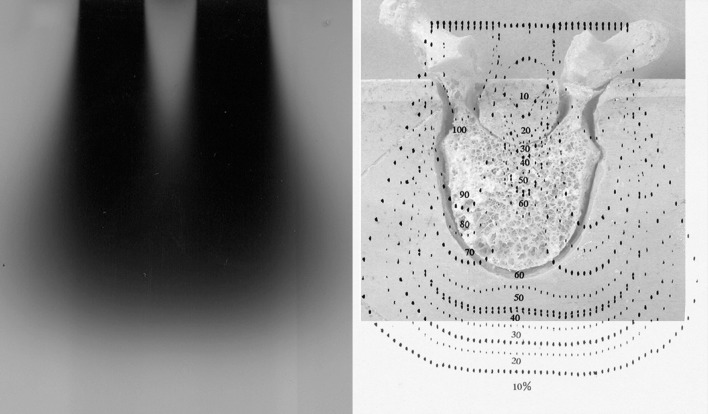
Simulation studies were undertaken to determine dose distribution in IORT, particularly to estimate irradiated dose in the spinal cord. Dose distributions for a human vertebra and phantom are presented in Fig. 2. In vivo measurements were also performed several times to confirm actual irradiated doses using a small film (GAF CHROMIC film type MD-55; Nuclear Associates, NY, USA) for dose measurement. Dose of IORT in these measurements was 20 Gy.
Fig. 2.
Representation of electron beam when lead shielding is used to protect the spinal cord (left). Dose distribution for cone size 4 × 6 cm, energy 16 MeV, shield width 10 mm, shield thickness 5 mm
Percentage dose in the spinal cord was 10–25% (equivalent to 2–8 Gy of fractionated external radiotherapy (FERT)) with a lead shield, and 90% (equivalent to 84 Gy of FERT) without lead shielding. In FERT, radiation myelopathy reportedly occurs in 0.2–0.5% of cases with irradiation at 50 Gy, and in 50% of cases at 68–73 Gy [6, 8, 9, 15]. Lead shielding is effective and absolutely indispensable for preventing radiation myelopathy.
Irradiated dose in vertebral tumors varies with location. Simulation studies indicated that percentage dose is minimized at the posterior edge of the vertebrae. In vivo measurements showed that the range of irradiated doses at the posterior edge is approximately 6.3 Gy (mid) to 35 Gy (lateral edge) in FERT.
A linear–quadratic model was used for dose conversions between IORT and FERT, using biological effective dose (BED):
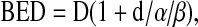 |
where D is the total dose, d single fraction dose, and α/β differs for each tissue. In the current study, α/β was three for spinal cord, a late-responding tissue, and ten for vertebral tumor, an early responding tissue [3]. Single fraction dose in FERT was 2 Gy.
IORT procedures
Indications for surgical intervention comprised neurological deficit and intractable pain not expected to be controlled by external radiotherapy or chemotherapy. Surgery was not indicated for patients displaying high surgical risk, particularly patients in poor general condition with poor prognosis due to poorly controlled primary lesions or metastases involving vital organs. If neurological status of a patient was worse than Frankel classification C (unambulatory) and progression was rapid, emergency surgery was performed.
If a tumor was expected to be hypervascular, preoperative embolization was performed to minimize intraoperative bleeding.
After posterior decompression (laminectomy and resection of epidural metastatic tumor where possible) and control of bleeding, patients were covered with sterile cloth and transferred from the operating room to the radiotherapy room (about 80 m). In the radiotherapy room, an appropriately sized sterile electron cone with a lead shield was connected to the electron beam generator, and placed precisely in the surgical field so that the beam covered the tumor, while the lead shield protected the spinal cord (Fig. 3). An electron beam was generated using a Microtron (Hitachi, Tokyo, Japan). Energy utilized depended on the depth of the lesion. Mean energy in this study was 15.9 MeV (range, 11–20 MeV). Mean dose of IORT was 20.7 Gy (range, 15–25 Gy). All IORT procedures took 40–50 min in the single case, including transfer, preparations and so on. The patients were carefully monitored continuously during transfer and IORT by experienced anesthesiologists.
Fig. 3.
Photographic (above) and schematic (right below) representation of intraoperative radiotherapy. Lead shielding to protect the spinal cord was connected to the electron beam generator (left below)
After IORT, patients were transferred back to the operating room. Internal fixation with instrumentation and posterolateral fusion with allograft was performed if necessary. A spinal instrument made of titanium (CD HORIZON or TSRH; Medtronic Sofamor Danek, Minneapolis, USA) was used because of the reduced interference with postoperative magnetic resonance imaging (MRI).
External radiotherapy
In addition to IORT, external radiotherapy was performed postoperatively in 34 cases, and preoperatively in 20 cases. Mean dose was 36 Gy (range, 12–60 Gy). Single fraction dose was generally 2 Gy.
In principle, if a patient did not receive preoperative radiotherapy, external radiotherapy at a dose of approximately 30 Gy was recommended after wound suture removal to increase local control. The dose of 30 Gy in addition to IORT was selected based on the tolerance dose of the spinal cord. However, consensus regarding optimum doses for such applications has yet to be reached.
Chemotherapy
The decision to perform adjuvant chemotherapy rested with the physicians who had managed the primary lesions. Of the 45 patients who received adjuvant chemotherapy, 12 received preoperative chemotherapy only, 15 received postoperative chemotherapy only, and 18 received both preoperative and postoperative chemotherapy. Chemotherapy was administered for breast cancer (n=13), lung cancer (n=9), colon cancer (n=4), renal cancer (n=4), malignant lymphoma (n=3), hepatoma (n=2), multiple myeloma (n=2), prostate (n=1), pancreas (n=1), stomach (n=1), esophagus (n=1), malignant melanoma (n=1), leiomyosarcoma of the thigh (n=1), squamous cell carcinoma of the external genitalia (n=1), and adenocarcinoma of the parotid gland (n=1).
Hormone therapy was administered for five patients with prostate cancer and two patients with breast cancer.
Clinical evaluation
Neurological function was evaluated using Frankel’s classification system [4], as follows:
complete motor and sensory loss;
complete motor loss but some sensation preserved;
some motor power preserved but of no functional use;
useful motor power including walking with or without aids;
no neurological symptoms.
Follow-up examinations, including plain radiography and enhanced MRI of the operated site, were performed every 3 months to detect local recurrence.
Results
For 57 patients, mean survival was 10.4 months after IORT (range, 2 weeks to 63 months). The remaining 17 patients were still alive after a mean follow-up period of 20 months (range, 1–65 months). Second surgery with IORT was undertaken in four cases for further spinal metastasis, and in one case for local recurrence.
Posterior decompression was performed without instrumentation in 33 cases, and with instrumentation in 46 cases. Mean duration of surgery was 5 h 10 min (range, 2 h 20 min to 9 h). Mean blood loss was 934 ml (range, 100–4340 ml).
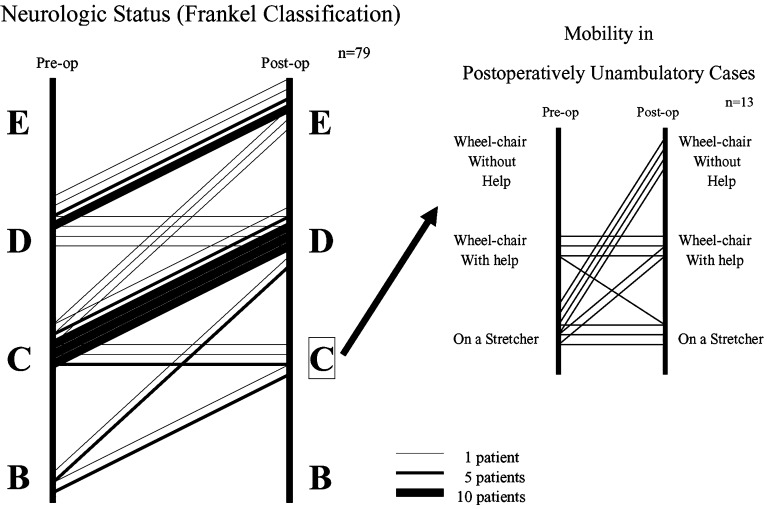
No cases demonstrated immediate neurological deterioration. Neurological improvements according to Frankel’s classification are presented in Fig. 4. At least one level of improvement was attained in 68 cases (86%). Of the 58 patients unable to walk preoperatively, 45 (78%) regained walking ability postoperatively. Mobility was improved in six of the 13 patients who did not regain ambulation, but was not improved in seven patients who experienced deteriorated general condition (Fig. 4).
Fig. 4.
Neurological improvement according to Frankel’s classification. At least one level of improvement was attained by 86% of all patients
Postoperatively, 66 patients were ambulatory. Of these, 64% were ambulatory by 6 months after surgery, and 36% remained ambulatory by 1 year after surgery. Mean duration of maintained walking ability was 10.3 months (range, 1–65 months). Causes of recurrent loss of walking ability comprised: deterioration of general condition (including metastasis to other organs, n=30), other spinal metastases (n=13), bone metastasis in lower extremities (n=2), radiation myelopathy (n=1), local recurrence (n=2), and compression fracture and kyphotic changes in the operated vertebra (n=3). Patients with compression fracture and kyphotic changes to the operated vertebra displayed metastasis in the thoracic vertebrae and were treated without instrumentation.
One patient displayed disseminated intravascular coagulation (DIC), and two developed postoperative pneumonia. These patients died 16–18 days postoperatively. No cases of surgical wound infection were encountered.
One patient displayed neurological improvement temporarily after surgery, followed with neurological deterioration into the preoperative status 3 months later. We could not find any substantial cause of this neurological deterioration and diagnosed as radiation myelopathy. The patient had received both preoperative external radiotherapy (60 Gy) and second IORT for metastasis in an adjacent vertebra.
Discussion
Surgery for spinal metastasis involves posterior decompression, posterior decompression with stabilization, and anterior resection or decompression with reconstruction. In many cases, tumor resection is incomplete and local recurrence may occur. Surgery combined with external radiotherapy may improve the therapeutic ratio, but outcomes remain somewhat unsatisfactory. IORT was applied in our series to minimize local recurrence. The IORT has previously been used to manage pancreatic, bladder, and colorectal cancer and malignant brain tumors. However, no reports of IORT used to manage spinal metastasis in a large series of patients have previously been published from other institutions [16].
The comparison of neurological results with the literature is difficult because of the many various factors, such as kinds of primary tumor, stages of progress and therapeutic histories. Moreover, concepts and indications are very different in each surgical method. For example, the anterior method is usually intended as total resection of metastatic lesion. The results of the anterior method are good, but its indication is usually limited to single and intra-compartment lesion. On the other hand, the posterior method is usually intended as decompression of the spinal cord, and its indication includes progressive and multi-level lesions. As for clinical evaluation, duration of walking ability is very important, nevertheless duration of walking ability was not mentioned in many previous reports of other surgical methods. Frankel’s classification is the only means for comparison, because neurological improvement was evaluated merely according to Frankel’s classification. Rates of neurological improvement according to Frankel’s classification in previous reports of these surgical methods have been approximately 30–40% in posterior decompression, 50–70% in posterior decompression and stabilization combined with external radiotherapy, and 70–80% in the anterior method [1, 5, 11, 13, 14, 18]. The 86% rate of neurological improvement achieved in the current study is not worse at least, considering that many patients displayed progressive and multi-level lesions. In particular, rate of local recurrence in the current study was 2.5%, representing an excellent result compared to previous reports (20–30%) [7, 10]. Because quantifying the effects directly attributable to IORT is difficult in the present study, the following study is required for revealing the conditions that need IORT and the appropriate indication of IORT.
Radiotherapy is necessary in the treatment of spinal metastasis, as radical excision is impossible in many cases. However, total dose is limited by the tolerance dose of the spinal cord. The common dose limit is about 45 Gy, and radiation myelopathy reportedly occurs with the following frequencies in FERT: 0.2–0.5% at 50 Gy; 5% at 57–61 Gy; and 50% at 68–73 Gy [6, 8, 9, 15]. In single IORT of 20 Gy, irradiated dose to the spinal cord is 2–8 Gy (in FERT equivalents). In cases where preoperative external radiotherapy has been administered, dose of IORT should be determined carefully with consideration of total dose. When postoperative external radiotherapy is performed, total dose might be decreased out of consideration for the irradiated dose in IORT (we recommend 30 Gy for most cases). In the current study, one case of radiation myelopathy was encountered. The patient had received high-dose preoperative external radiotherapy (60 Gy) and second IORT for metastasis in an adjacent vertebra. Lead shielding represents an effective preventative measure, but risk of radiation myelopathy should always be considered when performing IORT.
All IORT procedures, especially transfer from the operating room to the radiotherapy room, would increase the infection risk. The most important prophylaxis of infection is to perform all procedures smoothly and quickly, for which cooperation and training of all staff are necessary. Careful covering during transfer and thorough irrigation of the surgical field are also important for prophylaxis of infections. Fortunately, there were no cases of surgical wound infection in these 79 cases.
Two patients displayed local recurrence postoperatively. Primary tumor sites for the two cases were lung and malignant lymphoma, and both cases were treated using adjuvant chemotherapy and external radiotherapy. Both cases displayed large and progressive lesions, and recurrent growth to adjacent vertebrae and the paravertebral area.
Three patients regained ambulation postoperatively, then lost walking ability again due to compression fracture and kyphotic changes in vertebrae that underwent operation without instrumentation. Although no significant differences were observed between results for patients with or without instrumentation, we now add stabilization with instrumentation for all cases of metastases in thoracic vertebrae.
Conclusion
The IORT, combined with posterior surgery and FERT, might be one of the effective methods for local control of spinal metastasis and neurological improvement, especially in cases with progressive and multi-level lesions. This procedure has to be examined for its appropriate indication in the following study.
Acknowledgements
The submitted manuscript does not contain any information regarding medical devices or drugs. No funds were received in support of this work and no benefits in any form have been or will be received from commercial parties related directly or indirectly to the subject of this manuscript.
References
- 1.Bauer HC. Posterior decompression and stabilization for spinal metastases. Analysis of sixty-seven consecutive patients. J Bone Joint Surg Am. 1997;79:514–522. doi: 10.2106/00004623-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery. 1979;5:726–746. doi: 10.1227/00006123-197912000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Eric JH. Radiobiology for the radiologist. 5. Baltimore: Lippincott Williams & Wilkins; 2000. pp. 397–418. [Google Scholar]
- 4.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 5.Hatrick NC, Lucas JD, Timothy AR, et al. The surgical treatment of metastatic disease of the spine. Radiother Oncol Sep. 2000;56:335–339. doi: 10.1016/S0167-8140(00)00199-7. [DOI] [PubMed] [Google Scholar]
- 6.Jeremic B, Djuric L, Mijatovic L. Incidence of radiation myelitis of the cervical spinal cord at doses of 5500 cGy or greater. Cancer. 1991;68:2138–2141. doi: 10.1002/1097-0142(19911115)68:10<2138::AID-CNCR2820681009>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Manabe S, Tateishi A, Abe M, et al. Surgical treatment of metastatic tumors of the spine. Spine. 1989;14:41–47. doi: 10.1097/00007632-198901000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Marcus RB, Million RR. The incidence of myelitis after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1990;19:3–8. doi: 10.1016/0360-3016(90)90126-5. [DOI] [PubMed] [Google Scholar]
- 9.McCunniff A, Lliang MJ. Radiation tolerance of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1989;16:675–678. doi: 10.1016/0360-3016(89)90484-7. [DOI] [PubMed] [Google Scholar]
- 10.Missenard G, Lapresle P, Cote D. Local control after surgical treatment of spinal metastatic disease. Eur Spine J. 1996;5:45–50. doi: 10.1007/BF00307826. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls PG, Jarecky TW. Evaluate posterior decompression by laminectomy for malignant tumors of the spine. Clin Orthop. 1985;201:210. [PubMed] [Google Scholar]
- 12.Ortiz Gomez JA. The incidence of vertebral body metastases. Int Orthop. 1995;19:309–311. doi: 10.1007/BF00181116. [DOI] [PubMed] [Google Scholar]
- 13.Rompe JD, Hopf CG, Eysel P. Outcome after palliative posterior surgery for metastatic disease of the spine—evaluation of 106 consecutive patients after decompression and stabilisation with the Cotrel-Dubousset instrumentation. Arch Orthop Trauma Surg. 1999;119:394–400. doi: 10.1007/s004020050008. [DOI] [PubMed] [Google Scholar]
- 14.Sapkas G, Kyratzoulis J, Papaioannou N, et al. Spinal cord decompression and stabilization in malignant lesions of the spine. Acta Orthop Scand Suppl. 1997;275:97–100. doi: 10.1080/17453674.1997.11744756. [DOI] [PubMed] [Google Scholar]
- 15.Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093–1112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 16.Seichi A, Kondoh T, Hozumi T, et al. Intraoperative radiation therapy for metastatic spinal tumors. Spine. 1999;24:470–473. doi: 10.1097/00007632-199903010-00014. [DOI] [PubMed] [Google Scholar]
- 17.Tomita K, Kawahara N, Baba H, et al. Total en block spondylectomy. Spine. 1997;22:324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 18.Wetzel FT, Phillips FM. Management of metastatic disease of the spine. Orthop Clin North Am. 2000;31:611–621. doi: 10.1016/S0030-5898(05)70179-6. [DOI] [PubMed] [Google Scholar]
- 19.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15:1–4. doi: 10.1097/00007632-199001000-00001. [DOI] [PubMed] [Google Scholar]