Abstract
A non-linear 3-dimensional finite element pediatric lumbar spine model with vertebral growth plate and apophyseal bony ring was developed. Lumbar spondylolysis was simulated in the model. The Von Mises stresses in the structures surrounding the vertebral growth plate, including apophyseal bony ring and osseous endplate were calculated in various loading modes. Instantaneous axis of rotation (IAR) path from flexion to extension was also analyzed. The results were compared with those of the intact model and the literature. The IAR path was at the posterior disc-endplate space of the lower vertebra in the intact spine, and moved cranially towards the upper-posterior disc space in the lytic spine. This was in agreement with in vivo radiological data by Sakamaki et al. [19]. During various loading modes, stresses in the spondylolytic pediatric model were higher than that of the intact model; ranging from 1.1 to 6.0 times, with the highest value in extension at the growth plate. In conclusion, FE models indicate that stress concentrations in the lytic model increase at the growth plate which may lead to physis stress fracture leading to spondylolisthesis.
Keywords: Pediatric spine, Spondylolysis, Spondylolisthesis, Biomechanics, Finite element model, Growth plate
Introduction
Lumbar spondylolysis occurs in approximately 5–6% of the population [4]. Among them, 80% of the spondylolytic vertebrae show slippage to some extent. In general the percent slippage is not severe; only one-fourth of the vertebrae show more than 25% slippage. The progression from the spondylolysis to the spondylolisthesis generally occurs during adolescent growth periods, and is very rare thereafter [2, 4, 12, 16, 21, 22]. However, the pathomechanism of its prevalence in the growth period is still unclear.
In 1976, Farfan et al. [3] first hypothesized that pediatric spondylolisthesis may occur after epiphyseal separation. His theory was further supported by MRI findings of pediatric patients with isthmic spondylolisthesis [8]. Similar conclusions were reached by Sairyo and co-workers [9,15,16,17,19,20]. They concluded from these series of investigations that the isthmic olisthesis in children and adolescents occurred based on epiphyseal separation: and proposed that pediatric isthmic spondylolisthesis is a physis stress fracture of the vertebral body. The authors further hypothesized that biomechanical and kinematical alterations occur following spondylolysis in immature spines. Also, due to these alterations there is an increased stress seen at the growth plate inducing the physis stress fracture, eventually causing slippage.
In order to support the hypothesis, a biomechanical investigation to assess the stresses in structures surrounding the growth plate is needed. The study of the instantaneous axis of rotation (IAR) of the vertebral body can be useful to assess the kinematics of the lytic spine [19]. A three-dimensional finite element model [5,6,7,10,11,18] (FEM) of the pediatric lumbar spine was developed as described in Part I of this study. This model was used to predict the path of IAR were in flexion/extension, and stresses on the intact and spondylolytic pediatric spines in response to various loading modes.
Methods
Intact and spondylolysis finite element models
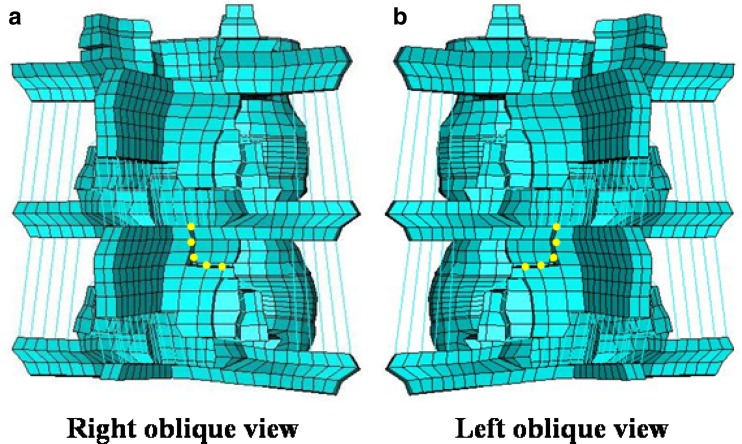
The details of the intact pediatric 3-dimensional lumbar spine model are provided in Part I of the study. It contains the growth plate layer and the apophyseal ring. This model was modified to simulate bilateral spondylolysis, Fig. 1. The dotted yellow thick lines at the pars interarticularis of L4 indicate the location of spondylolysis in this model. Pars fracture was simulated as a 1.0 mm gap by deleting the elements in that region.
Fig. 1.
Finite element model of the L3-L5 segment simulating bilateral spondylolysis at L4. The bilateral pars interarticularis was simulated as a gap of 1.0 mm, shown as dotted yellow lines
Load applications and stress analyses
To analyze stresses within various spine structures in response to various spinal motions, first an axial compressive pre-load was applied to mimic weight bearing in the standing posture. The value chosen was 351 N in proportion to the adult model. Then, a pure moment of 10 Nm was applied to simulate flexion, extension, lateral bending and axial rotation. Von Mises stresses in the intact and spondylolytic pediatric models at the various sites were compared. Commercially available FEM software ABAQUS CAE Version 6.0 was used for this analysis.
Calculation of IAR
The instantaneous locations of two points on L4 vertebra and L5 vertebra were calculated from 10 Nm flexion to 10 Nm extension positions in 1 Nm steps. The spatial data was used to compute IAR path as per the methodology reported in Panjabi et al [14].
Results
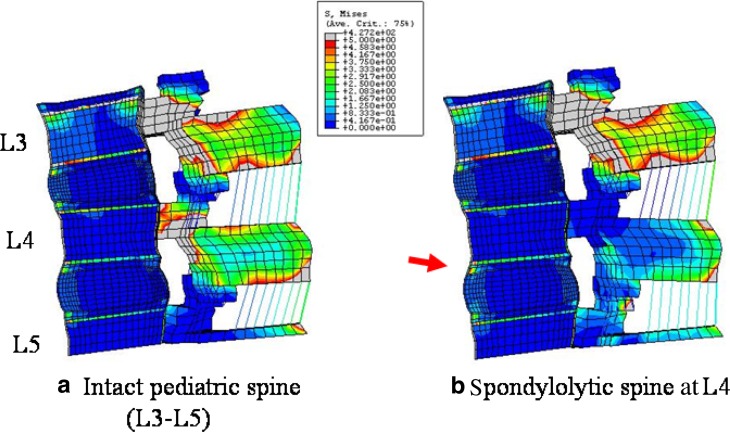
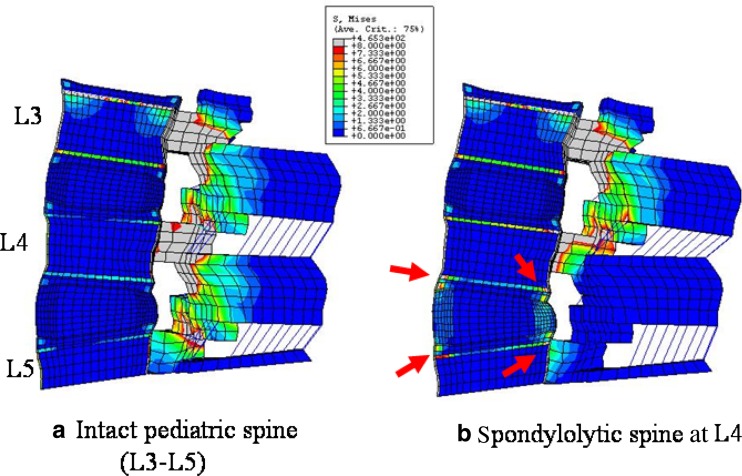
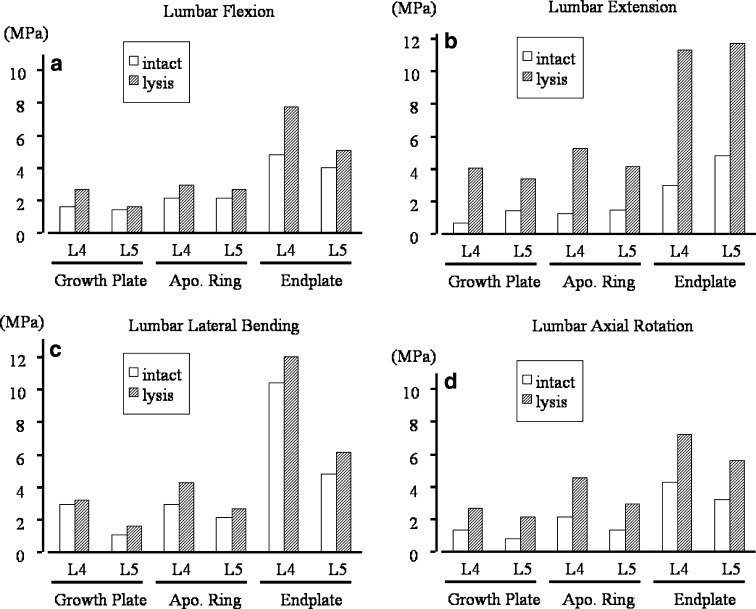
Higher stresses were observed in the spondylolytic pediatric model as compared to intact at the growth plate, apophyseal ring and osseous endplate in all the loading modes. In flexion the stress increased (<2 times) at the anterior growth plate in the spondylolytic pediatric spine, Fig. 2. Stress decreased at the site of defect (pars) when compared to the intact. In extension for the spondylolytic pediatric spine (Fig. 3), stresses increased at both the anterior and the posterior growth plate regions. The highest increase of about 2.4–6 times when compared to the intact was seen at posterior region (highlighted with arrows in Figs. 2, 3, Table 1). Figures 4a, b show the absolute stress values at the growth plate, apophyseal ring and endplate for flexion and extension, respectively.
Fig. 2.
Von Mises stress contour plot in flexion in the mid-sagittal sectional plane of (a) intact model and (b) spondylolysis model at L4. The various colors indicate the magnitude of the stresses. Gray and red color indicate higher stresses; whereas, blue and green, lower stresses. Overall, posterior structures (pedicle and facet) experience higher stresses compared to anterior structures (discs and vertebral body). Among the anterior structures, osseous endplate and apophyseal bony ring are highly stressed, shown with the aid of an arrow
Fig. 3.
Von Mises stress contour plot in extension the mid-sagittal sectional plane of (a) intact model and (b) spondylolysis model at L4. The various colors indicate the magnitude of the stress. Gray and red color indicate higher stresses; whereas, blue and green, lower stresses. Overall, posterior structures (pedicle and facet) experience higher stresses compared to anterior structures (discs and vertebral body). Among the anterior structures, osseous endplate and apophyseal bony ring are highly stressed in two models, shown with the aid of an arrow.
Table 1.
Stress ratios of lytic pediatric spine to intact pediatric spine model values (lytic/intact)
| Growth plate | Apo. ring | Endplate | ||||
|---|---|---|---|---|---|---|
| L4 | L5 | L4 | L5 | L4 | L5 | |
| Extension | 6.0 | 2.4 | 4.3 | 2.8 | 3.8 | 2.4 |
| Flexion | 1.3 | 1.2 | 1.3 | 1.2 | 1.2 | 1.1 |
| Lateral bending | 1.1 | 1.3 | 1.2 | 1.2 | 1.1 | 1.2 |
| Axial rotation | 1.8 | 2.7 | 1.7 | 2.3 | 1.6 | 1.8 |
In all lumbar motions, and in all structures around the L4-5 disc, stresses increased after spondylolysis when compared to the intact pediatric model. The highest increase of 6.0-fold was observed in extension at the L4 growth plate
Fig. 4.
Highest Mises stresses calculated at growth plate, apophyseal bony ring and osseous endplate in intact and spondylolytic spines. These structures around L4-5 disc space are analyzed, since spondylolysis mainly cause slippage at caudal adjoining disc level. During all lumbar motion of (a) extension, (b) flexion, (c) lateral bending, (d) axial rotation, the stresses increase after the lumbar spondylolysis when compared to intact. (Apo. Ring: apophyseal bony ring)
In lateral bending, the structures on the contralateral side showed increased stresses (<2 times) and the increase was more in the lytic pediatric spine compared to the intact, Fig. 4c. A similar situation was observed in the axial rotation mode, Fig. 4d.
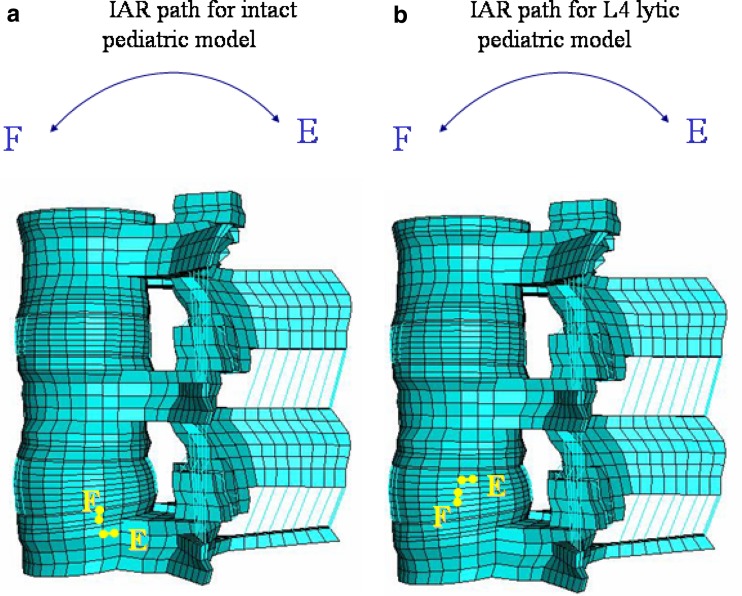
The IAR path was located at the posterior disc-endplate space of the lower vertebra in the intact spine. In the lytic pediatric spine it moved cranially towards the upper-posterior disc space, Fig. 5.
Fig. 5.
Path of instantaneous axis of rotation (IAR) from flexion to extension of L4 vertebral body in (a) intact and (b) spondylolysis model. In the intact spine model, the IAR of L4 locates one-third posterior in disc space, and it moves cranially after spondylolysis at L4
Discussion
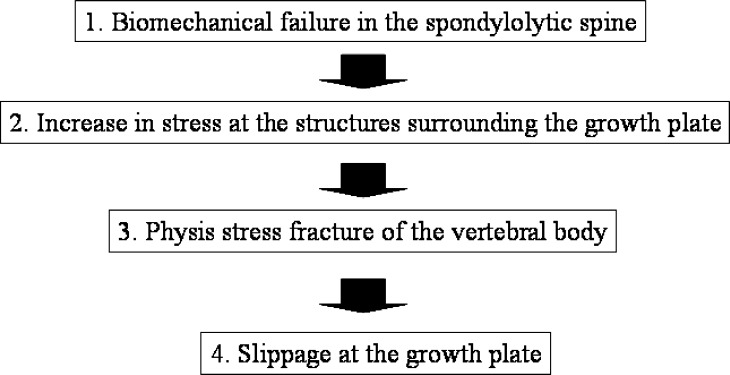
The slippage mechanism of the spondylolytic vertebrae in children and adolescents is still unclear. We hypothesized the mechanism based on Farfan’s theory, i.e. the vertebral slippage after the epiphyseal separation [3]. The theory seems reasonable because the slippage is most prevalent in children and adolescents [2, 4, 12, 16, 21, 22]. Also, in the pediatric immature spine, our previous studies revealed that the growth plate at the vertebral body was the weakest-link against anterior shear force [9, 11, 15]. Our hypothesis of the slippage mechanism of the spondylolytic pediatric spine is explained in Fig. 6. First, biomechanical alteration occurs in the spondylolytic pediatric spine. Then, stresses during lumbar motion increase at the growth plate. The accumulation of the stresses (cyclic loading) causes physis stress fracture, leading to spondylolisthesis at the growth plate. The present study attempted to prove the initial part of the hypothesis using the finite element model.
Fig. 6.
A flow chart explaining our hypothesis of the slippage mechanism at the growth plate in children and adolescents
The study on the kinematics of spondylolysis, using Roentgen stereophotogrammetric technique, by Axelsson et al. [1] stated that defects of pars interarticularis do not cause lumbar instability. However, one can conceive that kinematic alterations are likely to occur after the breakage of pars interarticularis even though the alterations may not lead any noticeable instability. Mihara et al. [13] conducted an experimental biomechanical study using fresh human cadaveric spines, and found hypermobility in flexion, extension and axial rotation following pars defect. Sakamaki et al. [19] measured IAR during flexion and extension on radiograms of patients, and found that the IAR moved cranially in pediatric spondylolytic patients when compared with normal children. In this study, we also analyzed the IAR theoretically using FEM to understand the kinematic alterations in the lytic pediatric spine. In the lytic pediatric spine the IAR moved cranially when compared to the intact spine, indicating the alteration of the spine. These results are in good agreement with the clinical study by Sakamaki et al. [19]. This data in a way validates the pediatric FEM model predictions, overcoming the limitation as stated in part one of this study.
We analyzed stresses at various structures surrounding the vertebral growth plate in the immature spine with or without bilateral pars defects, simulating pediatric lumbar spondylolysis. The stresses in all structures including the growth plate, apophyseal bony ring and endplate increased after spondylolysis in all loading modes. Extension had the highest stress increase, around six times at the growth plate when compared to the intact. During lumbar motion, stresses did not transmit through the pars interarticularis because of the defect, causing more stresses to transmit through the anterior compartment of the lytic pediatric spine.
The repeated occurrence of high stresses may cause physis stress fracture in the spondylolytic pediatric spine. We previously proved this part by radiological and histological study using the rat immature spine slippage model [17, 20]. The physis stress fracture occurred on the seventh day following the posterior destabilization not immediately after the surgery [15]. With this physis stress fracture in immature rat models, the cranial adjoining vertebra slipped forward. At the time of slippage, the disc was found to be intact histologically. Thus, it was revealed that in the immature spine the vertebral body could slip forward at the growth plate without any disc degeneration.
From the results it appears that the most detrimental lumbar motion for the slippage-spondylolysis seems to be extension. For the pediatric patients with lumbar spondylolysis, restriction of lumbar extension using a brace should be useful to prevent the vertebral physis injuries. In particular for the pediatric patients with vertebral cartilaginous stage [14] which is the most prone period for occurrence of slippage, such management would be beneficial to prevent further slippage.
In conclusion, the present FEM study showed that spondylolytic pediatric lumbar spine has altered biomechanics when compared to the intact model. The stresses surrounding the vertebral growth plate increase, when compared to the corresponding intact model. These stress concentrations may lead to physis stress fracture and eventually spondylolisthesis.
Footnotes
Part I of this article can be found at http://dx.doi.org/ 10.1007/s00586-005-1026-z
References
- 1.Axelsson P, Johnsson R, Stromqvist B. Is there increased intervertebral mobility in isthmic adult spondylolisthesis? A matched comparative study using roentgen stereophotogrammetry. Spine. 2000;25:1701–1703. doi: 10.1097/00007632-200007010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Dandy DJ, Shannon MJ. Lumbosacral subluxation (Group 1 spondylolisthesis) J Bone Joint Surg [Br] 1971;53:578–595. [PubMed] [Google Scholar]
- 3.Farfan HE, Osteria V, Lamy C. The mechanical etiology of spondylolysis and spondylolisthesis. Clin Orthop. 1976;117:40–55. [PubMed] [Google Scholar]
- 4.Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg [Am] 1984;66:699–707. [PubMed] [Google Scholar]
- 5.Goel VK, Lim TH, Gwon J, et al. Effects of rigidity of an internal fixation device. A comprehensive biomechanical investingation. Spine. 1991;16(Suppl):S155–S161. doi: 10.1097/00007632-199103001-00023. [DOI] [PubMed] [Google Scholar]
- 6.Goel VK, Monroe BT, Gilbertson LG, et al. Interlaminar shear stresses and laminae separation in a disc: Finite element analysis of the L3-4 motion segment subjected to axial compressive loads. Spine. 1995;20:689–698. [PubMed] [Google Scholar]
- 7.Goel V, Grauer J, Patel T, et al. Effects of Charite artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine. 2005;30:2755–2764. doi: 10.1097/01.brs.0000195897.17277.67. [DOI] [PubMed] [Google Scholar]
- 8.Ikata T, Miyake R, Katoh S, et al. Pathomechanism of sports-related spondylolisthesis in adolescents: radiographic and magnetic resonance imaging study. Am J Sports Med. 1996;24:94–98. doi: 10.1177/036354659602400117. [DOI] [PubMed] [Google Scholar]
- 9.Kajiura K, Katoh S, Sairyo K, et al. Slippage mechanism of pediatric spondylolysis: biomechanical study using immature calf spines. Spine. 2001;26:2208–2212. doi: 10.1097/00007632-200110150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Kong WZ, Goel VK. Ability of the finite element models to predict response of the human spine to sinusoidal vertical vibration. Spine. 2003;28:1961–1967. doi: 10.1097/01.BRS.0000083236.33361.C5. [DOI] [PubMed] [Google Scholar]
- 11.Konz RJ, Goel VK, Grobler LJ, et al. The pathomechanism of spondylolytic spondylolisthesis in immature primate lumbar spines in vitro and finite element assessments. Spine. 2001;26:E38–49. doi: 10.1097/00007632-200102150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Laurent LE, Einola S. Spondylolisthesis in children and adolescents. Acta Orthop Scand. 1961;31:45–64. doi: 10.3109/17453676108989297. [DOI] [PubMed] [Google Scholar]
- 13.Mihara H, Onari K, Cheng BC, et al. The biomechanical effects of spondylolysis and its treatment. Spine. 2003;28:235–238. doi: 10.1097/00007632-200302010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Panjabi MM, Goel VK, Walter SD, Schick S. Errors in the center and angle of rotation of a joint: an experimental study. J Biomech Eng. 1982;104:232–237. doi: 10.1115/1.3138354. [DOI] [PubMed] [Google Scholar]
- 15.Sairyo K, Goel VK, Grobler LJ, et al. The pathomechanism of isthmic lumbar spondylolisthesis. A biomechanical study in immature calf spines. Spine. 1998;23:1442–1446. doi: 10.1097/00007632-199807010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sairyo K, Katoh S, Ikata T, et al. Development of spondylolytic olisthesis in adolescents. Spine J. 2001;1:171–175. doi: 10.1016/S1529-9430(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 17.Sairyo K, Katoh S, Sakamaki T, et al. Slippage occurs following epiphyseal separation in immature spine and its occurrence is unrelated to disc degeneration. Spine. 2004;29:524–527. doi: 10.1097/01.BRS.0000106492.51581.9B. [DOI] [PubMed] [Google Scholar]
- 18.Sairyo K, Biyani A, Goel VK, et al. Pathomechanism of hypertrophy of ligamentum flavum: A multidisciplinary investigation by clinical, biomechanical, histological and biological assessment. Spine. 2005;30:2649–2656. doi: 10.1097/01.brs.0000188117.77657.ee. [DOI] [PubMed] [Google Scholar]
- 19.Sakamaki T, Katoh S, Sairyo K. Normal and spondylolytic pediatric spine movements with reference to instantaneous axis of rotation. Spine. 2002;27:141–145. doi: 10.1097/00007632-200201150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Sakamaki T, Sairyo K, Katoh S, et al. The pathogenesis of slippage and deformity in the pediatric lumbar spine: a radiographic and histologic study using a new rat in vivo model. Spine. 2003;28:645–650. doi: 10.1097/00007632-200304010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Saraste H. Long-term clinical and radiographical follow up of spondylolysisand spondylolisthesis. J Pediatr Orthop. 1987;7:931–938. [PubMed] [Google Scholar]
- 22.Seitsalo S, Osterman K, Hyvarinen H, et al. Progression of spondylolisthesis in children and adolescents: a long-term follow-up of 272 patients. Spine. 1991;16:417–421. doi: 10.1097/00007632-199104000-00004. [DOI] [PubMed] [Google Scholar]