Recent findings have increasingly demonstrated the importance of rare genetic variants in the etiology of schizophrenia (1). 22q11.2 deletion syndrome, or velocardiofacial syndrome (VCFS), is among the most common of these rare variants, accounting for about 1% to 2% of schizophrenia cases in the general population (2). VCFS is caused by a hemizygous deletion at chromosome 22q11.2, an area that encompasses approximately 40 genes, several of which are known to play a role in neuronal migration, myelination, and brain development (2). Longitudinal studies have found that approximately 30% of individuals with 22qDS develop psychotic illness in adolescence or early adulthood (3), offering the possibility of delineating a relatively homogenous developmental pathway to psychosis. Schizophrenia patients with VCFS have clinical profiles that are indistinguishable from schizophrenia patients without the deletion (4). However, whether the neurobiological predictors of psychosis in highly penetrant rare mutations such as VCFS are similar to those observed for idiopathic schizophrenia remains unresolved.
Until recently, the prevailing model for discovering the genetic basis of schizophrenia has focused on “common disease-common variants,” hypothesizing that the disorder arises from interactions between environmental effects and multiple common variants, each carrying modest risk for disease. However, recent findings have shifted our understanding of the genetic architecture of schizophrenia; taken together, these studies indicate that schizophrenia involves much greater genetic heterogeneity than was previously believed (1). Thus, a complementary approach focusing on highly penetrant rare variants is likely to yield new insights into schizophrenia pathophysiology. Recent findings support this notion, showing a disproportionate increase in rare mutations of genes involved in neurodevelopment in schizophrenia cases (5). The high odds ratios for certain loci in “genomic hotspots”—including the 22q11.2 locus—suggest enrichment of a causal variant (or variants) for the disorder in these regions. Thus, investigating intermediate traits (e.g., structural brain changes over time) in highly penetrant genetic subtypes of complex neuropsychiatric disorders such as VCFS can provide a unique opportunity to link genetic mechanisms directly to phenotypes associated with schizophrenia.
The report by Kates and colleagues in this issue of Biological Psychiatry exemplifies how this genetic subtype of schizophrenia can shed light on neurobiological pathways leading to the development of psychosis. Specifically, Kates et al. (6) amassed an impressive sample of longitudinal neuroimaging data on adolescents with VCFS, their unaffected siblings, and unrelated community control participants and then examined the neuroanatomic trajectories associated with the development of positive prodromal symptoms of psychosis. The large sample size and narrow age range used in this study address the limitations of the few previous longitudinal studies examining neuroanatomic predictors of psychosis in VCFS (e.g., Gothelf et al. [3]). Kates et al. find that, although progressive volume loss in multiple brain regions was associated with a general increase in symptom severity over the 3-year follow-up period, only decrements in temporal lobe gray matter and verbal IQ were uniquely predictive of increased severity of positive psychotic-like symptoms at the follow-up time point. Perhaps most interestingly, they used a receiver operating characteristic (ROC) analysis to determine the accuracy with which prodromal status in VCFS was predicted by neuroanatomic changes in brain regions significantly associated with prodromal symptom scores. These results indicate that 86% of the time, this change score would accurately identify a prodromal individual with VCFS. This analysis presents a novel way to look at predictive accuracy of neuroanatomic markers in the context of this disease model, in which larger effects can likely be identified because of reduced heterogeneity.
As the largest study to date to examine longitudinal brain changes as predictors of outcome in VCFS, this study provides novel information regarding neuroanatomic changes associated with the emergence of psychosis in this syndrome. These findings are highly consistent with prior cross-sectional work; for example, Bearden et al. (7) found that reduced temporal gray matter was associated with severity of thought problems (as measured by the Child Behavior Checklist) in nonpsychotic youth with VCFS. Further corroborating these findings, Chow et al. (8) directly compared brain structures of adults with the deletion with and without schizophrenia, finding that the expression of schizophrenia in adults with VCFS is associated with a selective reduction in gray matter in the superior temporal gyrus (STG). Although speculative, taken together, these find-ings suggest that temporal gray matter loss in adolescence may remain a stable and distinguishing characteristic associated with the expression of psychosis in VCFS.
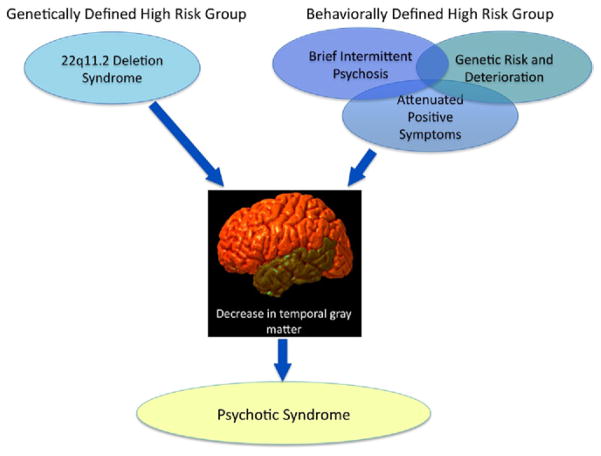
These findings converge remarkably with the extant literature on youth with clinical symptoms indicating high risk for developing psychosis. In particular, Takahashi et al. (9) recently reported that clinical high-risk youth who subsequently convert to psychosis show progressive gray matter decreases in superior temporal regions. Others have found baseline reductions in the temporal lobe in those who later develop psychosis (e.g., Pantelis et al. [10]), suggesting a regional pathological process that precedes the expression of overt psychosis. Findings of selective STG gray matter loss have previously been observed specifically in first-episode schizophrenia patients, but not in affective psychosis (11), and volume reduction of these regions, particularly in the left hemisphere, is associated with auditory hallucinations and thought-disorder severity (12). Collectively, these findings suggest that disruption of cortical regions specialized for speech and language may be implicated in disease pathogenesis (8) and thus may serve as a valuable phenotype for identifying increased risk for psychosis (see Figure 1).
Figure 1.
Converging predictors of psychotic symptoms in a behaviorally defined risk group (adolescents with clinical symptoms indicating high-risk status) and those at high risk by virtue of a specific genetic etiology (22q11.2 microdeletion). These two alternative approaches offer convergent evidence of potential neuroanatomic endophenotypes for psychosis.
The results from this study also leave many unanswered questions. First, none of the individuals in the published sample had converted to overt psychotic illness at the follow-up time point. Therefore, it will be important to map neuroanatomic trajectories similarly in older adolescents and young adults with VCFS, through the time period in which the onset of schizophrenia is most likely to occur. In addition, the underlying cellular mechanisms of the observed neuroanatomic changes are unknown. It has been hypothesized that dysfunction of glutamate synapses in this region may be related to neurotoxicity in early psychosis (9); to that end, the investigation of a single mutational model of schizophrenia confers particular advantages for conducting translational studies in animal models, in which the molecular, cellular, and synaptic basis of neuroanatomic changes can be directly probed.
Although many genes within the 22q11.2 locus are known to be involved in neurodevelopment and several have been implicated in schizophrenia risk, the exact mechanisms through which genetic variation— both within and outside of this genomic region—leads to variability in phenotypic expression in VCFS individuals remain to be determined. Studies in mouse models have provided compelling evidence that the 22q11.2 microdeletion causes dysregulation of brain microRNAs, which may contribute to schizophrenia susceptibility (2). However, the contribution of these genetic pathways to neuroanatomic alteration is not yet known and remains an important area for future investigation.
It has been hypothesized that schizophrenia is a constellation of multiple rare variants (1). Therefore, examining the most common of these rare, highly penetrant mutations—VCFS—may help delineate a homogenous pathway to psychosis. The new findings from Kates et al. featured in this issue offer compelling evidence for common neuroanatomic substrates underlying the development of psychotic symptoms in VCFS and idiopathic schizophrenia and thus represent a powerful example of how this line of inquiry can provide clues into the biological mechanisms underlying development of psychotic illness in the broader population.
Acknowledgments
This research was supported by the National Institute of Mental Health Grant No. RO1 MH085953 (to CEB) and T32MH073526-05 (to MJ).
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: One disorder, multiple mutations; one mutation, multiple disorders. Trends Genet. 2009;25:528–535. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: Linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- 4.Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R, et al. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 6.Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, et al. Neuroanatomic predictors to prodromal psychosis in velocardiofacial syndrome (22q11.2 deletion syndrome): A longitudinal study. Biol Psychiatry. 2011;69:945–952. doi: 10.1016/j.biopsych.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearden CE, van Erp TG, Monterosso JR, Simon TJ, Glahn DC, Saleh PA, et al. Regional brain abnormalities in 22q11.2 deletion syndrome: Association with cognitive abilities and behavioral symptoms. Neurocase. 2004;10:198–206. doi: 10.1080/13554790490495519. [DOI] [PubMed] [Google Scholar]
- 8.Chow EW, Ho A, Wei C, Voormolen EH, Crawley AP, Bassett AS. Association of schizophrenia in 22q11.2 deletion syndrome and gray matter volumetric deficits in the superior temporal gyrus [published online ahead of print March 1] Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2010.10081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 10.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: A cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 11.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]