INTRODUCTION
Anxiety disorders are the most common group of childhood psychiatric disorders, with almost one in three children having suffered from an anxiety disorder at some point during childhood or adolescence.1 Most anxiety disorders begin early in development, with a median age at onset of 12 years.1 Although significant advances have been made in identifying the best treatments for anxious children,2 little is known about the neural underpinnings of anxiety disorders. Identifying the neural basis of childhood anxiety disorders can guide development of prevention programs, a critical step in reducing these early-onset and highly prevalent disorders. This article reviews the available literature to provide a current understanding of the neural basis of childhood anxiety disorders. It reviews the development of normative fear and the development of the brain regions that subserve fear and anxiety. It further provides a comprehensive summary of relevant functional and structural neuroimaging studies, including studies examining children with anxiety disorders and children at-risk for developing anxiety disorders.
NORMATIVE DEVELOPMENT OF FEAR
Development of Fear
Fear is a normal and adaptive response to potential threat. Key components of the fear system mature early in development,3 and multiple periods of child development are marked by normative fears4 (see Ref.3 for a review). For example, most infants experience a period of stranger anxiety around 8 to 12 months of age, marked by wariness or distress around new people.5–8 Stranger anxiety is often followed by separation anxiety, typically evident around 10 to 18 months, and characterized by distress about being separated from the mother or father.6 Stranger and separation fears are thought to be protective, preventing the child from encountering harm during developmental periods marked by the onset of walking and increased exploration away from caregivers. For most children, these normative fears vanish by 2 to 3 years of age; however, for some, childhood is marked by the persistence of these fears and the development of new fears. Increases in the prevalence of anxiety disorders parallel decreases in normal fear, such that separation anxiety disorder typically arises relatively early in life, shortly after the age-typical diminution in normal separation anxiety. Similarly, social phobia typically arises in adolescence, around the time of age-typical decreases in normal social anxieties.
These normative fears are observed in most children and manifest across cultures,3 suggesting that fundamental aspects of human brain development sculpt developmental changes in fear. The universality of the development of early fears raises questions about the nature of relationships between brain development and fear. Although we expect that developmental changes in brain function relate to developmental changes in both normal and abnormal fears, the nature of these relationships largely remains unknown.
Fear Neurocircuitry
Research on rodents and nonhuman primates has identified the key components of fear circuitry.9–14 Using fear conditioning tasks in rodents, researchers have identified that the amygdala—a small subcortical collection of nuclei in the medial temporal lobe—is critical for the production of fear behaviors.9 In nonhuman primates, amygdala lesions result in significantly reduced fear behaviors,11,15 providing further support for the amygdala's role in the production of fear behaviors. However, some debate persists concerning the precise role of the amygdala in fear. Findings appear most consistent for stimulus-reinforcement learning, as it relates to fear in classical conditioning, in which the amygdala is necessary for such forms of learning. But the amygdala also supports stimulus-reinforcement learning as it relates to positive stimuli.16–18 Moreover, animals that suffer amygdala lesions still are capable of manifesting fear. In fact, under some circumstances, amygdala lesions actually can lead to increases in fear.19 Thus, the amygdala's role in fear may represent one prototypical function of a broader role in emotional processing.
The amygdala has received substantial attention as a core component of fear circuitry; however, other brain regions are also involved in fear and anxiety. For example, the bed nucleus of the stria terminalas (BNST)—a part of the “extended amygdala”—is involved in sustained fear reactions (in contrast to short-term or phasic fear responses) in rodents.10 These sustained reactions, which are elicited by less specific and less predictable threats, are maintained over time and are considered akin to anxiety in humans.20
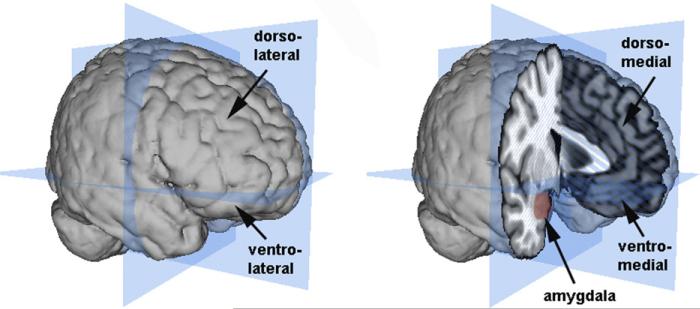
Another major component of the fear circuit is the prefrontal cortex (PFC), a brain region involved in both the automatic and effortful regulation of emotion. Within the prefrontal cortex, multiple regions are engaged during emotion processing and emotion regulation; however, as with research on the amygdala, debate persists about the nature of specific PFC contributions. Most current theories divide the primate PFC using two axes, one separating the medial from lateral PFC and another separating the dorsal from ventral PFC (Fig. 1).
Fig. 1.
Illustration of the amygdala and the major divisions of the PFC. The planes (in blue) show the major dorsal/ventral and anterior/posterior divisions of the brain. The lateral PFC is shown on the left and the medial PFC and amygdala are shown on the right. Brain images and surface constructions were created using Mango (Research Imaging Center, UTHSCSA; http://ric.uthscsa.edu/mango/mango.html) and a Montreal Neurological Institute standard brain.
Of the multiple PFC brain regions, the ventromedial PFC (vmPFC) is most strongly implicated in anxiety. The vmPFC plays a critical role in the inhibition of conditioned fear expression14,21 and extinction of conditioned fear,22–24 which can be viewed as two instances of emotion regulation.23,25 Evidence also exists showing that vmPFC activity in some specific locales correlates positively with negative emotions,26,27 suggesting that at least some particular portions of the vmPFC may drive negative emotions. These multiple roles may reflect different functions of subregions of the vmPFC. For example, some research suggests that in rodents the prelimbic component of vmPFC supports fear-related behaviors whereas the infralimbic component of vmPFC suppresses fear-related behaviors. However, because the majority of this research has examined rodents, issues of cross-species differences in PFC function preclude firm conclusions in humans.
In humans, other research focuses on lateral and dorsal regions of the PFC. The ventrolateral PFC (vlPFC) has been implicated in emotion regulation,28,29 which may reflect the role of this region in attention control. The dorsomedial PFC (dmPFC) is engaged during the intentional control of emotional reactions,23,30–33 which may reflect the broader role of the dmPFC in monitoring. Finally, the dorsolateral PFC (dlPFC) is implicated in intentional emotion regulation,33–35 which may relate to the dlPFC's role in cognitive control and executive function.
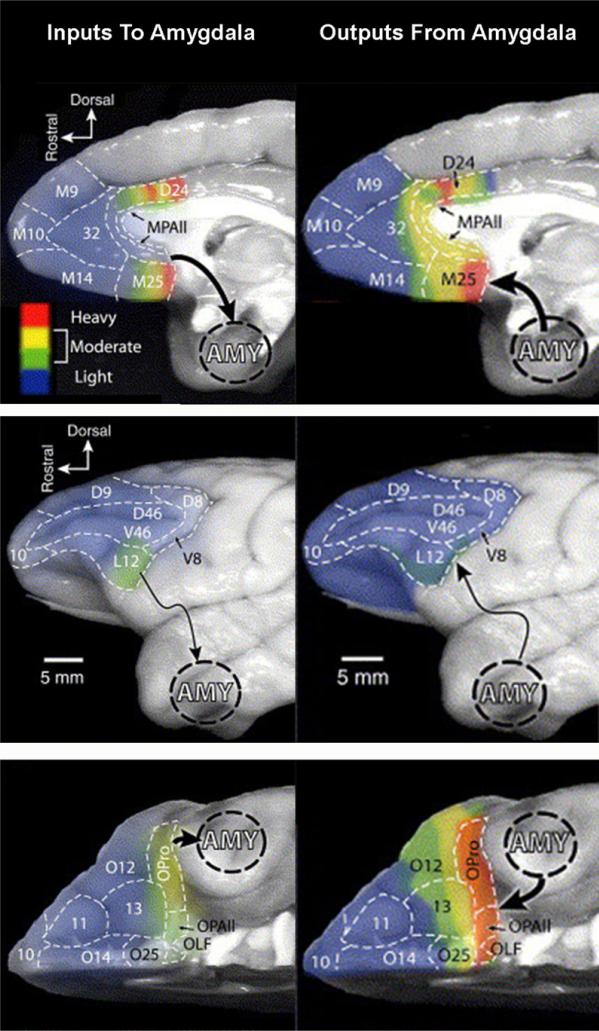
Neuronal tracer studies in nonhuman primates provide evidence of anatomic connections between the amygdala and multiple regions of the PFC (for reviews see Ref.35–37). The amygdala has strong, bidirectional connections to medial prefrontal regions along the anterior cingulate cortex surrounding the corpus callosum, extending from ventral regions (subgenual cingulate cortex) to rostral (pregenual cingulate) and dorsal (supragenual cingulate) regions (Fig. 2). The amygdala also has dense projections to the vlPFC,38 – 40 but these connections are more unilateral and are mostly ascending from vlPFC to the amygdala.38 – 42 In contrast, the amygdala appears to have relatively few connections to frontal regions of the PFC (frontal pole) or dlPFC regions.38,40–42 Ongoing research continues to refine understandings of these connections, both from anatomic and functional perspectives. Given that the majority of this research has focused on mature organisms and that connections continue to change across development, understandings of these connections remain even less precisely specified in immature organisms. For example, although it is clear that children and adolescents are capable of utilizing all portions of the PFC to perform at least some of the functions for which adults utilize the PFC, the precise timing when specific PFC functions and associated connections mature in humans remains unclear.
Fig. 2.
Degree of labeling intensity in PFC neurons for both inputs to the amygdala (left column) and outputs from the amygdala (right column). Rows show different surface views: medial surface (top), lateral surface (middle), and ventral surface (bottom). (Adapted from Ghashghaei H, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 2007;34:905–23; with permission.)
PFC connections to the amygdala synapse in multiple regions. However, interest focuses primarily on γ-aminobutyric acid-ergic (GABAergic) neurons, emphasizing an inhibitory role for the PFC over amygdala function.41,43,44 At least in some contexts, neuroimaging studies in humans show an inverse relationship between the amygdala and multiple PFC regions including vmPFC,45,46 vlPFC,32,47,48 dmPFC,23,34,49–54 and dlPFC.25,34 Particularly for the vlPFC and vmPFC, in which PFC–amygdala connections have been mapped, these findings suggest inhibitory input; however, such findings remain indirect.
In summary, the amygdala and PFC are key components of human fear neurocircuitry. The amygdala and PFC are interconnected with the PFC modulating amygdala responses. To date, the majority of our knowledge about the neural bases of anxiety comes from functional neuroimaging studies in adults (for a meta-analysis see Ref.55). Results from these studies suggest that adult anxiety disorders may be characterized by brain dysfunction resulting in “too much gas and not enough brakes”; that is, the fear production system is too strong and the fear regulation system is too weak.
Development of Fear Circuitry
The developmental trajectories of both the amygdala and PFC are consistent with this idea of an imbalance between the fear production and fear regulation systems. The amygdala is functional early in development,56 shortly after birth in humans.57 Studies of amygdala lesions occurring early versus late in development provide critical information about the development of amygdala function. In rhesus monkeys, early lesions (2 weeks) result in a decreased fear to threatening objects during multiple stages of development,19,58,59 demonstrating that early damage to the amygdala has an early and sustained impact on nonsocial fear processing. In contrast, amygdala lesions in neonatal rhesus monkeys result in increased fear during nonthreatening social interactions at both 6 to 8 months of age19 and 12 months of age,60 suggesting a different early role for the amgydala in social processing. In adult rhesus monkeys, amygdala lesions produce decreased fear to threatening objects11 and decreased fear during both nonthreatening and threatening social interactions.11,15 Thus, the amygdala's role in nonsocial fear is consistent across development, with both early and late amygdala lesions similarly decreasing fear to threatening objects. However, the role of the amygdala in the development of social fear is more complex, pointing to possible developmental differences in the contribution of the amygdala to social behaviors.
Evidence from human studies of amygdala lesions also indicates developmental effects; for example, individuals with early amygdala lesions fail to show the normal emotional enhancement of memory and understanding of others’ emotional states.61,62 Developmental functional magnetic resonance imaging (fMRI) studies of amygdala responses to human expressions of fear (“fear faces”) may provide clues about amygdala development (see Ref.63 for a review). When viewing fear faces, adult humans show an increase in amygdala activation relative to amygdala activation during a baseline condition, such as viewing a fixation cross or blank screen.16–18 Increased amygdala activation likely represents the salience of the stimulus64 – 66 because the amygdala responds robustly to negative and positive stimuli,16 as well as novel67 stimuli. Preliminary studies in children and adolescents suggest that amygdala activation to fear faces in children is similar to adults, but intriguingly is heightened in adolescents45,68,69 (although not all studies find this developmental effect70). This heightened fear response in adolescence is consistent with heightened emotion recognition accuracy during this period.70 Together, findings from lesion and neuroimaging studies demonstrate that the amygdala is critical for the normal development of the fear system.
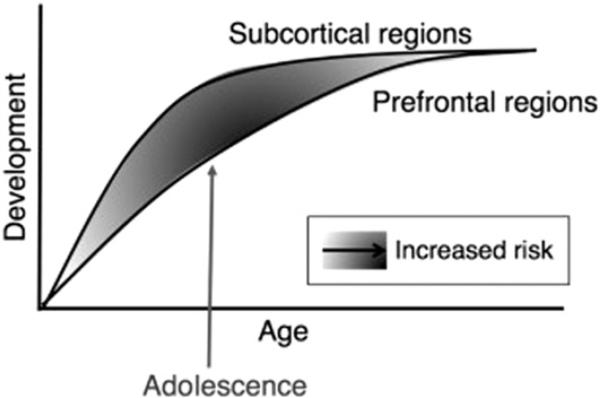
Both the ventral and dorsal regions of the PFC are among the last brain regions to mature, undergoing significant structural and functional changes during development.71–76 fMRI studies comparing different age groups provide evidence of differences across between children, adolescents, and adults in activation in the vlPFC,69,77 vmPFC,78,79 dmPFC,70,79–81 and dlPFC.72,79,82,83 These findings are consistent with behavioral data showing increasing skill with age for both emotional tasks45,84 and cognitive tasks.84 – 87 PFC development is relatively linear with age, and the development of the PFC provides a mechanism for dampening amygdala hyper-responsivity through increased emotion regulation abilities. Casey and colleagues88,89 provide an integrative developmental theory that postulates that the increased emotionality common during adolescence results from the imbalance between patterns of amygdala and PFC development (Fig. 3). According to this model, during adolescence amygdala function is enhanced relative to PFC function, resulting in an overcontribution of the amygdala to adolescent emotions and behavior. As PFC development catches up during early adulthood, emotions and behavior are stabilized. This amygdala–PFC imbalance may contribute to the increased prevalence of anxiety disorders during early adolescence.
Fig. 3.
Model illustrating the imbalance between the early maturation of subcortical regions, such as the amygdala, and late maturation of PFC regions during adolescence. The shaded areas indicate degree of amygdala–PFC imbalance. (From Somerville LH, Jones RM, Casey B. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn 2010;72:124–33; with permission.)
Attentional Modulation of the Amygdala
Attention contributes to fear processing and the amygdala's response to stimuli is modulated by attention. Multiple studies have demonstrated that amygdala activity varies as a function of task demands.34,68,90–93 Neuroimaging studies using a passive viewing task—in which attention is not specifically constrained by the task—show greater amygdala activation than studies that use a specific task, such as gender discrimination or emotion rating.16,17 Reduced amygdala activation during tasks likely reflects the effects of emotion regulation brain regions that are engaged during the tasks.34,90–93 However, it should also be noted that attention is not required in all circumstances for amygdala response to emotional stimuli—for example, amygdala activation to fearful faces can occur in the absence of attention, potentially when the fearful faces are masked and not consciously detected94—although debate remains surrounding this point.95
The modulating role of attention is especially important for understanding anxiety disorders, given that attentional processes are altered in children and adults with anxiety disorders.96,97 In anxiety disorders, a commonly studied attentional process is attentional bias. Attentional bias is often measured using the “dot probe task.” In that task, individuals fixate on a central point on the screen and are then shown a threatening and a neutral image on opposite sides of the screen. After the brief stimulus presentation, a dot is presented on one side of the screen and individuals must press a button as soon as they detect the dot. A shorter latency to detect the dot that appears in the same position as the threatening stimulus is evidence of attentional vigilance to threat. Anxious individuals are more likely to show attentional bias to threat, such as aversive images, fear faces, or angry faces (see Refs.96,98 for reviews).
In fMRI studies of anxiety, a limitation of passive viewing studies (studies that do not require the subject to perform a task) is that observed amygdala differences may be a consequence of differences in attention. Controlling for attentional differences using a task can help to dissect differences in amygdala activation caused by anxiety from those caused by attention. Thus, neuroimaging findings need to be considered within the context of the attention requirements of various study designs.
NEUROIMAGING STUDIES OF FEAR-BASED ANXIETY DISORDERS: GENERALIZED ANXIETY DISORDER, SOCIAL PHOBIA, SEPARATION ANXIETY DISORDER
Although we assume that differences in brain function underlie childhood anxiety, little research has focused on identifying the underlying neural causes. Studies of adult anxiety have provided critical information about possible brain dysfunction in childhood anxiety disorders; however, we do not know whether the differences found in adults reflect the underlying causes of anxiety or are the consequence of anxiety. Thus, studies in children with anxiety or at high-risk for developing anxiety are critical for uncovering the neural causes of anxiety.
Findings from fMRI studies of adults with anxiety disorders55 provide the initial candidate brain regions for studies of childhood anxiety—the amygdala and PFC. Most neuroimaging studies of child anxiety have used the same experimental tasks previously used to study anxious adults. However, several groups are developing new tasks that are especially valid for studying children and adolescents, such as the “chat room” task (see section on Social Phobia). While this is an exciting time in the evolution of our understanding of the neural bases of childhood anxiety disorders, we still have much to discover.
Studies of children with anxiety disorders provide a direct approach to identifying the neural bases of anxiety disorders. An equally important approach is to study traits that are associated with high-risk for developing anxiety.99 Advances in neuroscience methods, such as neuroimaging and genetics, provide new opportunities to link neurobiological measures to dimensional traits in individuals with and without anxiety disorders. Among various high-risk traits, a commonly studied trait is behavioral inhibition— or inhibited temperament—a trait characterized by wary or avoidant responses to novel people, places, or things.100 Behavioral inhibition emerges in infancy,101,102 is heritable,103 and is associated with a distinct physiologic profile.104–107 Inhibited children have a four-fold increase in risk for developing any anxiety disorder, with specific risk for social phobia.108–110 Studies of young adults who were inhibited as children provide evidence for amygdala dysfunction111–114 which is best characterized as a failure of the amygdala to habituate normally to repeated presentations of faces,114,115 resulting in a sustained amygdala response.112 Recent studies provide initial evidence for both structural116 and functional differences in the PFC.117 Thus, studying traits associated with anxiety provide a promising approach to identifying the structural and functional correlates of anxiety.
This section reviews and integrates functional and structural neuroimaging findings in generalized anxiety disorder, social phobia, and separation anxiety disorder, as well as the associated high-risk traits.
Generalized Anxiety Disorder
Functional MRI
Childhood generalized anxiety disorder (GAD) is characterized by excessive anxiety and worry about a variety of events and situations, difficulty controlling the anxiety, and presence of one or more physical symptoms.118 To date, the majority of studies in GAD have focused on the amygdala. The most consistent finding in childhood GAD is increased amygdala activation during viewing of negative emotional expressions. Increased amygdala activation has been reported across multiple study designs including passive viewing of fear faces,119 subjective ratings of fear,120,121 viewing masked angry faces during a dot-probe task,122 and viewing both subsequently remembered and forgotten faces during a face memory task.123 In one of the studies, amygdala activation to fear faces also correlated with anxiety severity,119 suggesting a direct link between amygdala response and anxiety. It should be noted that one study failed to find differences in amygdala activation when viewing masked angry faces during the dot-probe task.124
Multiple studies also report differences in PFC activation to negative emotions in GAD. For example, anxious children had increased activation during subjective ratings of fear, relative to healthy controls, in the vlPFC120,121 and dmPFC.120 In another study, adolescents with GAD showed increased activation in the vlPFC relative to healthy controls when viewing masked angry faces in a dot-probe task; but, within the GAD adolescents, degree of vlPFC activation correlated negatively with anxiety severity, suggesting a regulatory role.124 However, not all studies report PFC differences.122
Children with GAD also show differences in functional connectivity between the amygdala and other brain regions. For example, when making subjective ratings of fear faces, anxious children showed increased functional connectivity between the amygdala and insula, relative to healthy controls,120 and the degree of connectivity correlated positively with anxiety symptoms. In another study, healthy controls showed an inverse functional connectivity between the amygdala and vlPFC that was diminished in the children with GAD.122
Several studies have examined the neural correlates of treatment effects in childhood GAD. A preliminary study examined the association between pretreatment differences in brain function with symptom improvement following treatment with medication or cognitive behavior therapy in adolescents with GAD.125 Pretreatment amygdala activation was associated with less clinical improvement after treatment. A second study examined the neural correlates of treatment response in adolescents with GAD.126 Successful treatment with either cognitive behavioral therapy (CBT) or fluoxetine was associated with increased activation to angry faces in the right vlPFC, a region associated with emotion regulation.
In summary, children with GAD consistently show increased amygdala activation across a variety of tasks. Although several studies reported PFC differences, the direction of the effect was not consistent. It is important to note that task differences likely impact the direction of the PFC effect (increase vs decrease) when comparing individuals with anxiety relative to healthy controls. For example, because healthy controls do not typically show an attentional bias, it is unlikely that they will engage attentional or regulatory regions. In this case, PFC activation should be higher in the anxious group because they have an attentional bias, but increased activation should not be interpreted as “better” function. To understand how PFC dysfunction relates to anxiety, it is critical to examine the association between PFC activation and anxiety severity or symptom improvement. Relatively lower vlPFC activation was associated with higher anxiety severity and lower symptom improvement with treatment, suggesting dysfunction in vlPFC regulation of the amygdala contributes to GAD anxiety symptoms.
Structural MRI
Findings of structural differences in the amygdala are less consistent. A study of adolescents with GAD showed larger right amygdala volume relative to controls127; however, another study of adolescents with anxiety disorders (predominantly GAD) found smaller left amygdala volume.128 Sample sizes in both studies were relatively small; moreover, methods in the two studies also were quite different. Therefore, larger samples studied comprehensively will be useful in resolving the conflicting amygdala results. To date, only one study has examined PFC volume and found no differences in adolescents with GAD compared to controls in PFC gray matter volume or white matter volume.129 Thus, support for structural differences in childhood GAD remains weak.
At-risk children
Trait anxiety reflects a temperamental trait characterized by persistent anxiety and worry and is therefore very similar to GAD. Generally, higher trait anxiety is associated with increased risk for GAD; however, a child can have high trait anxiety but not meet diagnostic criteria for GAD. Telzer and colleagues130 demonstrated that trait anxiety in healthy children and adolescents corresponded to both a behavioral attentional bias toward angry faces and increased activation in the right dlPFC. When viewing all faces (regardless of emotion), higher trait anxiety was associated with increased vlPFC activation.
Krain and colleagues131 examined intolerance of uncertainty—the tendency to react negatively to situations that are uncertain—a major symptom of GAD.132,133 In healthy adolescents and anxious adolescents (GAD, social phobia, or both), higher intolerance of uncertainty was associated with increased activation in the amygdala, vmPFC and dmPFC during decision making in the context of absolute uncertainty (50/50 chance of being right). Within only the anxious adolescents, there was substantial variability in both intolerance of uncertainty and brain function. Anxious adolescents with high intolerance of uncertainty had increased amygdala and orbitofrontal cortex activation, whereas anxious adolescents with low intolerance of uncertainty had decreased activation in these same brain regions. These findings point to the importance of examining specific traits or symptoms of anxiety even within individuals with an anxiety diagnosis.
Social Phobia
Functional MRI
Among the anxiety disorders, social phobia (SP) is very common, second in prevalence only to specific phobias.1 Blair and colleagues134 explored brain function differences in adolescents with SP relative to healthy controls during an emotional face processing task. Adolescents with SP had increased activation in the amygdala and vmPFC when viewing fear faces. In addition, social anxiety severity correlated with vmPFC activation to both angry and fearful faces in the anxious group. The findings in adolescents were similar to results in adults with SP compared to controls, suggesting that the neural substrates of adult SP are already present in adolescence.
The emotion processing tasks commonly used to study brain function in anxiety disorders use both human faces and negative emotional expressions—both of which are particularly salient for SP—however, emotion processing tasks may not tap into other key factors that elicit SP, such as social-evaluative processes. Recently, several groups have begun to develop ecologically valid experimental tasks. Guyer and colleagues135 have developed a “chat room” task to engage concerns about peer evaluation. In this task, adolescents rate pictures of other adolescents based on their desire to chat with them in a future encounter. During a second visit, adolescents rate how much they think the other adolescents want to chat with them. Anxious adolescents showed increased activation in the amygdala and vmPFC when rating how much the other adolescents would want to chat with them; interestingly, activation was increased only when viewing the adolescents that the subjects had previously rated as low desirability. Anxious adolescents, but not healthy controls, showed functional connectivity between the amygdala and vlPFC, with higher anxiety associated with stronger functional connectivity.
At-risk children
Several studies in children and adolescents have examined traits relevant to SP, such as behavioral inhibition. Inhibited children and adolescents are typically shy, cautious, and reserved and are at significantly increased risk for developing social phobia.108,110,136 Behavioral inhibition is observable across development and can be measured using a variety of methods including direct observation, parent report, current self-report, and retrospective self-report. The most commonly used method for identifying behavioral inhibition in children is direct observation of behavior and using measured with laboratory assessments consisting of unfamiliar objects, unfamiliar peers, and unfamiliar adults.100,101,106,137 Laboratory assessments are considered by many to be the gold standard because they provide objective assessments of behavior; but, behavioral assessments are time-consuming, expensive, and may not reflect real-world behaviors. Parent-report and self-report questionnaires provide efficient and economical assessments of behavior across a wide variety of situations; but, questionnaires are subject to a variety of reporter biases, including over-reporting and under-reporting. Although there is evidence for moderate convergence across the two methods,138 it is important to consider methodologic issues when interpreting study results.
Studies of young adults who were inhibited as children demonstrate that behavioral inhibition is associated with amygdala hyperactivity111–114,117 and dmPFC hypoactivity117; however, only a handful of studies have examined brain function in inhibited children and adolescents. Perez-Edgar and colleagues139 compared neural responses during emotion processing in adolescents who were either characterized as inhibited or noninhibited as young children. The emotion processing task included a passive viewing (no task) condition and several attention conditions in which subjects subjectively rated emotional or nonemotional characteristics of the faces. In the inhibited adolescents, amygdala activation to fear faces was increased, relative to the noninhibited adolescents, when attention was focused on rating the emotion but decreased when the fear faces were passively viewed. These results suggest that inhibited adolescents have different neural responses to emotional faces and that the neural responses are modulated by attention and emotional state.
An important contribution of high-risk trait studies is the discovery that behaviorally inhibited adolescents are not only sensitive to threatening stimuli, but are also sensitive to reward. In an initial behavioral study, shy (inhibited) adolescents had faster reaction times during reward conditions in the Monetary Incentive Delay (MID) task, suggesting increased reward sensitivity.140 Using fMRI, Guyer and colleagues141 compared neural responses in the striatum (including the nucleus accumbens, caudate, and putamen) during the MID task in inhibited, relative to noninhibited, adolescents. Inhibited adolescents showed significantly greater striatal activation to increasing amounts of incentives (gain or loss) relative to the noninhibited adolescents. In a subsequent fMRI study, Bar-Haim and colleagues142 tested the effects of reward while controlling for possible effects due to performance evaluation by including a noncontingent motor task. Inhibited adolescents showed increased activation in the nucleus accumbens when reward outcome was contingent on their response but did not show differences in the noncontingent condition. These studies demonstrate that behaviorally inhibited adolescents have increased sensitivity during performance-dependent rewarding tasks, in addition to increased sensitivity to threat. This pattern of increased sensitivity to both threatening and rewarding stimuli is remarkably similar to increases seen normally during adolescence, consistent with an imbalance between development of the amygdala and PFC.
In summary, studies of children with SP or children at-risk for developing SP demonstrate increased amygdala activation to anxiogenic stimuli and demonstrate increased striatal activation to rewarding stimuli, suggesting a general heightened reactivity. In the PFC, childhood SP was associated with increased vmPFC activation. Although more studies of PFC function are needed, increased the observed vmPFC activation may result from specific regions of the vmPFC that drive amygdala activation and negative emotions.
Separation Anxiety Disorder
Separation anxiety disorder (SAD) is characterized by anxiety caused by separation from the child's home or caretakers that causes impairment because it leads to avoidance of separation. School refusal and difficulty sleeping alone are common symptoms of SAD. Although no study to date has used neuroimaging methods to probe brain structure or function in SAD, one study included a continuous measure of separation anxiety. In that study, amygdala activation to fear faces was examined in healthy children.143 Continuous measures of anxiety symptoms were assessed using the Multidimensional Anxiety Scale for Children (MASC144). Higher separation anxiety symptoms were associated with increased amygdala activation to fear faces, providing preliminary evidence for the amygdala as one neural basis of separation anxiety disorder.
Summary
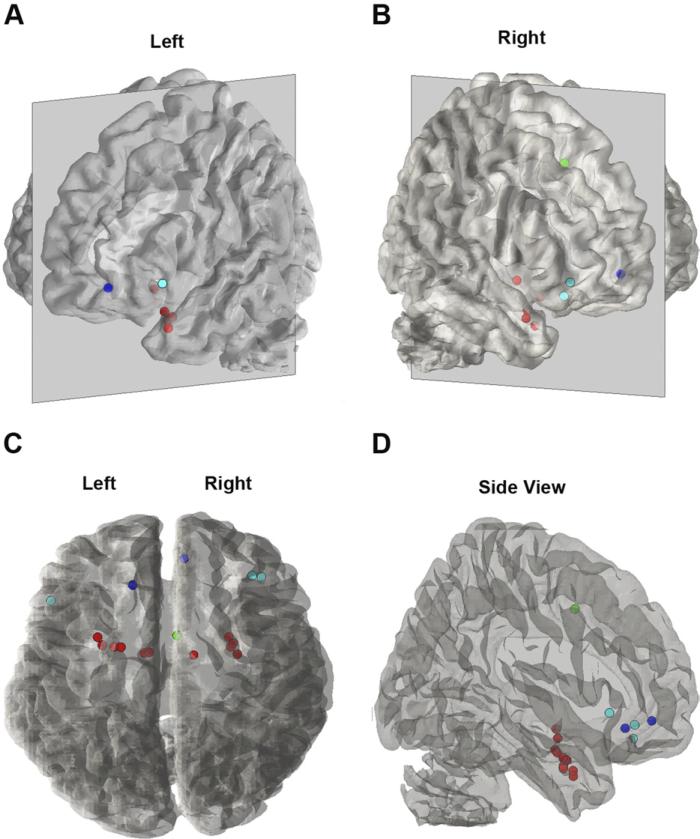
In summary, children and adolescents with GAD, SP, SAD, or high-risk traits show dysfunction in the amygdala and multiple PFC brain regions during experimental tasks probing fear neurocircuitry (Fig. 4). These findings are consistent with the results of a recent meta-analysis that reports that childhood anxiety (GAD, OCD, PTSD) is associated with alterations in the amygdala, vlPFC, and dmPFC.145 Across the studies, amygdala activation was increased in anxious children and adolescents, consistent with findings in anxious adults. Increased amygdala activation was also demonstrated in high-risk individuals, suggesting that amygdala hyper-reactivity is a heritable trait that confers risk for developing anxiety. In the PFC, the most common finding was increased activation, not a lack of PFC activation as predicted by developmental evidence for increasing emotion regulation and PFC activation with age. These preliminary findings are intriguing and suggest several possibilities. Group differences in PFC activation may reflect differential effects of the tasks on the two groups; examination of the relationship between anxiety severity and PFC function is important for understanding the role of the PFC. Also, increased PFC activation in anxious children may reflect differences in the duration of amygdala–PFC processing. Whereas both anxious and nonanxious children may initially engage the PFC during emotional tasks, the PFC may effectively inhibit amygdala activation quickly in nonanxious children, but not in the anxious children. Thus, anxious children may continue to engage the PFC in an attempt to inhibit amygdala response, with the effect of greater activation over the task. Alternatively, increased PFC activation could be driving increased amygdala activation, as suggested by some findings of coactivation of these regions in adults.26 Functional differences may be caused by underlying structural differences, but to date there are too few structural studies to draw any conclusions. Thus, although the foundation has been laid, much research is needed to understand the precise nature of the brain dysfunction that gives raise to childhood anxiety.
Fig. 4.
Peak activations from studies of children and adolescents with GAD, SP, or SAD (relative to healthy controls). Seventeen peaks from nine studies are illustrated on a 3-D transparent brain. Four perspectives are shown: (A) left lateral view, (B) right lateral view, (C) top view, and (D) side view. Dot colors represent different brain regions: red = amygdala (n = 11); dark blue = vmPFC (n = 2); light blue = vlPFC (n = 3); green = dmPFC (n = 1). Images were created using the plot_points_on_suface Matlab script included in the Multilevel Kernel Density Analysis toolbox (Tor Wager; http://wagerlab.colorado.edu/tools/).
NEUROIMAGING STUDIES OF OBSESSIVE–COMPULSIVE DISORDER
Childhood-onset obsessive– compulsive disorder (OCD) can be considered to be distinct from the other childhood anxiety disorders.146 Instead of having fear at its core, childhood OCD is defined by recurrent obsessions and compulsions that are time consuming or cause marked distress or significant impairments in daily function.118 Childhood-onset OCD, relative to adult-onset OCD, is characterized more by motor and vocal tics and the presence of comorbid diagnoses such as Tourette syndrome and attention-deficit/hyperactivity disorder.147,148 In addition, the prevalence of OCD is substantially lower than for the other anxiety disorders (see the article by Franklin and colleagues elsewhere in this issue for further exploration of this topic).149
Although fear is not a defining feature of OCD, some early theories about the neural substrates of OCD posited amygdala deficits150,151 with the amygdala having a role in maintaining compulsive behaviors. Like the other anxiety disorders, OCD has been conceptualized as an imbalance between a subcortical hyper-responsivity and cortical hyporesponsivity. In the case of OCD, the hyper-responsivity has been hypothesized to arise from the basal ganglia, with hyporesponsivity in multiple PFC regions, including the orbitofrontal cortex (OFC) and the anterior cingulate cortex (ACC). Deficits in these regions support the notion of OCD as a disorder of overmonitoring and difficulty inhibiting responses. Neuroimaging findings to date suggest that dysfunction in cortico–striato–thalamic circuit may underlie OCD.152,153
Relative to the other childhood anxiety disorders, OCD has received significantly more neuroimaging research attention. A relatively large and growing number of neuroimaging studies have examined both functional and structural correlates of childhood OCD. In light of this volume and the broad focus of the current review, only general conclusions can be provided. Despite the many challenges of studying childhood OCD—including small sample sizes, different experimental tasks, and medication status—some common trends are emerging (for extensive review please see Refs.154–158).
Functional MRI
Early neuroimaging research in childhood OCD has not shown evidence of increased amygdala activation in response to either contamination or symmetry provocations.159 A common probe of amygdala function used to study other anxiety disorders is the presentation of pictures of human emotional expressions. As reviewed earlier, anxious children often show increased amygdala activation when viewing fear faces; however, in children with OCD, amygdala activation is decreased, relative to healthy controls.160 This result is in contrast to other studies in the other childhood anxiety disorders, providing further evidence for a distinction between OCD and the other disorders.
To examine cortico–striato–thalamic dysfunction Woolley and colleagues161 tested response inhibition. During successful response inhibition, OCD was associated with decreased activation in the OFC, thalamus, and basal ganglia (caudate head, putamen, globus pallidus). During unsuccessful response inhibition OCD was associated with decreased medial PFC activation. To isolate the contributions of cognitive flexibility deficits to OCD, Britton and colleagues162 tested cognitive inflexibility while controlling for motor behavior. Children with OCD, relative to controls, had decreased OFC activation during set shifting, but there were no differences in the striatum, dmPFC, or dlPFC. Caudate activation was positively correlated with a behavioral measure cognitive flexibility in healthy controls, but negatively correlated in OCD. Findings from these studies are consistent with a meta-analysis of functional brain differences found that childhood OCD was associated with functional differences in the frontal and parietal cortices, striatum, and thalamus.145 Together, these fMRI studies provide preliminary evidence that children with OCD have dysfunction in the OFC, thalamus, and basal ganglia.
The first neuroimaging studies to examine the effect of treatment on brain function in children with OCD used regional cerebral blood flow (rCBF) measures. Diler and colleagues163 compared rCBF at baseline and after 12 weeks of treatment with paroxetine. At baseline, children with OCD had increased blood flow in the caudate, dlPFC, and ACC; blood flow in these regions was reduced following treatment. A subsequent study failed to find changes in rCBF using clomipramine.164 Using fMRI, Lazaro and colleagues165 examined the effect of treatment on brain activation during a serial reaction time task in treatment-naïve children with OCD. At baseline, the serial reaction time task produced increased activation in the caudate, middle frontal gyrus, and inferior parietal lobe; total OCD scores correlated with activation in the nucleus accumbens and superior parietal lobe and obsession scores correlated with activation in the ACC. After 6 months of fluoxetine treatment and significant clinical improvement, activation in the insula and putamen were decreased during performance of the serial reaction time task.
Structural MRI
Investigations of differences in amygdala volume have been inconsistent. An initial study found no amygdala volume differences in medication-naïve children with OCD152; however, a later study reported an initial amygdala asymmetry in OCD (left larger than right) relative to controls.151
Several structural studies have found evidence that children with OCD have larger volumes in the putamen166–168; however, two studies reported either smaller volumes or no differences in the putamen169,170 and another study reported smaller volume in the globus pallidus.170 Other structural differences include larger volume in the thalamus,171 caudate,168 and cerebellum.172 In the PFC, the most consistent structural finding to date is that childhood OCD is associated with larger gray-matter volume in the ACC, a finding that has been replicated in multiple samples using both manual tracing152,166,170 and voxel-based morphometry162 methods. However, one study reported areas of smaller gray matter volume in the ACC.167 Larger gray-matter volume has also been reported in the OFC162,166 and larger gray matter volume correlated with higher symptom severity.166 Other PFC areas that show differences in OCD include the medial frontal gyrus162,167 and inferior frontal gyrus.162
Several studies have examined treatment effects on brain structure. For the amygdala, one study reported that an initial amygdala asymmetry was normalized with selective serotonin reuptake inhibitor (SSRI) treatment.151 In another study, thalamic volume normalized with treatment and decreased volume with treatment was associated with reduced OCD symptom severity.171 However, in another study no changes in thalamic volume were seen with CBT, even though symptom severity reduced with treatment.173
IMPLICATIONS FOR RESEARCH AND CLINICAL PRACTICE
Using functional and structural neuroimaging methods to isolate the neural underpinnings of childhood anxiety disorders is still in its infancy, with the first childhood anxiety fMRI study conducted a little over a decade ago. Given this relatively short period of time, significant progress has been made in identifying possible sources of brain dysfunction contributing to childhood anxiety. In the fear-based anxiety disorders (GAD, SP, SAD), neuroimaging studies have reported brain dysfunction in both the amygdala and multiple regions of the PFC. In OCD, the basal ganglia, orbitofrontal cortex, and anterior cingulate cortex are emerging as key regions of structural and functional deficits.
Studies of children and adolescents at high-risk for developing an anxiety disorder have contributed substantially to our current knowledge of possible neural substrates of anxiety disorders. Given the National Institute of Mental Health's new emphasis on dimensional traits—the Research Domain Criteria (RDoC)—we expect that more neuroimaging studies will begin to study high-risk traits, such as behavioral inhibition. The use of dimensional approaches may be especially useful for childhood anxiety disorders given that many children have subsyndromal symptoms or symptoms across multiple anxiety disorders.174
Although progress has been made, many important questions remain. Some important future areas for research include:
Measure individual differences in developmental brain trajectories to identify the neural underpinnings of trajectories that result in childhood anxiety.
Study the developmental brain trajectories of children with anxiety disorders, because not all anxious children become anxious adults. Identifying the brain and behavioral trajectories associated with reduction in anxiety may inform novel targets for treatment.
Increase focus on isolating the neural bases of dimensional traits associated with risk for developing anxiety, such as behavioral inhibition, trait anxiety, and intolerance of uncertainty.
Collect measures of behavior and arousal, in addition to functional neuroimaging measures, to provide greater clarity about the meaning of differences in brain activation in anxious children.
Develop new functional tasks to examine the relative contributions of the amygdala and the PFC to anxiety and to identify the sequelae of individual differences in the amygdala–PFC imbalance.
Test the effects of pharmacologic and nonpharmacologic treatments on the developing brain. Research is greatly needed to identify whether treatment success is associated with changes in brain function and/or development.
The current findings from the functional and structural neuroimaging studies reviewed in this article suggest that anxious children and adolescents have dysfunction in amygdala–PFC neurocircuitry. While it is likely that common treatments for childhood anxiety—such as CBT and selective serotonin reuptake inhibitors (SSRIs)—act on this circuit, empirical evidence is still needed. Importantly, identification of dysfunction in the amygdala–PFC neurocircuitry may suggest new avenues for the development of novel therapies. For example, evidence of dysfunction in PFC-dependent attentional processes has led to the development and testing of attention retraining therapies.97,175 In addition, new pharmacotherapies or behavioral therapies can capitalize on neuroscience findings by targeting emotion regulation abilities in anxious children.
KEY POINTS.
Development of fear is a normative process; normal development of the fear system goes awry in children who develop anxiety disorders and dysfunction in the amygdala-prefrontal cortex fear circuitry is likely.
In the fear-based anxiety disorders, neuroimaging studies have reported brain dysfunction in both the amygdala and multiple regions of the PFC.
In OCD, neuroimaging studies have reported functional and/or structural anomalies in the basal ganglia, orbitofrontal cortex and anterior cingulate cortex.
Functional and structural neuroimaging studies have contributed to the significant progress made in identifying possible neural substrates of childhood anxiety disorders.
Acknowledgments
The preparation of this manuscript was partially supported by Award No. K01-MH083052 to Jennifer Urbano Blackford from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Mental Health or the National Institutes of Health.
Footnotes
The authors have nothing else to disclose.
REFERENCES
- 1.Merikangas KR, He Jp, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–66. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gullone E. The development of normal fear: a century of research. Clin Psychol Rev. 2000;20(4):429–51. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 4.Scarr S, Salapatek P. Patterns of fear development during infancy. Merrill Palmer Q. 1970;16(1):53–90. [Google Scholar]
- 5.Waters E, Matas L, Sroufe LA. Infants’ reactions to an approaching stranger: description, validation, and functional significance of wariness. Child Dev. 1975;46(2):348–56. [PubMed] [Google Scholar]
- 6.Thompson RA, Limber SP. Social anxiety in infancy: stranger and separation reactions. In: Leitenberg H, editor. Handbook of social and evaluation anxiety. Plenum Press; New York: 1992. pp. 85–137. [Google Scholar]
- 7.Campos JJ, Emde RN, Gaensbauer T, et al. Cardiac and behavioral interrelationships in the reactions of infants to strangers. Dev Psychol. 1975;11(5):589–601. [Google Scholar]
- 8.Gaensbauer TJ, Emde RN, Campos JJ. Stranger distress: confirmation of a developmental shift in a longitudinal sample. Percept Mot Skills. 1976;43(1):99–106. [Google Scholar]
- 9.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 10.Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans Biol Sci. 1997;352(1362):1675–87. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–15. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–9. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16(6):723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Emery NJ, Capitanio JP, Mason WA, et al. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav Neurosci. 2001;115(3):515–44. [PubMed] [Google Scholar]
- 16.Costafreda SG, Brammer MJ, David AS, et al. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–30. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 19.Prather MD, Lavenex P, Mauldin-Jourdain ML, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106(4):653–8. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 20.Davis M, Walker DL, Miles L, et al. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quirk GJ, Likhtik E, Pelletier JG, et al. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23(25):8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Diekhof EK, Geier K, Falkai P, et al. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58(1):275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 24.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–41. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109(4):681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 28.Phan KL, Fitzgerald DA, Nathan PJ, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Goldin PR, McRae K, Ramel W, et al. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 31.Kober H, Barrett LF, Joseph J, et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 34.Ochsner KN, Bunge SA, Gross JJ, et al. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 35.Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Amaral DG, Price JL, Pitkanen A, et al. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- 39.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363(4):615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 40.Ghashghaei H, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34(3):905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 42.Porrino LJ, Crane AM, Goldmanrakic PS. Direct and indirect pathways from the amygdala to the frontal-lobe in rhesus-monkeys. J Comp Neurol. 1981;198(1):121–36. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- 43.Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13(4):489–U112. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 45.Hare TA, Tottenham N, Galvan A, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnstone T, van Reekum CM, Urry HL, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delgado MR, Nearing KI, LeDoux JE, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banks SJ, Eddy KT, Angstadt M, et al. Amygdala–frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 50.Hariri AR, Mattay VS, Tessitore A, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 51.Sarinopoulos I, Grupe DW, Mackiewicz KL, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20(4):929–40. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Killgore WDS, Yurgelun-Todd DA. Cerebral correlates of amygdala responses during non-conscious perception of facial affect in adolescent and pre-adolescent children. Cogn Neurosci. 2010;1(1):33–43. doi: 10.1080/17588920903243957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlund MW, Siegle GJ, Ladouceur CD, et al. Nothing to fear? Neural systems supporting avoidance behavior in healthy youths. NeuroImage. 2010;52(2):710–9. doi: 10.1016/j.neuroimage.2010.04.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benes FM. Development of the corticolimbic system. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. Guilford Press; New York: 1994. pp. 176–206. [Google Scholar]
- 57.Clancy B, Finlay BL, Darlington RB, et al. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28(5):931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bliss-Moreau E, Toscano JE, Bauman MD, et al. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Dev Psychobiol. 2010;52(5):487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bliss-Moreau E, Toscano JE, Bauman MD, et al. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience. 2011;178:123–32. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bauman MD, Lavenex P, Mason WA, et al. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16(8):1388–411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 61.Shaw P, Lawrence EJ, Radbourne C, et al. The impact of early and late damage to the human amygdala on ’theory of mind’ reasoning. Brain. 2004;127:1535–48. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- 62.Shaw P, Brierley B, David AS. A critical period for the impact of amygdala damage on the emotional enhancement of memory? Neurology. 2005;65(2):326–8. doi: 10.1212/01.wnl.0000168867.40688.9b. [DOI] [PubMed] [Google Scholar]
- 63.Somerville LH, Fani N, Clure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36(4):408–28. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ewbank MP, Barnard PJ, Croucher CJ, et al. The amygdala response to images with impact. Soc Cogn Affect Neurosci. 2009;4(2):127–33. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santos A, Mier D, Kirsch P, et al. Evidence for a general face salience signal in human amygdala. NeuroImage. 2011;54(4):3111–6. doi: 10.1016/j.neuroimage.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 66.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14(4):303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 67.Blackford JU, Buckholtz JW, Avery SN, et al. A unique role for the amygdala in novelty detection. NeuroImage. 2010;50(3):1188–93. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guyer AE, Monk CS, Clure-Tone EB, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–82. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20(1):420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 70.Williams LM, Brown KJ, Palmer D, et al. The mellow years? Neural basis of improving emotional stability over age. J Neurosci. 2006;26(24):6422–30. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luna B, Thulborn KR, Munoz DP, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13(5):786–93. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 73.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 74.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–58. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- 76.Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17(2):243–50. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc Cogn Affect Neurosci. 2009;4(4):387–98. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hooper CJ, Luciana M, Conklin HM, et al. Adolescents’ performance on the Iowa gambling task: implications for the development of decision making and ventrome-dial prefrontal cortex. Dev Psychol. 2004;40(6):1148–58. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- 79.Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perlman SB, Pelphrey KA. Regulatory brain development: balancing emotion and cognition. Soc Neurosci. 2010;5(5–6):533–42. doi: 10.1080/17470911003683219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yurgelun-Todd DA, Killgore WDS. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci Lett. 2006;406(3):194–9. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 82.Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci U S A. 2002;99(20):13336–41. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lau JY, Britton JC, Nelson EE, et al. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108(11):4500–5. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prencipe A, Kesek A, Cohen J, et al. Development of hot and cool executive function during the transition to adolescence. J Exp Child Psychol. 2011;108(3):621–37. doi: 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Levin HS, Culhane KA, Hartmann J, et al. Developmental-changes in performance on tests of purported frontal-lobe functioning. Dev Neuropsychol. 1991;7(3):377–95. [Google Scholar]
- 86.Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: a dimensional and developmental study. Dev Neuropsychol. 2004;26(2):571–93. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- 87.Welsh MC, Pennington BF, Groisser DB. A normative developmental-study of executive function—a window on prefrontal function in children. Dev Neuropsychol. 1991;7(2):131–49. [Google Scholar]
- 88.Casey BJ. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52(3):225–35. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Somerville LH, Jones RM, Casey B. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72(1):124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lange K, Williams LM, Young AW, et al. Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry. 2003;53(3):226–32. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- 91.Lieberman M, Inagaki T, Tabibnia G, et al. Subjective responses to emotional stimuli during labeling, reappraisal and distraction. Emotion. 2011;11(3):468–80. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 93.Taylor SF, Phan KL, Decker LR, et al. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18(3):650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 94.Whalen PJ, Rauch SL, Etcoff NL, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bar-Haim Y, Lamy D, Pergamin L, et al. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 97.Fox NA, Pine DS. Temperament and the emergence of anxiety disorders [abstract]. J Am Acad Child Adolesc Psychiatry. 2012;51(2):125–8. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review. 2010;30:203–16. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merikangas KR, Avenevoli S, Dierker L, et al. Vulnerability factors among children at risk for anxiety disorders. Biol Psychiatry. 1999;46(11):1523–35. doi: 10.1016/s0006-3223(99)00172-9. [DOI] [PubMed] [Google Scholar]
- 100.Garcia-Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Dev. 1984;55:1005–19. [Google Scholar]
- 101.Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 1996;67(2):523–40. [PubMed] [Google Scholar]
- 102.Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Dev. 1998;69(6):1483–93. [PubMed] [Google Scholar]
- 103.Robinson JL, Reznick JS, Kagan J, et al. The heritability of inhibited and uninhibited behavior—a twin study. Dev Psychol. 1992;28(6):1030–7. [Google Scholar]
- 104.Fox NA, Henderson HA, Marshall PJ, et al. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 105.Hirshfeld-Becker DR, Biederman J, Rosenbaum JF. Behavioral inhibition. In: Morris TL, March JS, editors. Anxiety disorders in children and adolescents. 2nd ed. Guilford Press; New York: 2004. pp. 27–58. [Google Scholar]
- 106.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58(6):1459–73. [PubMed] [Google Scholar]
- 107.Kagan J, Reznick JS, Snidman N, et al. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988;59(6):1580–9. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 108.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48(9):928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Essex MJ, Klein MH, Slattery MJ, et al. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167(1):40–6. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38(8):1008–15. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 111.Blackford JU, Avery SN, Shelton RC, et al. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blackford JU, Avery SN, Cowan RL, et al. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc Cogn Affect Neurosc. 2011;6(5):621–9. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwartz CE, Wright CI, Shin LM, et al. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300(5627):1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 114.Schwartz CE, Kunwar PS, Greve DN, et al. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.96. http://dx.doi.org/10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed]
- 115.Blackford JU, Allen AH, Cowan RL, et al. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nsr078. http://dx.doi.org/10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed]
- 116.Schwartz CE, Kunwar PS, Greve DN, et al. Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Arch Gen Psychiatry. 2010;67(1):78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clauss JA, Cowan RL, Blackford JU. Expectancy and temperament modulate amygdala and dorsal anterior cingulate responses to fear faces. Cogn Affect Behav Neurosci. 2011;11(1):13–21. doi: 10.3758/s13415-010-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (text revision) 4th edition American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 119.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–63. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 120.McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 121.Beesdo K, Lau JYF, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66(3):275–85. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roberson-Nay R, McClure EB, Monk CS, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biol Psychiatry. 2006;60(9):966–73. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 124.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 125.McClure E, Adler A, Monk C, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007;191(1):97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 126.Maslowsky J, Mogg K, Bradley BP, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20(2):105–11. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Bellis MD, Casey BJ, Dahl RE, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48(1):51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 128.Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57(9):961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 129.De Bellis MD, Keshavan MS, Shifflett H, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002;51(7):553–62. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- 130.Telzer EH, Mogg K, Bradley BP, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79(2):216–22. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krain AL, Gotimer K, Hefton S, et al. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63(6):563–8. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 132.Gentes EL, Ruscio AM. A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive-compulsive disorder. Clin Psychol Rev. 2011;31(6):923–33. doi: 10.1016/j.cpr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 133.Dugas MJ, Marchand A, Ladouceur R. Further validation of a cognitive-behavioral model of generalized anxiety disorder: diagnostic and symptom specificity. J Anxiety Disord. 2005;19(3):329–43. doi: 10.1016/j.janxdis.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 134.Blair KS, Geraci M, Korelitz K, et al. The pathology of social phobia is independent of developmental changes in face processing. Am J Psychiatry. 2011;168:1202–9. doi: 10.1176/appi.ajp.2011.10121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guyer AE, Lau JYF, Clure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65(11):1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hirshfeld-Becker DR, Biederman J, Henin A, et al. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28(3):225–33. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 137.Gest SD. Behavioral inhibition: stability and associations with adaptation from childhood to early adulthood. J Pers Soc Psychol. 1997;72(2):467–75. doi: 10.1037//0022-3514.72.2.467. [DOI] [PubMed] [Google Scholar]
- 138.Bishop G, Spence SH, McDonald C. Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Dev. 2003;74(6):1899–917. doi: 10.1046/j.1467-8624.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- 139.Perez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35(4):1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hardin MG, Perez-Edgar K, Guyer AE, et al. Reward and punishment sensitivity in shy and non-shy adults: relations between social and motivated behavior. Pers Indiv Differ. 2006;40(4):699–711. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bar-Haim Y, Fox NA, Benson B, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20(8):1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. NeuroReport. 2005;16(15):1671–5. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 144.March JS, Parker JDA, Sullivan K, et al. The multidimensional anxiety scale for children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–65. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 145.Mana S, Martinot MLP, Martinot JL. Brain imaging findings in children and adolescents with mental disorders: a cross-sectional review. Eur Psychiatry. 2010;25(6):345–54. doi: 10.1016/j.eurpsy.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 146.Stein DJ, Fineberg NA, Bienvenu OJ, et al. Should OCD be classified as an anxiety disorder in DSM-V? Depress Anxiety. 2010;27(6):495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- 147.Kalra SK, Swedo SE. Children with obsessive-compulsive disorder: are they just “little adults”? J Clin Invest. 2009;119(4):737–46. doi: 10.1172/JCI37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fireman B, Koran LM, Leventhal JL, et al. The prevalence of clinically recognized obsessive-compulsive disorder in a large health maintenance organization. Am J Psychiatry. 2001;158(11):1904–10. doi: 10.1176/appi.ajp.158.11.1904. [DOI] [PubMed] [Google Scholar]
- 149.Lewinsohn PM, Hops H, Roberts RE, et al. Adolescent psychopathology .1. Prevalence and incidence of depression and other DSM-III-R disorders in high-school-students. J Abnorm Psychol. 1993;102(1):133–44. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- 150.Rauch SL, Jenike MA. Neurobiological models of obsessive-compulsive disorder. Psychosomatics. 1993;34(1):20–32. doi: 10.1016/S0033-3182(93)71924-6. [DOI] [PubMed] [Google Scholar]
- 151.Szeszko PR, Robinson D, Alvir JMJ, et al. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56(10):913–9. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- 152.Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biol Psychiatry. 1998;43(9):623–40. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- 153.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23(3):563–86. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 154.MacMaster FP, O'Neill J, Rosenberg DR. Brain imaging in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1262–72. doi: 10.1097/CHI.0b013e318185d2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fitzgerald KD, MacMaster FP, Paulson LD, et al. Neurobiology of childhood obsessive-compulsive disorder. Child Adolesc.Psychiatr Clin N Am. 1999;8(3):533–75. [PubMed] [Google Scholar]
- 156.Huyser C, Veltman DJ, de Haan E, et al. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev. 2009;33(6):818–30. doi: 10.1016/j.neubiorev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 157.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166(6):664–74. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20(4):1251–83. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gilbert AR, Akkal D, Almeida JR, et al. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2009;48(9):936–44. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- 160.Britton JC, Stewart SE, Killgore WD, et al. Amygdala activation in response to facial expressions in pediatric obsessive-compulsive disorder. Depress Anxiety. 2010;27(7):643–51. doi: 10.1002/da.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Woolley J, Heyman I, Brammer M, et al. Brain activation in paediatric obsessive-compulsive disorder during tasks of inhibitory control. Br J Psychiatry. 2008;192(1):25–31. doi: 10.1192/bjp.bp.107.036558. [DOI] [PubMed] [Google Scholar]
- 162.Britton JC, Rauch SL, Rosso IM, et al. Cognitive inflexibility and frontal-cortical activation in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):944–53. doi: 10.1016/j.jaac.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Diler RS, Kibar M, Avci A. Pharmacotherapy and regional cerebral blood flow in children with obsessive compulsive disorder. Yonsei Med J. 2004;45(1):90–9. doi: 10.3349/ymj.2004.45.1.90. [DOI] [PubMed] [Google Scholar]
- 164.Castillo ARGL, Buchpiguel CA, de Araujo LASB, et al. Brain SPECT imaging in children and adolescents with obsessive-compulsive disorder. J Neural Transm. 2005;112(8):1115–29. doi: 10.1007/s00702-004-0240-x. [DOI] [PubMed] [Google Scholar]
- 165.Lazaro L, Caldu X, Junque C, et al. Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: a functional MRI study. J Psychiatr Res. 2008;42(13):1051–9. doi: 10.1016/j.jpsychires.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 166.Szeszko PR, Christian C, MacMaster F, et al. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: an optimized voxel-based morphometry study. Am J Psychiatry. 2008;165(10):1299–307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- 167.Gilbert AR, Keshavan MS, Diwadkar V, et al. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: a preliminary voxel-based morphometry study. Neurosci Lett. 2008;435(1):45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zarei M, Mataix-Cols D, Heyman I, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70(11):1083–90. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 169.Rosenberg DR, Keshavan MS, Ohearn KM, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54(9):824–30. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 170.Szeszko PR, MacMillan S, McMeniman M, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161(6):1049–56. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- 171.Gilbert AR, Moore GJ, Keshavan MS, et al. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch Gen Psychiatry. 2000;57(5):449–56. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- 172.Tobe RH, Bansal R, Xu DR, et al. Cerebellar morphology in burette syndrome and obsessive-compulsive disorder. Ann Neurol. 2010;67(4):479–87. doi: 10.1002/ana.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosenberg DR, Benazon NR, Gilbert A, et al. Thalamic volume in pediatric obsessive-compulsive disorder patients before and after cognitive behavioral therapy. Biol Psychiatry. 2000;48(4):294–300. doi: 10.1016/s0006-3223(00)00902-1. [DOI] [PubMed] [Google Scholar]
- 174.Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–5. doi: 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hakamata Y, Lissek S, Bar-Haim Y, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68(11):982–90. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]