Abstract
Traumatic brain injury (TBI) survivors experience long-term post-traumatic morbidities. In diffuse brain-injured rats, a chronic sensory sensitivity to whisker stimulation models the agitation of TBI survivors and provides anatomical landmarks across the whisker-barrel circuit to evaluate post-traumatic neuropathology. As a consequence of TBI, acute and chronic microglial activation can contribute to degenerative and reparative events underlying post-traumatic morbidity. Here we hypothesize that a temporal sequence of microglial activation states contributes to the circuit pathology responsible for post-traumatic morbidity, and test the hypothesis by examining microglial morphological activation and neuroinflammatory markers for activation states through gene expression and receptor binding affinity. Adult male, Sprague-Dawley rats were subjected to a single moderate midline fluid percussion (FPI) or sham injury. Microglial activation was determined by immunohistochemistry, quantitative real-time PCR and receptor autoradiography in the primary somatosensory barrel field (S1BF) and ventral posteromedial nucleus of the thalamus (VPM) at 7 and 28 days following FPI. Morphological changes indicative of microglial activation, including swollen cell body with thicker, shrunken processes, were evident in S1BF and VPM at 7 and 28 days post-injury. Principally at 7 days post-injury in VPM, general inflammatory gene expression (MHC-I, MHC-II, translocator protein 18 kDa [TSPO]) is increased above sham level and TSPO gene expression confirmed by receptor autoradiography. Further, CD45, a marker of classical activation, and TGF-βI, an acquired deactivation marker, were elevated significantly above sham at 7 days post-injury. Daily administration of the anti-inflammatory ibuprofen (20 mg/kg, i.p.) significantly reduced the expression of these genes. Evidence for alternative activation (arginase 1) was not observed. Thus, these data demonstrate concomitant classical activation and acquired deactivation phenotypes of microglia in diffuse TBI in the absence of overt contusion or cavitation. Anti-inflammatory treatment may further alleviate the neuropathological burden of post-traumatic inflammation.
Keywords: classical activation, alternate activation, anti-inflammatory, neuroplasticity
Introduction
Diffuse traumatic brain injury (TBI) arises from abrupt acceleration and deceleration forces found in motor vehicle accidents and contact sports concussion. These external forces primarily impact gray-white matter interfaces, the axon hillock and the blood brain barrier; which result in mechanically sheared axons, vasculature and membranes (Graham et al., 1995, Povlishock and Katz, 2005, Farkas and Povlishock, 2007). Immediate prolonged unconsciousness associated with diffuse TBI is not necessarily accompanied by an intracranial mass lesion and accounts for two-thirds of all TBI (Graham et al., 2002). The vast numbers of patients that survive a brain injury can develop varying degrees of post-traumatic morbidities, including cognitive, social, emotional and sensory deficits (McAllister, 1992, Millis et al., 2001, Povlishock and Katz, 2005, Yeates et al., 2008).
Post-TBI morbidities are brought on, if not exacerbated, by the secondary molecular, biochemical and cellular events that compound the neuronal, glial and vascular injuries across multiple brain areas (Povlishock and Katz, 2005, Farkas and Povlishock, 2007). In diffuse brain injury modeled by midline fluid percussion injury (FPI) these secondary cascades can occur in the absence of overt edema, hemorrhage or cavitation (Kelley et al., 2007). In the spared tissue of the thalamocortical relays mediating whisker somatosensation, including somatosensory barrel field (S1BF) and ventral posteromedial nucleus (VPM) of thalamus, axonal transport is disrupted (Kelley et al., 2006), neurons atrophy (Lifshitz et al., 2007, Lifshitz and Lisembee, 2012), microglia classically activate (Kelley et al., 2007), glutamate neurotransmission becomes hypersensitive (Thomas et al., 2012) and neurons show hyperactivation with whisker stimulation (Hall and Lifshitz, 2010). These neuropathological changes along the whisker circuit correlate with the robust avoidance and apprehensive behavioral responses to whisker stimulation (increased sensory sensitivity) over 1 month in FPI-injured animals (McNamara et al., 2010, Learoyd and Lifshitz, 2012).
An intensely investigated feature of diffuse TBI is the prominent histological evidence for microglial activation (Lenzlinger et al., 2001, Morganti-Kossmann et al., 2007, Venkatesan et al., 2010). Currently, the range and extent of microglial activation states are being investigated in various neurological conditions, including spinal cord injury, Alzheimer’s disease and TBI (Grossman et al., 2003, Colton et al., 2006, Bye et al., 2007, Donnelly and Popovich, 2008, Farfara et al., 2008, Popovich and Longbrake, 2008, Venkatesan et al., 2010). For simplicity, specific cellular functions have been associated with particular activation states (Gordon, 2003, Chen and Guilarte, 2008, Graeber, 2010), however it is more likely that functions overlap. Broadly, microglia can function to regulate both degenerative and reparative events in the injured and recovering brain. As such, microglia have been reported to exist in a state of dynamic equilibrium between a classical and alternative activated state following an insult, contributing to seemingly contradictory cellular processes (Popovich and Longbrake, 2008). Discrete signals in the pathological microenvironment induce action of resident microglia (Gordon, 2003, Mantovani et al., 2004, Colton et al., 2006, Popovich and Longbrake, 2008, Colton and Wilcock, 2010). Classically activated microglia participate in the host defense system as a part of innate and adaptive immunity, of which phagocytosis by macrophages is a primary role (Ransohoff and Cardona, 2010, Prinz et al., 2011). Microglial activation and their progression towards phagocytotic macrophages can lead to progressive and cumulative neuronal cell loss, as demonstrated by the toxicity associated with lipopolysaccaride (LPS) injection (Gao et al., 2002, Ling et al., 2006). Alternative activated microglia are purported to promote neuroplasticity and axonal regeneration, in addition to the monitoring and pruning of synapses (Gordon, 2003, Chen and Guilarte, 2008). For example, experimental spinal cord injury revealed microglia expressing genes linked to the alternative activated state in the presence of IL-4, which resulted in longer distance axon projections and promoted axon outgrowth overcoming chondroitin sulfate proteoglycan (CSPG) inhibition (Kigerl et al., 2009), however morphological features of alternative activated microglia have yet to be defined. Microglia with an acquired deactivation phenotype express TGF-βI and likely down-regulate the inflammatory response (Mantovani et al., 2004, Cullheim and Thams, 2007). In diffuse brain injury, the relative contributions of activated microglia phenotypes remain unknown.
Injury-induced behavioral deficits are likely due to interrupted neural network structure and function (Ghajar and Ivry, 2008). Microglial activation can modify neural networks in multiple ways: promoting neuronal cell death, influencing neural circuitry by neuroplastic reorganization or synaptic stripping (reviewed in (Perry and O’Connor, 2010). In this study, for the first time, we survey the time course of microglial activation states during which late-onset sensory sensitivity develops and the consequence of inhibiting classical activation after experimental diffuse TBI.
Materials and Methods
Surgical Preparation and Fluid Percussion Brain Injury
Adult male Sprague-Dawley rats (350–375 g) were subjected to midline fluid percussion injury (FPI) consistent with methods described previously (Lifshitz et al., 2007, Lifshitz, 2008, Hosseini and Lifshitz, 2009, McNamara et al., 2010). Briefly, rats were anesthetized with 5% isoflurane in 100% O2 prior to the surgery and maintained at 2% isoflurane via nose cone. During surgery, animals’ body temperature was maintained with a Deltaphase® isothermal heating pad (Braintree Scientific Inc., Braintree, MA). In a head holder assembly (Kopf Instrument, Tujunga, CA), a midline scalp incision exposed the skull. A 4.8-mm circular craniotomy was performed (centered on the sagittal suture midway between bregma and lambda) without disrupting the underlying dura or superior sagittal sinus. An injury hub was fabricated from the female portion of a Luer-Loc needle hub, which was cut, beveled, and scored to fit within the craniotomy. A skull screw was secured in a 1-mm hand-drilled hole into the right frontal bone. The injury hub was affixed over the craniotomy using cyanoacrylate gel and methyl-methacrylate (Hygenic Corp., Akron, OH) was applied around the injury hub and screw. The incision was sutured at the anterior and posterior edges and topical Lidocaine ointment was applied. Animals were returned to a warmed holding cage and monitored until ambulatory (approximately 60–90 min).
For injury induction, animals were re-anesthetized with 5% isoflurane 60–90 min after surgery to standardize anesthesia levels at the time of injury. The dura was inspected through the injury-hub assembly, which was then filled with physiological saline and attached to the male end of the fluid percussion device (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA). As rat’s reflexive responses returned, a moderate injury (1.9–2.0 atm) was administered by releasing the pendulum onto the fluid-filled cylinder. Animals were monitored for the presence of a forearm fencing response and the return of the righting reflex as indicators of injury severity (Hosseini and Lifshitz, 2009). Sham animals were connected to the FPI device, but the pendulum was not released. The injury-hub assembly was removed en bloc, integrity of the dura was observed, bleeding was controlled with Gelfoam (Pharmacia, Kalamazoo, MI) and the incision was stapled. Moderate brain-injured animals had righting reflex recovery times that averaged 6 minutes, and sham-injured animals recovered within 15 seconds. After recovery of the righting reflex, animals were placed in a warmed holding cage before being returned to the vivarium. In our hands, one of twenty five brain-injured animals dies within three days from consequences of pulmonary edema. Surgical recovery was monitored post-operatively for three days, for which no overt differences (e.g. weight, coat, movement, grooming) were observed between animals. Staples were removed 7–10 days post-injury as needed. Experiments were conducted in accordance with NIH and institutional guidelines concerning the care and use of laboratory animals. Adequate measures were taken to minimize pain or discomfort. Animal numbers are reported in the figure legends for each study.
Tissue preparation and Immunohistochemistry
At designated time points post-injury, sham and brain-injured rats were overdosed with sodium pentobarbital (200 mg/kg i.p) and transcardially perfused with 0.9% sodium chloride, followed by a fixative solution containing 4% paraformaldehyde. Following decapitation, the heads were stored in a fixative solution containing 15% sucrose for 24 h, after which the brains were removed, placed in fresh fixative, and shipped for histological processing to Neuroscience Associates Inc. (Knoxville, TN). The rat brains were embedded into a single gelatin block (Multiblock® Technology; Neuroscience Associates). Individual cryosections containing all the rat brains were mounted and immunostained for ionized calcium binding adaptor molecule 1 (Iba-1), to identify all microglia. The Immunostained slides were analyzed in our laboratory using an Olympus AX80 Automatic Research microscope with attached DP70 digital camera.
Radioligand binding
Uninjured sham and brain-injured animals were euthanitized by decapitation, and then the brains were removed and flash frozen in an isopentane-dry ice slurry. The brains were stored at −80°C until further processing. Brains were processed on a cryostat (Lecia CM1850, Nussloch, Germany) into a series of 16-μm-thick sections. The translocator protein 18 kDa (TSPO) receptor densities were measured using [3H]-PK-11195 autoradiography, as previously described (Little et al., 1998, Kelso et al., 2009, van Bregt et al., 2012). A ligand concentration of 1 nM ([3H]-PK-11195 specific activity 85.5 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA, USA) was used for incubation. RayMax Beta High Performance Autoradiography Film (ICN Biomedicals, Aurora, Ohio) was used to visualize ligand binding. Radioactive rat brain tissue standards were included in each film cassette. Exposure time was optimized at 29 days. All films were processed using Kodak GBX developer. Binding data were analyzed using NIH image v1.59 on a Power Macintosh connected to a Sony XC-77 CCD camera via a Scion LG-3 frame-grabber. The areas of interest were outlined manually to include primary somatosensory barrel cortex and ventral posterior medial nucleus of the thalamus. Mean staining density was calculated for each individual section in triplicate, across 3 to 14 coronal sections (S1BF: 9 to 14 sections, VPM: 3–10 sections) (approximately Bregma −0.48 mm through −4.20 mm for S1BF and −2.76 mm through −4.08 mm for VPM) to obtain an overall mean receptor binding density for each animal.
Quantitative Real-time PCR (qRT-PCR)
At 7 or twenty-eight days after sham or brain injury, animals were euthanitized by an overdose of sodium pentobarbital (200 mg/kg, i.p.) and transcardially perfused with cold phosphate buffered saline (PBS). Brains were removed and sliced into 2 mm thick coronal sections. A 1 mm diameter biopsy was collected (approximately 2–4 mg) from somatosensory barrel field (S1BF) and ventral posterior medial nucleus (VPM) of one hemisphere. The biopsy was stored in RNlater® solution (Qiagen Corp) at −20°C for later extraction of mRNA.
The mRNA was isolated (RNeasy, Qiagen Corp), quantified (NanoDrop ND-1000 spectrophotometer), converted to complementary DNA (cDNA; High Capacity RNA-to-cDNA Kit, Applied Biosystems Inc.) and then used as a template for commercially-available gene expression assays (Taqman® Gene Expression Assay, Applied Biosystem), according to manufacturer’s protocols. Injury-related neuroinflammation and neuroplasticity gene expression (Table 1) was quantified in triplicate (StepONE Plus, Applied Biosystems) according to manufacturer’s protocols. Within each animal, relative gene expression was normalized to the 18s rRNA endogenous control, which shows minimal expression variation after fluid percussion injury (Cook et al., 2009, Harris et al., 2009). The average threshold cycle of each animal was then normalized to the sham group, and analyzed using the 2−ΔΔCT method (Livak and Schmittgen, 2001), which relates gene expression to the PCR cycle number at which the fluorescence signals exceed a threshold above baseline.
Table 1.
Detail of commercially available primers used in quantitative real-time PCR.
| Gene name | Gene symbol | Assay ID | Category |
|---|---|---|---|
| 18s rRNA | 18s rRNA | 4352930E | Endogenous control |
| Major histocompatibility complex class I beta-2 microglobulin | MHC I | Rn00560865_m1 | Microglia Activation |
| Major histocompatibility complex class II, DM α | MHC II | Rn01768597_m1 | Microglia Activation |
| Translocator protein 18 kDa | TSPO | Rn00560892_m1 | Microglia Activation |
| Tumor necrosis factor α | TNFα | Rn99999017_m1 | Classical Activation |
| Protein tyrosine phosphatase receptor type C | CD45 | Rn00709901_m1 | Classical Activation |
| Transforming growth factor β1 | TGF-βI | Rn99999016_m1 | Acquired Deactivation |
| Transforming growth factor β-receptor II | TGF-βRII | Rn00579682_m1 | Acquired Deactivation |
| Arginase 1 | Arg 1 | Rn00567522_m1 | Alternative Activation |
| Growth associated protein 43 | GAP43 | Rn00567901_m1 | Neuroplasticity |
| Synaptophysin | Syn | Rn00561986_m1 | Neuroplasticity |
Anti-inflammatory Administration
Additional animals were generated to analyze the effect of anti-inflammatory drug administration on gene expression after a moderate midline FPI. At 30 minutes post-FPI, uninjured and brain-injured animals were treated with an initial dose of vehicle (saline) or ibuprofen (20 mg/kg, i.p.) and then daily for 7 days. Ibuprofen at this dose inhibits cyclooxygenase 2 (COX2) activity and reduces inflammation (Fu et al., Wang et al.). At 7 days post-injury, tissue biopsies of the VPM thalamus were taken from coronal brain sections, as above. Gene expression of TSPO, CD45, TGF-βI, arginase 1, growth associated protein 43 (GAP43), and synaptophysin were quantified using real-time quantitative PCR, as described above.
Statistical Analysis
Peirce’s criterion was used to identify outliers in the data sets (Ross, 2003). All statistical assessments were performed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test using GraphPad Prism (v. 5.04) statistical software. In the anti-inflammatory treatment study, to maximize statistical power and reduce type I error, sham-saline treated and sham-ibuprofen treated groups were combined into a common sham group after confirming no significant differences between the two groups by student t-test. Results were considered significant when p < 0.05. All data are presented as mean ± standard error of the mean (SEM).
Results
Transient S1BF and sustained VPM microglial activation after diffuse brain injury
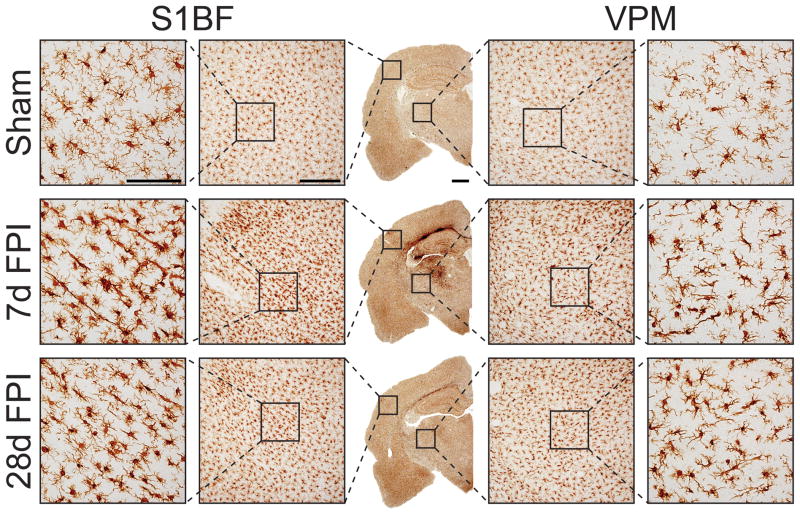
The discrete late-onset sensory sensitivity to whisker stimulation that develops after diffuse brain injury in the rat has guided our temporal evaluation to the primary somatosensory barrel cortex (S1BF) and ventral posterior medial (VPM) nucleus of the thalamus as the cortical and thalamic relays of the whisker barrel circuit (McNamara et al., 2010, Hinzman et al., 2012, Learoyd and Lifshitz, 2012). To characterize microglia activation following moderate midline FPI, we visualized morphological change in microglia by ionized calcium binding adaptor molecule (Iba-1) immunohistochemistry (Figure 1). In uninjured sham brains, Iba-1 positive microglia were spread evenly across the S1BF and VPM. At higher magnification, microglia in sham brains were characterized by small spherical cell bodies from which several primary processes with ramifications at many branch points emanated. In contrast, microglia from 7 days post-injury brains showed more intense staining in S1BF and VPM. Throughout the whisker barrel circuit, microglia of brain-injured animals exhibited swollen cell bodies and fewer, thicker, ramified processes, which are distinctive signs of activated microglia (Graeber, 2010). The apparent increased staining density after brain injury could be a consequence of cellular swelling or migration. Additionally, microglia in the brain-injured cortex appeared elongated perpendicular to the dural surface. Although a reduction of Iba-1 staining intensity occurred between 7 and 28 days post-injury, intense Iba-1 staining in both the VPM and S1BF was elevated in comparison to sham.
Figure 1.
Microglia were labeled with immunohistochemistry for ionized calcium binding adaptor molecule (Iba-1). The center column illustrates the distribution of microglia activation at low magnification. Compared to the uninjured sham (top row), increased staining is observed across the cortex, hippocampus and deep into the thalamus at 7 days post-injury (middle row). At 28 days post-injury (bottom row), Iba-1 staining becomes less extensive. Highly ramified microglia with spherical cell bodies in sham animals become less ramified with swollen or stretched cell bodies by 7 days post-injury (see first and last columns). Microglia show increased ramification in the cortex by 28 days post-injury, but to lesser extent compared to 7 days post-injury. Images are representative of three animals per time point. Scale bars are 1 mm, 500 μm, and 100 μm for photomicrographs on increasing magnification.
Morphologically, microglia remained swollen with fewer ramifications at 7 days post-injury. However, at this time point, microglia with an apparent resting morphology could be identified within fields of activated microglia. The morphology of microglia in the VPM and S1BF indicated that these cells were activated over the 28 day post-injury time course, as has been previously described for in experimental diffuse and focal brain injury (Soares et al., 1995, Lenzlinger et al., 2001, Bye et al., 2007, Kelley et al., 2007, Morganti-Kossmann et al., 2007, Semple et al., 2010a, Semple et al., 2010b, Ziebell and Morganti-Kossmann, 2010, Hutson et al., 2011, Ziebell et al., 2011).
Inflammatory Genes Indicate Microglial Activation After Diffuse Brain Injury
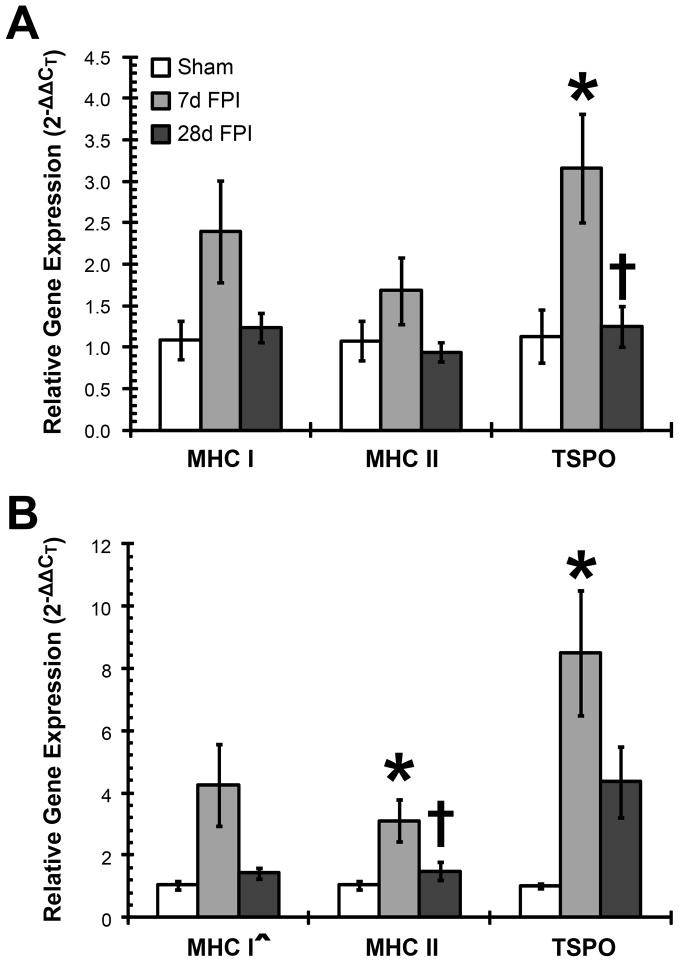
Microglial activation in the S1BF and VPM is further confirmed by transient increases in expression of activated microglia genes, including major histocompatibility complex I (MHC I), major histocompatibility complex II (MHC II) and translocator protein 18 kDa (TSPO). Firstly, in the S1BF, diffuse brain injury transiently, but not significantly, elevated gene expression of MHC I (F(2,13) = 2.587; p = 0.1133) and MHC II (F(2,13) = 1.766; p = 0.2096) compared to uninjured sham control (Figure 2A). Gene expression of TSPO increased significantly by 7 days post-injury compared to sham values, and then at 28 days post-injury decreased significantly from 7 days post-injury to sham values (F(2,11) = 6.209; p = 0.0157; Figure 2A). Secondly, in the VPM, diffuse brain injury significantly increased gene expression of MHC I (F(2,14) = 3.801; p = 0.0480), MHC II (F(2,15) = 5.321; p = 0.0179) and TSPO (F(2,10) = 6.526; p = 0.0154) compared to uninjured sham control (Figure 2B). At 28 days post-injury, relative gene expression was reduced to levels not significantly different from sham.
Figure 2.
Increased gene expression of microglia markers in brain-injured animals indicated microglial activation at 7 days post-injury. Overall significant changes in gene expression of MHC-I, MHC-II and TSPO are observed in thalamus. (A) In the S1BF, TSPO gene expression was increased significantly in fluid percussion brain-injured (FPI) animals at 7 days post-injury compared to sham animals, and then decreased to sham levels by 28 days post-injury; this decrease was significantly different to levels at 7 days post-injury. (B) In the VPM, MHC-II and TSPO were increased significantly by 7 days following FPI compared to sham. The gene expression in 28 days post-injured animals remained elevated, but it was no longer significantly different to sham-injured animals. *, p < 0.05 compared to sham-injured animals; †, p < 0.05 compared to 7 days post-injured animals; ^, p < 0.05 for the one-way ANOVA without post-hoc analysis showing significance. Means are from 3–6 animals per time point. MHC-I: major histocompatibility complex I, MHC-II: major histocompatibility complex II, TSPO: translocator protein 18 kDa.
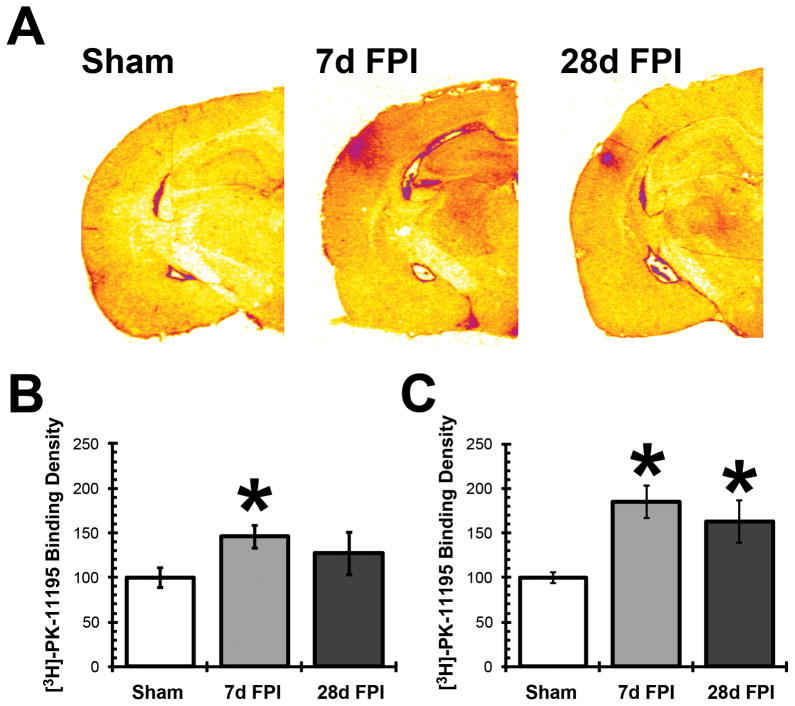
To further demonstrate the post-injury time course of microglial activation observed by Iba-1 staining and gene expression, [3H]-PK-11195 radioligand binding assays were conducted, which measure TSPO receptor density on activated microglia. Autoradiographs on coronal sections of uninjured and injured rat brains showed increased TSPO binding density, especially prominent in the S1BF and VPM (Figure 3A). In S1BF, radiolabeled ligand binding increased significantly at 7 days post-injury compared to sham animals (F(2,9) = 5.366; p = 0.0292); this effect was no longer significant at 28 days post-injury (Figure 3B). In VPM, radiolabeled ligand binding was significantly increased at both 7 days and 28 days post-injury compared to sham animals (F(2,9) = 19.965; p = 0.0005; Figure 3C).
Figure 3.
Diffuse brain injury increased microglial translocator protein 18 kDa (TSPO) receptor binding. (A) Autoradiographs of [3H]PK-11195 radioligand receptor binding to TSPO in sham, 7 day, and 28 day fluid percussion injured (FPI) rats brain (n = 3–5). [3H]PK-11195 binding showed increased TSPO protein density at 7 days post-injury in the S1BF and VPM, indicating microglial activation in these two regions. At 28 days after FPI, [3H]PK-11195 binding above sham levels was observed in the S1BF and VPM, but this was less extensive compared to 7 day FPI animals. (B, C) The quantification of [3H]PK-11195 binding by optical densities indicated a significant increase in TSPO protein density by 7 days post-FPI in both the S1BF (B) and VPM (C). By 28 days post-FPI, the TSPO protein density remained significantly elevated in the thalamus. *, p < 0.05 compared to sham-injured animals. Means are from 3–4 animals per time point.
Classical activation and acquired deactivation but no alternative activation are observed after diffuse brain injury
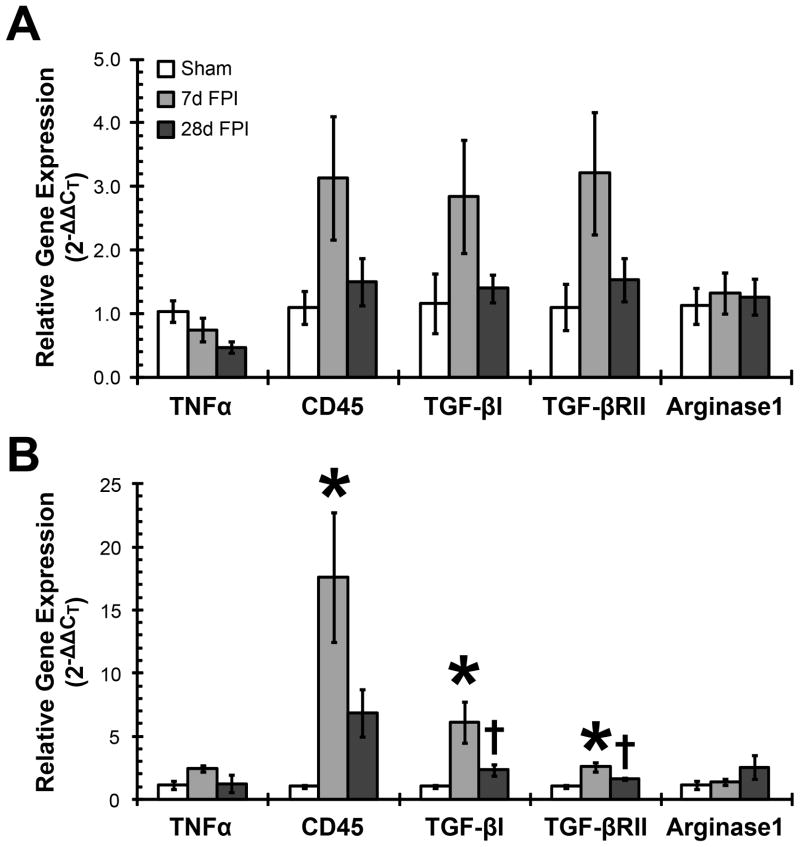
Changes in morphology and gene expression of activated microglia markers signal a change in the functional state of microglia in S1BF and VPM after FPI. In order to better understand the nature of microglial activation following diffuse brain injury, we quantified the post-traumatic time course of gene expression associated with three distinctive microglial activation states: classical activation (measured by TNFα and CD45), acquired deactivation (measured by TGF-βI and TGF-βRII) and alternative activation (measured by Arginase 1). Classical activation and acquired deactivation gene expression was increased transiently at 7 days post-injury, which was similar to the gene expression of more general microglia activation markers (Figure 2). TNFα gene expression in the S1BF decreased over time post-injury, however this was not significant (F(2,12) = 2.833; p = 0.0983). In contrast, the S1BF gene expression of CD45 (F(2,12) = 2.305; p = 0.1422), TGF-βI (F(2,12) = 1.735; p = 0.2178), and TGF-βRII (F(2,12) = 2.128; p = 0.1618) showed a transient increase in gene expression 7 days post-injury compared to sham animals, which returned towards sham levels by 28 days post-injury (Figure 4A), but these changes were not statistically significant. No change in arginase 1 gene expression was measured (F(2,11) = 0.1088; p = 0.8978).
Figure 4.
Elevated neuroinflammatory gene expression in the cortex (A) and thalamus (B) 7 days after fluid percussion brain injury (FPI). TNFα and CD45 represent classical activation of microglia. TNFα showed regionally distinct, but non-significant changes in gene expression over 28 days post-injury. CD45 showed the highest gene expression levels of all genes by 7 days post-injury particularly in the thalamus, which subsided by 28 days post-injury. TGF-βI and TGF-βRII gene expression represent acquired deactivation of microglia. TGF-βI and TGF-βRII showed increased gene expression at 7 days post-injury compared to sham. Significant increases were found in VPM samples. Microglial deactivation gene expression subsided to sham levels by 28 days post-injury. Arginase 1 represents alternative activated microglia gene expression and showed no injury-induced changes in gene expression. *, p < 0.05 compared to sham-injured animals; †, p < 0.05 compared to 7 days post-injured animals. Means are from 3–6 animals per time point.
When analysis of these genes was conducted in the VPM, a similar trend in expression was observed (Figure 4B). A non-significant, transient increase in TNFα gene expression was observed over the time course post-injury compared to uninjured sham animals (F(2,9) = 2.235; p = 0.1629). CD45 gene expression showed a significant 17 fold increase at 7 days post-injury compared to uninjured sham (F(2,10) = 5.678; p = 0.0225), indicating classical microglial activation. In addition, diffuse brain injury resulted in significantly increased TGF-βI (F(2,15) = 6.131; p = 0.0013) and TGF-βRII (F(2,14) = 8.995; p = 0.0031) gene expression at 7 days post-injury compared to sham, indicating acquired deactivation of microglia. By 28 days, CD45, TGF-βI, and TGF-βRII gene expression was reduced to levels above, but not significantly different from, sham animals. These results were similar to the results for MHC I, MHC II and TSPO gene expression in brain-injured VPM (Figure 2B). There was no change in arginase 1 gene expression after brain injury compared to sham in the VPM (F(2,9) = 1.613; p = 0.2519).
Anti-inflammatory treatment attenuates injury-induced inflammatory gene expression in the thalamus
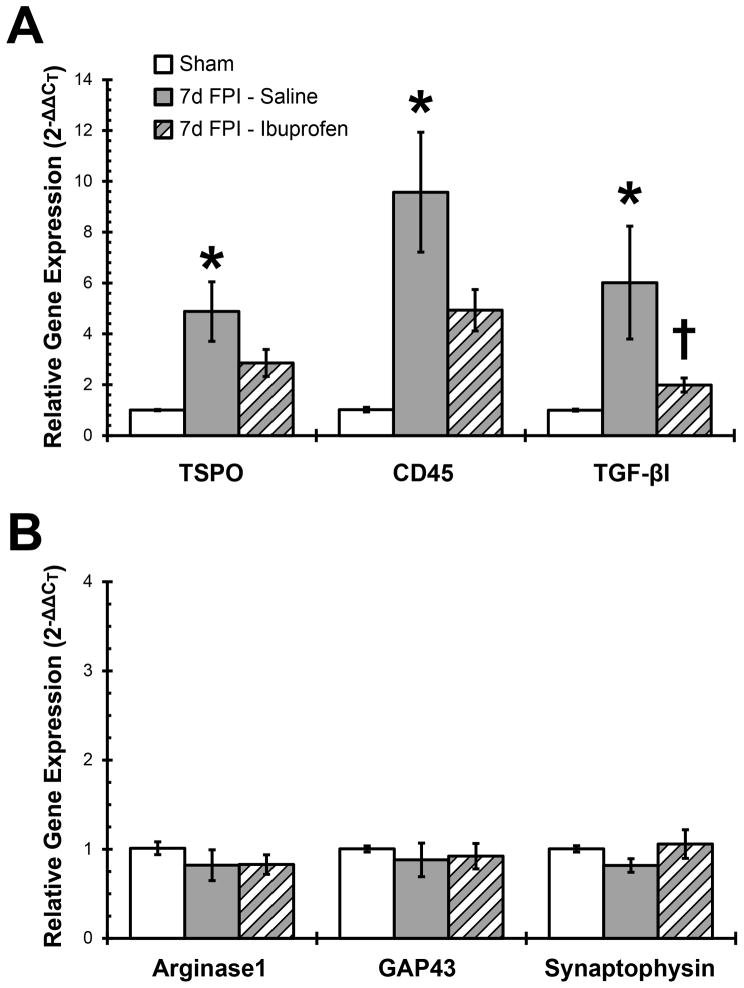
Histological, receptor binding and gene expression were significantly altered in the diffuse-injured VPM, which increases the probability of detecting a treatment effect in this region. Classical activation and acquired deactivation gene expression predominate the microglial phenotype in the diffuse-injured thalamus at 7 days post-injury. We sought to modulate injury-induced gene expression by the sustained administration of the non-steroidal anti-inflammatory ibuprofen, given that the majority of human TBI consists of mild to moderate diffuse brain injury for which self-medication may be the primary treatment. Uninjured sham and brain-injured animals were treated daily for 7 days with intraperitoneal ibuprofen or saline to blunt the peak of microglial activation. The sham saline-treated and sham ibuprofen-treated groups were combined into one sham group after confirming no significant differences between the two groups by student t-test.
Injury-induced increases in classical and acquired deactivation gene expression in the VPM were responsive to anti-inflammatory treatment (Figure 5A). The fourfold significant increase in TSPO gene expression in brain-injured saline treated animals was attenuated by the ibuprofen treatment to a level where no significant difference was detected compared to the sham group (F(2,14) = 6.754; p = 0.0088). Similar results were obtained for CD45 (F(2,13) = 8.160; p = 0.0051) and TGF-βI (F(2,12) = 4.844; p = 0.0287) gene expression which increased significantly in saline-treated brain-injured animals and was attenuated with ibuprofen treatment. Only those genes that showed an injury-induced increase in gene expression were quantified in response to anti-inflammatory treatment.
Figure 5.
Anti-inflammatory treatment attenuates diffuse brain injury-induced increases in inflammatory gene expression in the thalamus. For 7 days after sham control or fluid percussion brain injury (FPI), rats (n=5–6 per group) were administered ibuprofen (20 mg/kg, i.p.) or saline and gene expression in the thalamus quantified. (A) Gene expression for classical (TSPO and CD45) and acquired deactivation (TGF-βI) microglial activation markers were significantly increased at 7 days after FPI. Ibuprofen treatment reduced TSPO, CD45 and TGF-βI gene expression to levels no longer significantly different from sham. (B) Alternative activation (arginase 1) or neuroplasticity (GAP43 and synaptophysin) gene expression was not affected by brain injury or ibuprofen treatment in the thalamus. The sham saline-treated and sham ibuprofen-treated groups were combined into a single sham group after confirming no significant difference between groups. *, p < 0.05 compared to sham-injured animals; †, p < 0.05 compared to 7 day saline-treated brain-injured animals. Means are from 3–6 animals per time point.
Alternative activated microglial have been reported to contribute to axon growth and synaptic regeneration in the injured CNS (Colton and Wilcock, 2010). However, anti-inflammatory treatment did not adversely affect gene expression for the alternative activation marker arginase 1 (F(2,14) = 0.6358; p = 0.5441), axon growth marker GAP43 (F(2,15) = 0.2062; p = 0.8159) or synapse quantification marker synaptophysin (F(2,14) = 1.272; p = 0.3107; Figure 5B).
Discussion
We investigated a time course of inflammatory gene expression relative to microglia activation states and the consequence of inhibiting classical microglial activation after experimental diffuse TBI. Similar studies have been previously published for focal and mixed models of experimental brain injury (Wei et al., 2009, Rojo et al., 2011), however limited data on microglial activation states based on genetic expression has been published for diffuse brain injury. In animal models of Alzheimer’s disease and spinal cord injury, similar gene expression studies have been conducted to dissect the spatio-temporal contribution of microglial activation states (Colton et al., 2006, Kigerl et al.). In the present communication, we present the location and timing of microglia activation followed by gene expression for classical activation, acquired deactivation and alternative activation. Further, we tested the potential of ibuprofen to inhibit this inflammatory response. We show microglia are predominantly classically activated, a state which is responsive to over-the-counter anti-inflammatory treatment. In these results, the VPM showed more genes with significant gene expression than the S1BF, despite morphological activation in both regions.
Of principal importance, diffuse TBI results in circuit disruption, without contusion, cavitation or tissue destruction. Over time post-injury, the diffuse-injured brain is preserved for repeated analysis of homotypic tissue; whereas focal injuries necessitate isolation of core and penumbral tissues. Here, we focus on the primary somatosensory barrel cortex (S1BF) and ventral posterior medial (VPM) nucleus of the thalamus, as these regions have selective vulnerabilities and roles in whisker somatosensation (Lifshitz et al., 2007, Hall and Lifshitz, 2010, McNamara et al., 2010, Lifshitz and Lisembee, 2012, Thomas et al., 2012). To date, we have shown tissue damage that includes acute cellular swelling, likely as a result of an injury induced neurochemical storm (Lifshitz et al., 2007, Lifshitz and Lisembee, 2012) and concomitant neuropathology by accumulation of silver stain (Lifshitz and Lisembee, 2012). Following diffuse TBI, similar cellular cascades that lead to neuropathology can activate microglia from a resting phenotype (Kelley et al., 2007). Resting microglia with small cell bodies and highly ramified filamentous processes rapidly become activated as indicated by swollen cell bodies, with fewer, less-branched processes. One extreme morphological phenotype is the elongated rod morphologies seen perpendicular to the dural surface of the S1BF at 7 days post-injury, which resemble previously described rod microglia (Graeber, 2010). Once the temporal profile of morphological differences was documented in the S1BF and VPM, the remainder of the present study design was to classify microglia activation based on gene expression.
To identify pathways involved in microglial activation after diffuse brain injury, we quantified gene expression for general inflammatory markers (MHC I, MHC II, TSPO). In all conditions, gene expression was elevated at 7 days post-injury and resided by 28 days post-injury. TSPO expression was significantly elevated in both the S1BF and VPM at 7 days post-injury. Since TSPO has been implicated in early inflammation and later repair (Chen and Guilarte, 2008), we explored spatiotemporal expression by radiolabeled ligand binding. Receptor binding densities followed gene expression in both S1BF and VPM, possibly as a result of increased activation or microglial proliferation and migration into these areas. We favor microglial proliferation and migration, due to morphologically condensed microglial covering the entire diffuse-injured brain, including the substantia nigra (van Bregt et al., 2012). Microglia migrating to vulnerable areas could contribute to circuit reorganization by clearing degenerating synapses or participating in the frank stripping of synapses (Neumann et al., 2009, Perry and O’Connor, 2010). In diffuse brain injury, this role is not only plausible, it is possible as no overt cavitation or tissue loss occurs in the presence of neuropathological change (Lifshitz et al., 2007, Hall and Lifshitz, 2010, Lifshitz and Lisembee, 2012).
Within the first week after diffuse brain injury, neuroinflammation was elevated significantly above sham levels and subsided by 1 month. We chose time points that have previously been shown to align with the development and manifestation of sensory sensitivity after diffuse brain injury (McNamara et al., 2010, Learoyd and Lifshitz, 2012). Classically activated microglia in neurotrauma have been associated with higher phagocytotic activity and reactive oxygen species (ROS) production (Boekhoff et al., 2011), thereby increasing oxidative stress, inducing neurotoxicity and propagating secondary neurodegenerative cascades. Activated microglia can degrade the extracellular matrix, promote the retraction of dystrophic axons and destabilize synapses (del Zoppo et al., 2007 berg, 2007, stroke, Horn et al., 2008, Perry and O’Connor, 2010 synaptic stripping), even in the absence of focal tissue loss. Here, we report microglial activation that primarily indicated classical (TNFα, CD45) and acquired deactivation (TGF-β1, TGF-βRII), with little evidence for the alternative activation pathway (Arginase 1). The gene expression results indicate a predominant influence of classical activation in the diffuse-injured CNS, consistent with studies in spinal cord injury, where CD45 surface protein expression was increased (Boekhoff et al., 2011), but arginase 1 gene expression was only transiently up-regulated (Kigerl et al., 2009). The possibility exists for alternative activated microglia to be evident before, between or after the time points currently examined. After spinal cord injury, for example, arginase 1 gene expression was found to be transiently increased three days after injury but subside by 7 days after injury (Kigerl et al., 2009). Similarly there was no sustained increase in inflammatory gene expression, rather injury-induced increases in gene expression returned to baseline levels. However, histological evidence is present to support long term microglial activation and ongoing inflammatory processes. Morphological changes are insufficient in determining the mode of microglial activation, since microglia alternatively activated by IL-4 and classically activated by IFNγ showed similar morphologies (Colton et al., 2006, Colton and Wilcock, 2010). Sustained protein expression could maintain morphological activation, which remains to be explored.
Diffuse brain injury initiates a balanced inflammatory response, as evidenced by concomitant classical activation (TNFα, CD45) and acquired deactivation (TGF-βI, TGF-βRII) of microglia. The functional activation of opposing processes obscures the true magnitude of inflammation after diffuse brain injury, as acquired deactivation can suppress classical activation and vice versa (Colton, 2009, Colton and Wilcock, 2010). In fact, TGF-βI and TGF-βRII gene activation may explain the attenuated TNFα gene expression (Colton and Wilcock, 2010). Reinstatement of a homeostatic balance of microglial activation appears to be underway by 28 days post-injury as gene expression returns to sham levels.
Microglial activation can exist in more than one state, including proinflammatory, anti-inflammatory, or ptotrophic (Lai and Todd, 2008). When activated, microglia can initiate, if not aggravate, neuronal injury, as demonstrated in experimental brain and spinal cord injury (Morganti-Kossmann et al., 2002, Popovich and Longbrake, 2008). On the other hand, resident microglia and blood monocytes have been proposed as essential for promoting recovery after neurotrauma (Rapalino et al., 1998, Benowitz and Yin, 2007, Kigerl et al., 2009). In general, our knowledge of microglial activation in experimental TBI is limited, as the complexity associated with exploring all combinations of models, time points and brain regions. Additionally, commonly studied focal models of brain injury are confounded by necrotic tissue which cavitates within days post-injury, leaving little time or tissue to examine the long term inflammatory consequences. The possibility exists for microglial activation and migration to significantly influence secondary cascades leading to neurological circuit disruption, reorganization and repair (Neumann et al., 2009, Perry et al., 2010).
In our laboratory, we explore pathophysiology of the somatosensory whisker-barrel circuit after diffuse brain injury in the rat. For this reason, the present communication focused on the VPM and S1BF. As previously described, our model of diffuse brain injury results in atrophy (but not loss) of VPM thalamic and S1BF cortical neurons (Lifshitz et al., 2007, Lifshitz and Lisembee, 2012), acute hypoactivation followed chronic hyperactivation of thalamic and cortical neurons (Hall and Lifshitz, 2010) and hyperexcitability of pre-synaptic glutamate terminals (Hinzman et al., 2012, Thomas et al., 2012). Over 1 month post-injury, pathophysiological consequences of diffuse brain injury develop into the behavioral expression of sensory sensitivity to whisker stimulation (McNamara et al., 2010, Learoyd and Lifshitz, 2012, Thomas et al., 2012). Here our pathological examination extended to microglia, where injury-induced activation of microglia was seen as a swollen cell body with less ramified processes. This morphological change in microglial cells co-localized to areas with significantly increased TSPO expression compared to uninjured sham animals. Thus, in the same functionally relevant circuit, diffuse brain injury results in axotomy, hyperactivation and chronic microglia activation. Whether microglial activation precedes, coincides with or follows neural injury remains to be determined, however all three likely contribute to the development of sensory sensitivity.
In the natural course of diffuse brain injury, no clear relationship emerged between the gene expression of classical, alternative activation and acquired deactivation. To determine a relationship between modes of inflammatory gene expression, we have further examined gene expression in ibuprofen-treated animals after diffuse TBI. As an over-the-counter anti-inflammatory agent, ibuprofen can be taken by those suffering a mild TBI who do not seek medical attention. Chronic administration (4 months) of ibuprofen (20 mg/kg/day) worsened cognitive outcomes after experimental brain injury, without effecting cortical tissue loss (Browne et al., 2006). On the other hand, acute ibuprofen administration (10–50 mg/kg for 6 days) showed decreased inflammation after forebrain ischemia (Patel et al., 1993, Park et al., 2005). In the current study, daily ibuprofen treatment for 7 days was predicted to attenuate classical activation and uncover alternative activated microglia. In the thalamus, ibuprofen treatment reduced injury-induced inflammation by attenuating microglial activation (TSPO), classical activation (CD45) and acquired deactivation (TGF-βI) gene expression. However, the anti-inflammatory treatment had no influence on uncovering alternative activation of microglia, which has been linked to neuroplasticity (Gordon, 2003, Henkel et al., 2009, Kigerl et al., 2009). We have previously documented a significant decrease in the thalamus at 7 days post-injury of both GAP-43 and synaptophysin (Hall and Lifshitz, 2010). In the current study, gene expression in saline-treated animals was decreased, but failed to reach significance. Furthermore, ibuprofen treatment had no additional impact on neuroplastic gene expression. Prominent roles for alternative activated microglia may depend on injury model, brain region and severity, as has been demonstrated for neuroplastic gene expression in the hippocampus (Griesbach et al., 2002) and pro-inflammatory gene expression in graded in vitro hypoxia (Lai and Todd, 2008). Regardless, ibuprofen may prove effective in modulating classical activation; the long term consequences of acute anti-inflammatory treatment on behavioral outcome remain to be determined.
In conclusion, these experiments identified morphological and gene expression alterations in microglia after experimental diffuse brain injury. In particular, activated microglia were observed in the S1BF and VPM out to 28 days post-injury. Gene and protein neuroinflammatory markers were more robust in the VPm than the S1BF. The VPM of the somatosensory whisker circuit exhibited regional activation, primarily in classical activation and acquired deactivation. Ibuprofen administration attenuated inflammatory gene activation, which implicates a therapeutic role for ibuprofen in injury-induced inflammation over a defined post-injury period.
Highlights.
Morphologically activated microglia seen up to 28 days after diffuse brain injury.
Inflammatory genes acutely elevated in thalamus & cortex after diffuse TBI.
Anti-inflammatory genes acutely elevated after diffuse TBI.
Translocator protein 18 kDa receptor binding validated qPCR results.
Acute ibuprofen administration attenuated classical inflammatory genes in the VPM.
Acknowledgments
With generous thanks to: With generous thanks to: Amanda M. Lisembee for preparing the diffuse brain-injured rats; Deanne Hopkins for conducting the radioligand binding experiments; and Kelley D. Hall for assistance with the ibuprofen administration. Supported, in part, by NIH NINDS R01 NS065052, NIH NICHD R01 HD061996, Kentucky Spinal Cord and Head Injury Research Trust (KSCHIRT) 7-11 and NIH NINDS P30 NS051220.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz LI, Yin Y. Combinatorial treatments for promoting axon regeneration in the CNS: strategies for overcoming inhibitory signals and activating neurons’ intrinsic growth state. Developmental neurobiology. 2007;67:1148–1165. doi: 10.1002/dneu.20515. [DOI] [PubMed] [Google Scholar]
- Boekhoff TM, Ensinger EM, Carlson R, Bock P, Baumgartner W, Rohn K, Tipold A, Stein VM. Microglial Contribution to Secondary Injury Evaluated in a Large Animal Model of Human Spinal Cord Trauma. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1821. [DOI] [PubMed] [Google Scholar]
- Browne KD, Iwata A, Putt ME, Smith DH. Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats. Exp Neurol. 2006;201:301–307. doi: 10.1016/j.expneurol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204:220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacology & therapeutics. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS & neurological disorders drug targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Cook NL, Vink R, Donkin JJ, van den Heuvel C. Validation of reference genes for normalization of real-time quantitative RT-PCR data in traumatic brain injury. J Neurosci Res. 2009;87:34–41. doi: 10.1002/jnr.21846. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Thams S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev. 2007;55:89–96. doi: 10.1016/j.brainresrev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas O, Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JJ, Diesl V, Wittmann T, Morrison DC, Ryan JL, Vogel SN, Follettie MT. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. Journal of leukocyte biology. 2002;72:1234–1245. [PubMed] [Google Scholar]
- Ghajar J, Ivry RB. The predictive brain state: timing deficiency in traumatic brain injury? Neurorehabilitation and neural repair. 2008;22:217–227. doi: 10.1177/1545968308315600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH, Nicoll JA, Maxwell WL, Gennarelli TA. The nature, distribution and causes of traumatic brain injury. Brain pathology (Zurich, Switzerland) 1995;5:397–406. doi: 10.1111/j.1750-3639.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Graham DI, Gennarelli TA, McIntosh TK. Trauma. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. Vol. 7. London: Arnold Publishers; 2002. pp. 823–898. [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Gomez-Pinilla F. Alterations in BDNF and synapsin I within the occipital cortex and hippocampus after mild traumatic brain injury in the developing rat: reflections of injury-induced neuroplasticity. J Neurotrauma. 2002;19:803–814. doi: 10.1089/08977150260190401. [DOI] [PubMed] [Google Scholar]
- Grossman R, Shohami E, Alexandrovich A, Yatsiv I, Kloog Y, Biegon A. Increase in peripheral benzodiazepine receptors and loss of glutamate NMDA receptors in a mouse model of closed head injury: a quantitative autoradiographic study. Neuroimage. 2003;20:1971–1981. doi: 10.1016/j.neuroimage.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Hall KD, Lifshitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res. 2010;1323:161–173. doi: 10.1016/j.brainres.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JL, Reeves TM, Phillips LL. Injury modality, survival interval, and sample region are critical determinants of qRT-PCR reference gene selection during long-term recovery from brain trauma. J Neurotrauma. 2009;26:1669–1681. doi: 10.1089/neu.2009.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: the good, the bad, and the resting. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2009;4:389–398. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- Hinzman J, Thomas TC, Quintero J, Gerhardt G, Lifshitz J. Disruptions in the Regulation of Extracellular Glutamate by Neurons and Glia in the Rat Striatum Two Days after Diffuse Brain Injury. J Neurotrauma. 2012 doi: 10.1089/neu.2011.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini AH, Lifshitz J. Brain injury forces of moderate magnitude elicit the fencing response. Med Sci Sports Exerc. 2009;41:1687–1697. doi: 10.1249/MSS.0b013e31819fcd1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. J Neurotrauma. 2011;28:1783–1801. doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Farkas O, Lifshitz J, Povlishock JT. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol. 2006;198:350–360. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Scheff SW, Pauly JR, Loftin CD. Effects of genetic deficiency of cyclooxygenase-1 or cyclooxygenase-2 on functional and histological outcomes following traumatic brain injury in mice. BMC neuroscience. 2009;10:108. doi: 10.1186/1471-2202-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia. 2008;56:259–270. doi: 10.1002/glia.20610. [DOI] [PubMed] [Google Scholar]
- Learoyd AE, Lifshitz J. Comparison of rat sensory behavioral tasks to detect somatosensory morbidity after diffuse brain-injury. Behav Brain Res. 2012;226:197–204. doi: 10.1016/j.bbr.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Molecular neurobiology. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Fluid Percussion Injury. In: Jun Chen ZCX, Xu Xiao-Ming, Zhang Jhon H, editors. Animal Models of Acute Neurological Injuries. Totowa, NJ: The Humana Press, Inc; 2008. [Google Scholar]
- Lifshitz J, Kelley BJ, Povlishock JT. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J Neuropathol Exp Neurol. 2007;66:218–229. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Lisembee AM. Neurodegeneration in the somatosensory cortex after experimental diffuse brain injury. Brain structure & function. 2012;217:49–61. doi: 10.1007/s00429-011-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, McFinton PR, Dalack GW, Cook EH, Jr, Cassin BJ, Watson SJ. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Archives of general psychiatry. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am. 1992;15:395–413. [PubMed] [Google Scholar]
- McNamara KC, Lisembee AM, Lifshitz J. The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J Neurotrauma. 2010;27:695–706. doi: 10.1089/neu.2009.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Jr, Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Current opinion in critical care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain: a journal of neurology. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EM, Cho BP, Volpe BT, Cruz MO, Joh TH, Cho S. Ibuprofen protects ischemia-induced neuronal injury via up-regulating interleukin-1 receptor antagonist expression. Neuroscience. 2005;132:625–631. doi: 10.1016/j.neuroscience.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Patel PM, Drummond JC, Sano T, Cole DJ, Kalkman CJ, Yaksh TL. Effect of ibuprofen on regional eicosanoid production and neuronal injury after forebrain ischemia in rats. Brain Res. 1993;614:315–324. doi: 10.1016/0006-8993(93)91050-3. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature reviews Neurology. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Perry VH, O’Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nature reviews Neuroscience. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehab. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nature neuroscience. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature medicine. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Rojo DR, Prough DS, Falduto MT, Boone DR, Micci MA, Kahrig KM, Crookshanks JM, Jimenez A, Uchida T, Cowart JC, Hawkins BE, Avila M, DeWitt DS, Hellmich HL. Influence of stochastic gene expression on the cell survival rheostat after traumatic brain injury. PloS one. 2011;6:e23111. doi: 10.1371/journal.pone.0023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SM. Peirce’s criterion for the elimination of suspect experimental data. J Eng Technol. 2003;20:38–41. [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010a;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiology of disease. 2010b;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TC, Hinzman JM, Gerhardt GA, Lifshitz J. Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J Neurotrauma. 2012;29:187–200. doi: 10.1089/neu.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bregt DR, Thomas TC, Hinzman JM, Cao T, Liu M, Bing G, Gerhardt GA, Pauly JR, Lifshitz J. Substantia nigra vulnerability after a single moderate diffuse brain injury in the rat. Exp Neurol. 2012;234:8–19. doi: 10.1016/j.expneurol.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan C, Chrzaszcz M, Choi N, Wainwright MS. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J Neuroinflammation. 2010;7:32. doi: 10.1186/1742-2094-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Budel S, Baughman K, Gould G, Song KH, Strittmatter SM. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma. 2009;26:81–95. doi: 10.1089/neu.2007.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HH, Lu XC, Shear DA, Waghray A, Yao C, Tortella FC, Dave JR. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. J Neuroinflammation. 2009;6:19. doi: 10.1186/1742-2094-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates GN, Gracey F, McGrath JC. A biopsychosocial deconstruction of “personality change” following acquired brain injury. Neuropsychol Rehabil. 2008;18:566–589. doi: 10.1080/09602010802151532. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Bye N, Semple BD, Kossmann T, Morganti-Kossmann MC. Attenuated neurological deficit, cell death and lesion volume in Fas-mutant mice is associated with altered neuroinflammation following traumatic brain injury. Brain Res. 2011;1414:94–105. doi: 10.1016/j.brainres.2011.07.056. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]