Abstract
Purpose
In the last decade, a growing scientific and medical interest has emerged toward cardiovascular effects of dietary nitrite and nitrate; however, many questions concerning their mode of action(s) remain unanswered. In this review, we focus on multiple mechanisms that might account for potential cardiovascular beneficial effects of dietary nitrite and nitrate.
Results
Beneficial changes to cardiovascular health from dietary nitrite and nitrate might result from several mechanism(s) including their reduction into nitric oxide, improvement in endothelial function, vascular relaxation, and/or inhibition of the platelet aggregation. From recently obtained evidence, it appears that the longstanding concerns about the toxicity of oral nitrite or nitrate are overstated.
Conclusion
Dietary nitrite and nitrate may have cardiovascular protective effects in both healthy individuals and also those with cardiovascular disease conditions. A role for nitrite and nitrate in nitric oxide biosynthesis and/or in improving nitric oxide bioavailability may eventually provide a rationale for using dietary nitrite and nitrate supplementation in the treatment and prevention of cardiovascular diseases.
Keywords: Cardiovascular disease, Diet, Endothelial dysfunction, Nitric oxide, Nitrite, Nitrate
Introduction
Scientific and medical interest in cardiovascular health benefits of fruit- and vegetable-rich diets has grown exponentially in recent years, due to compelling epidemiological evidence showing that consumption of fruits and vegetables might reduce the risk of cardiovascular diseases [1–3]. Understanding the precise factors in these foods that account for cardiovascular protection is a major focus of experimental and clinical research for many years. Over the past two decades, several factors in these foods have been identified to have favorable effects on cardiovascular health, although many questions concerning their relevant contribution remain unanswered [4–6]. Of late, there is great interest in the nitrite and nitrate content of these foods in improving cardiovascular health due to improved understanding of the role of endogenous nitrite and nitrate in the maintenance of vascular homeostasis [7–13]. Over the past decade, several in vitro and in vivo animal model studies [14–26] and a few human studies [15, 27–30] have provided evidence that the nitrite and nitrate content of the fruits and vegetables could contribute to their cardiovascular health benefits, although many questions concerning their mode of action(s) remain unanswered. The major focus of this paper will be to provide a review of mechanisms that might account for the potential cardiovascular beneficial effects of dietary nitrite and nitrate. These include enhancement in nitric oxide bioavailability in the vasculature, dilation of the vasculature (vasodilator effects), and inhibition of platelet aggregation. In addition, this paper briefly discusses data from animal and human studies investigating the effects of nitrite and nitrate supplementation in the treatment and prevention of myocardial ischemia and hypertension.
Sources of nitrite and nitrate
Endogenous nitrite and nitrate originate from two potential sources [7–13]. First, oxidation of NO produced in vivo in biological fluids and cells. NO is synthesized in vivo from the amino acid l-arginine by the nitric oxide synthase (NOS) enzymes in the presence of molecular oxygen (O2) and several cofactors. These cofactors include nicotinamide adenine dinucleotide phosphate (NADPH), flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), tetrahydrobiopterin (BH4), and calmodulin and calcium. Three isoforms of NOS have been identified: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS). Initially, eNOS was generally considered as the sole (major) source of NO in the vasculature. NO is labile with a short half-life in biological fluids [31]. Once synthesized, NO gets rapidly oxidized to nitrate by oxyhemoglobin and to nitrite in the plasma in a reaction catalyzed at least in part by the copperstorage plasma protein ceruloplasmin [32, 33]. Indeed, l-arginine-eNOS pathway has been reported to contribute to ~70% of plasma nitrite [34–36]. Second, diet is also the major potential source of endogenous nitrite and nitrate. For the average population, the range of dietary nitrite intake has been reported to be 0–20 mg per day, whereas for dietary nitrate, it ranges from 53 to 300 mg per day [37]. Most dietary nitrate (80–95%) exposure comes from plant foods (mostly vegetables and a few fruits), whereas most dietary nitrite comes from nitrite food additives and added nitrite in processed, cured meats (~39%) and baked goods and cereals (~34%) [13, 37]. In addition, drinking water can also contain considerable amounts of nitrate (usually <10 mg/L in the absence of bacterial contamination), although in many countries the levels are strictly regulated. On the other hand, systemic nitrite can also be derived from the reduction of nitrate, originated either from NO oxidation or from the diet, to nitrite by two sources. First, nitrate can be reduced to nitrite by commensal facultative anaerobic bacteria present in the oral cavity (i.e., mouth) and possibly in the gastrointestinal tract [38–40]. For instance, in a recent cross-over designed study involving seven healthy human volunteers, rinsing the mouth with antibacterial mouthwash has been shown to abolish conversion of nitrate into nitrite in saliva and to markedly reduce increase in plasma nitrite following a dietary nitrate load (10 mg/kg of sodium nitrate dissolved in water) [40], suggesting that oral bacteria plays a critical role in nitrate reduction into nitrite, and, conversely, contributes to systemic nitrite levels. Indeed, it has been known for several years that bacteria use nitrate as an alternative electron acceptor to oxygen during respiration and a fraction of the nitrate anions present in saliva is converted in the mouth to nitrite under the influence of bacterial enzymes [38, 39]. Second, but only recently recognized, possibility for systemic nitrate reduction to nitrite is nitrate reductase enzymes present in the tissues such as lung, liver, heart, and kidney [41]. For instance, in a recent study, Jansson et al. [39] demonstrated that mammalian tissues reduce inorganic nitrate to nitrite and that this effect is significantly inhibited by allopurinol [41], suggesting that molybdenum-based oxidoreductase enzymes such as xanthine oxidase present in mammalian tissues may catalyze, at least in part, the nitrate reduction to nitrite and, conversely, contribute to systemic nitrite levels.
Pharmacokinetics of nitrite and nitrate
An important factor influencing the interpretation of the biological effects of nitrite and nitrate is their systemic concentration. Emerging data from human and animal studies suggest that nitrite and nitrate derived from both endogenous (i.e., oxidation of endogenously produced NO) and exogenous (i.e., dietary intake) sources contribute to steady-state systemic nitrite and nitrate concentrations [7–13]. In general, plasma concentrations obtained of nitrite and nitrate range between 0.01 and 0.6 µM and 20–40 µM, respectively [7–13, 32, 42–44]. The concentrations of nitrite in rat vascular tissue (10 ± 1 µM) have been reported to be much higher than that observed in the plasma [45]. Dietary nitrate is readily absorbed from the stomach and proximal small intestine into the blood and mixes with NO-derived nitrate. Although much of the nitrate ingested (65–70%) is excreted in the urine, part of it (~25%) accumulates greatly in saliva after active uptake by the salivary glands [38, 46– 48]. This nitrate is rapidly reduced to nitrite by bacteria in the mouth, and part of nitrite then formed is absorbed in the gut and enters the systemic circulation [39, 48]. Bartholomew and Hill [47] demonstrated that urinary nitrate reaches a maximum concentration in 4–6 h after ingestion of nitrate and returns to the baseline value within 24 h. Similar results were also reported by Pannala et al. [49]. Pannala et al. demonstrated that high dietary nitrate (< 3.65 mg/kg body weight or ~ 250 mg nitrate) intake leads to a ~4 to sevenfold increase in plasma, urinary, and salivary nitrate content in healthy human volunteers. In this study, nitrate intake increased urinary nitrate excretion from 53 ± 4 mg on day 1 (low-nitrate day) to 223 ± 13, 241 ± 15, and 243 ± 16 mg on days 2, 3, and 4, (high-nitrate days), respectively [49]. Interestingly, in the later study, high dietary nitrate intake also increased nitrite concentration in urine (~23 µg pre-intake to ~39 µg post-intake) and saliva (0.27 mM pre-intake vs. 1.8 mM at 2 h post-intake) but not in the plasma (78 ± 4 nM pre-intake vs. 90 ± 3 nM post-nitrate intake) [49]. In contrast, in a recent study, Kapil et al. [28] demonstrated that plasma nitrite concentration was significantly elevated at ~1.5 h, peaked at ~2.5 h (increased from ~0.5 µM to ~1.5 µM), sustained to 6 h, and remained elevated than baseline values at 24 h after potassium nitrate (KNO3 capsules, 24 mmol) supplementation. This study also demonstrated that plasma nitrate concentrations peaked at 3 h and steadily decreased thereafter but remains significantly elevated than baseline values at 24 h after ingestion of 24 mmol KNO3. In this study, plasma nitrate concentration elevated above baseline by ~35-, 27-, and 7-fold after administration of 24, 12, and 4 mmol of KNO3, respectively. Furthermore, Kapil et al. [28] also demonstrated that plasma nitrate concentrations were elevated much sooner than plasma nitrite concentrations after ingestion of KNO3 (30 min vs. 1.5 h), reflecting the use of bacterial reduction of nitrate to nitrite in the oral cavity.
Mechanisms by which nitrite and nitrate could improve cardiovascular health
When considering how nitrite and nitrate can improve cardiovascular health, it is important to consider recent insights into the pathophysiology of the cardiovascular system. The human cardiovascular system is composed of the heart, the blood vessels (or vasculature), and the cells and plasma of the blood. The walls of blood vessels consist three distinctive layers (from inside to outside): tunica intima (made of endothelial cells), tunica media (made of vascular smooth muscle cells), and tunica adventitia (made of connective tissue). The endothelial cell layer, referred to as the endothelium, separates rest of the vessel wall from the circulating blood. The endothelium plays an important role in the maintenance of vascular homeostasis, achieved through release of a variety of local vascular mediators [50–56]. Among these mediators, endothelium-derived NO is essential for the maintenance of vascular homeostasis due to its actions on both smooth muscle cells and circulating blood cells (platelets, monocytes, etc.) [50–56]. Endothelium-derived NO has been reported to control vascular tone, smooth muscle cell proliferation and growth, platelet activity and aggregation, leukocyte trafficking, expression of certain adhesion molecules, and inflammation [50–56]. Thus, alterations in endothelium-derived NO bioavailability can result in endothelial dysfunction, broadly defined as a failure of the endothelium to perform its physiological functions, leading to impaired vascular homeostasis and thus to the pathogenesis and clinical expression of a variety of cardiovascular diseases. Indeed, studies over the past two decades have suggested that cardiovascular risk factors including hypertension, diabetes mellitus, physical inactivity, postmenopausal state, smoking, aging, inflammation, dyslipidemia, and hypercholesterolemia are all associates with endothelial dysfunction due to reduced NO bioavailability in the vasculature [50–56]. Thus, restoration of or improving NO bioavailability in the vasculature has become a major goal of therapy in cardiovascular disease states. On the other hand, endothelial dysfunction causes a decrease in dilation of the vasculature, favors platelet adherence and activation leading to thrombosis, and increases vascular inflammation and proliferation of smooth muscle cells (hypertrophy), all of which could act to increase the risk of cardiovascular events. Prospective studies over the past two decades have shown that treatment modalities that can target one or more of the manifestations of endothelial dysfunction are likely to improve cardiovascular health. Beneficial changes to cardiovascular health from these interventions might result from promotion of vascular relaxation, inhibition of vasoconstriction, inhibition of platelet aggregation, reduction in the production of free radicals, or other mechanisms that protect the endothelium from the injury [50–56]. Relevant to the current review, there is increasing evidence that nitrite and nitrate in the diet can improve NO bioavailability in the vasculature, exert vasodilation, inhibit platelet aggregation, and conversely, improve cardiovascular health.
Improving NO bioavailability in the vasculature
Most studies to date have demonstrated that the protective effects of nitrite were attenuated or prevented by NO scavengers such as carboxy-PTIO and hemoglobin, suggesting that the beneficial effects of nitrite and nitrate were mediated at least in part, if not fully, by improved NO bioavailability. Emerging data suggest that the nitrite and nitrate in the diet could improve NO bioavailability through several mechanisms (discussed below) and, conversely, improved NO bioavailability could well contribute to the ability of nitrite and nitrate to improve cardiovascular health. When discussing the effect of nitrate, the results must be considered in relation to nitrite (i.e., nitrate should be viewed as a precursor of nitrite), because nitrate is first reduced to nitrite before entering the systemic circulation [38–41].
Nitrite and nitrate reduction to NO in the vasculature
Nitrite and nitrate can improve NO bioavailability in the vasculature by their reduction to NO in mammalian tissues including blood [7–13]. NO generation from one-electron reduction of inorganic nitrite was first described in stomach in 1994 [57, 58]. Protonation of inorganic nitrite in acidic gastric juice to form nitrous acid (HNO2), which then decomposes to NO and other nitrogen oxides, has been implicated in NO generation from inorganic nitrite [57, 58]. Soon after the discovery of gastric NOS-independent NO generation, Zweier et al. [59] showed that NO can also be generated in ischemic and acidic heart by direct reduction of nitrite to NO, providing the first evidence for NOS enzyme-independent production of systemic/vascular NO. In subsequent studies, numerous pathways that can catalyze nitrite and nitrate reduction to NO in vivo have been identified and the list is growing fatter. These include deoxyhemoglobin [29], deoxymyoglobin [60], xanthine oxidoreductase [61], cytochrome P450 enzymes [62], mitochondrial respiratory chain enzymes [63], aldehyde oxidase [64], carbonic anhydrase [65], acidic disproportionation [57, 58], and reducing agents (e.g., ascorbate, polyphenols) [66, 67]. These pathways have previously been reviewed [68], and a detailed discussion of these pathways is beyond the scope of this review. However, at this point of time, the relative contribution of each individual pathway to NO release from nitrite and nitrate is poorly understood. To our knowledge, so far only one study has addressed this issue [69]. In addition, whether or not all these pathways catalyze physiological NO generation from nitrite and nitrate is still a topic of debate due to their increased activity in the presence of very low pH and O2 (i.e., ischemic) environment. Nevertheless, their participation in the states of hypoxia/ischemia and acidosis is now well recognized and supported by a number of studies [7–13, 20, 68, 70]. While the controversy exists, several studies measuring arterial-to-venous gradients for nitrite suggest that nitrite is also metabolized to NO across physiological pH and oxygen gradients, as evidenced by large arterial-to-venous gradients for nitrite anions across human forearm circulation [29, 32, 71]. For example, Cosby et al. [29] demonstrated increased NO formation in the blood, as measured by the rate of formation of ironnitrosylated hemoglobin (HbNO), during nitrite infusion at rest and under exercise stress. On the other hand, emerging data show that NO generation from nitrite and nitrate is indeed an alternative source of bioactive NO in vivo in addition to that released from the endothelium and plays a key role in the maintenance of steady-state physiological NO levels [21, 22]. For example, nitrite supplementation (50 mg/L, 1 week in drinking water) has recently been shown to restore impaired NO homeostasis in eNOS-deficient mice [21]. However, at this point in time, there have been no direct experimental measurements in vivo of the amount of NO generated through nitrite and nitrate reduction.
Nitrite and nitrate as substrate to eNOS
Classically, endothelium-derived NO is synthesized from the amino acid l-arginine by the eNOS enzyme. This reaction requires the presence of molecular O2, and thus, eNOS-derived NO synthesis would be expected to cease to exist when the molecular O2 levels falls below the threshold levels (e.g., myocardial ischemia). Indeed, impaired endothelial NO production has been described in ischemic tissues [72, 73]. However, recent experimental data suggest that eNOS can generate NO in the absence of molecular O2 by utilizing nitrite as substrate, indicating that NO generation from nitrite and nitrate by eNOS could well contribute to improved vascular NO bioavailability in hypoxic/ischemic conditions [74, 75]. For example, using recombinant eNOS holoenzyme and its oxygenase domain, Gautier et al. [74] demonstrated that eNOS reduces nitrite ions to NO under anoxia. In this study, the release of NO from nitrite at physiological pH is observed electrochemically as well as with optical and electron paramagnetic resonance (EPR) spectroscopy. In another study, Vanin et al. [75] demonstrated that immortalized murine microvascular endothelial cells release significant quantities of NO under anoxic conditions. In this study, by utilizing multiple experimental approaches including pharmacological inhibition of eNOS and estimation of nitrite levels, the authors confirmed that the NO is released from nitrite reduction by eNOS. The later study also demonstrated that the magnitude of the anoxic NO production was significantly higher than basal normoxic yield, suggesting that the nitrite reductase activity of eNOS is relevant for fast NO delivery in hypoxic vascular tissues.
Improving endothelium-derived NO bioavailability (endothelial function)
An important mechanism of impaired NO bioavailability in the vasculature is endothelial dysfunction [50–56]. Although the cause of endothelial dysfunction remains unclear, increased production of reactive oxygen species (ROS) represents the primary problem [50–56]. ROS, in particular superoxide anions, rapidly react with NO released from the endothelium to form peroxynitrite, leading to reduced bioavailability of endothelium-derived NO. Mitochondria are an important source of ROS overproduction in cardiovascular pathological states [76–78]. Nitrite has been shown to inhibit mitochondrial ROS generation, achieved through S-nitrosation of mitochondrial respiratory chain complex I enzyme [23, 24]. Attenuation in mitochondrial ROS generation may well contribute to improvement in endothelial function. In this regard, mitochondria-targeted antioxidants such as MitoQ10 have been demonstrated to improve endothelial function [78]. Nitrite inhibition of ROS production could also lead to preservation of NO production from the classical eNOS-l-arginine pathway because ROS can readily oxidize BH4, an essential cofactor for eNOS, into dihydrobiopterin (BH2) [79, 80]. Relative BH4 deficiency is a significant contributor to endothelial dysfunction and can be specifically rescued by restoration of BH4 in relation to eNOS function. Indeed, in a recent study, dietary nitrite ingestion (50 mg per liter of sodium nitrite in drinking water) has been reported to increase BH4 levels in mice fed with high-cholesterol diet [16]. In this study, nitrite administration also increased BH4-to-BH2 ratio (evidence for inhibition of BH4 oxidation) in high-cholesterol-fed mice indicating that nitrite is actually antioxidant rather than prooxidant. On the other hand, in a recent study, dietary nitrite ingestion (150 mg per liter of sodium nitrite in drinking water) has been shown to attenuate microvascular inflammation in high-cholesterol-diet-fed mice [16]. Emerging data suggest that inflammation, as evidenced by increased expression of pro-inflammatory cytokines such as C-reactive protein (CRP), associates with endothelial dysfunction [81, 82]. Putting together, these findings suggest the possibility that the ability of nitrite and nitrate to modulate systemic inflammation may contribute at least in part to improvement in endothelium-derived NO bioavailability (i.e., endothelial function).
Vasodilation
Vasodilation refers to the opening of the blood vessels resulting from relaxation of smooth muscle cells within the blood vessel wall. Vasodilation plays a key role in regulation of blood flow and blood pressure, and thus in the control of cardiovascular system. Therefore, abnormal vasodilation would contribute to development and progression of cardiovascular events. Indeed, impaired dilation of the blood vessels (vasculature) has been demonstrated in a number of cardiovascular disease states including hypertension and atherosclerosis [50–56]. In addition, pharmacological agents such as calcium channel blockers and angiotensin-converting enzyme inhibitors have been suggested to achieve cardiovascular protection through improving vasodilation [83, 84]. Substantial data now demonstrate that nitrite and nitrate relax vascular smooth muscle both in vitro and in vivo [7–13, 15, 25, 27, 29], indicating that vasodilation might contribute at least in part to cardiovascular beneficial effects of dietary nitrite and nitrate. For example, infusion of inorganic nitrite (400 nM, at 1 mL/min for 5 min) has been shown to significantly increase fore-arm blood flow in healthy humans at rest and during exercise stress [29]. In another study, inorganic nitrite inhalation (300 mg in 5 mL buffered saline for 20 min) was found to exert potent and selective vasodilation of pulmonary circulation in newborn lambs [25]. Indeed, the vasodilatory properties of pharmacological doses of exogenous nitrite have been known for more than half a century [85, 86]. The mechanisms underlying smooth muscle relaxant effect of nitrite and nitrate remain unclear, but appear to involve their reduction to NO and thus activation of soluble guanylyl cyclase (sGC) in smooth muscle cells (classical NO/sGC/cGMP pathway) or direct activation of sGC in smooth muscle cells (at higher concentrations) [87, 88]. For instance, Laustiola et al. [88] demonstrated that sodium nitrite induces vascular smooth muscle relaxation in rat mesenteric arteries via stimulation of cGMP production, and this effect was augmented in the presence of exogenous guanosine triphosphate (GTP). In addition, in the mid-1970s and early 1980s, supra-physiological concentrations of nitrite were shown by Arnold et al. [89] and Gruetter et al. [90] to activate sGC and vasodilate vascular smooth muscle preparations. An additional potential mechanism for the vasorelaxant effect of nitrite is the release of cyclooxygenase (COX)-derived products from the endothelium. In this regard, Pinder et al. [91] demonstrated that nitrite-induced relaxation in endothelium-intact vessels, but not endothelium-denuded vessels, was inhibited significantly by the nonspecific COX inhibitor indomethacin. On the other hand, substituting endotheliumderived NO with organic nitrates (e.g., nitroglycerin) that release NO to cause vasodilation has been an important strategy in the pharmacotherapy of cardiovascular diseases for more than a century. However, the development of nitrate tolerance and increased formation of peroxynitrite under oxidative stress limits their continuous clinical application. In 1908 and 1930, two studies have investigated the effects of inorganic nitrite on the development of tolerance and compared it with nitroglycerin [92, 93]. These studies demonstrated that repeated oral administration of nitrite (150 mg to 300 mg, 2–3 times a day) similarly lowered systolic blood pressure over a period of 6 days, indicating that nitrite does not induce tolerance. Consistent with these findings, in a recent study, Dejam et al. [15] also reported that the vasodilatory effects of inorganic nitrite are not susceptible to tolerance, which sets this anion apart from the organic nitrates.
Inhibition of platelet aggregation
Platelet adhesion and aggregation are important events in the pathogenesis of cardiovascular diseases. For instance, abnormal platelet activation and adhesion can result in thrombosis and thus vessel occlusion within the coronary artery leading to myocardial infarction. Therefore, inhibiting platelet aggregation is an important strategy in the prevention and treatment of cardiovascular diseases. A growing weight of evidence suggests that nitrite and nitrate reduce platelet aggregation [26, 30, 94], indicating that inhibition of abnormal platelet aggregation may contribute at least in part to cardiovascular benefits of dietary nitrite and nitrate. For instance, a single bolus supplementation with 2 mM (10.1 mg) potassium nitrate has been demonstrated to inhibit platelet aggregation by up to 70% [94]. In another study, acidified sodium nitrite has been demonstrated to significantly inhibit platelet aggregation in a dose-dependent manner in cat platelet-rich plasma [26]. Indeed, in a recent study, modest dietary supplementation with nitrate (beetroot juice) has been shown to inhibit ex vivo platelet aggregation in healthy volunteers [30]. The precise mechanism(s) of this inhibition has still to be clarified. However, it is hypothesized that NO generated from nitrite and nitrate in the acidic stomach will be absorbed systemically, by nitrosating thiol groups which in the acidic stomach will be mainly in the reduced form, and exert an inhibitory effect on platelet aggregation [95]. Nevertheless, the above studies have shown that nitrite and nitrate have inhibitory effects on platelet activation which may actively contribute to cardiovascular health promoting properties.
Cardiovascular protection by nitrite and nitrate: human and animal studies
The usage of inorganic nitrate for the treatment of cardiovascular conditions (angina and digital ischemia) has been harnessed since medieval times as evidenced by a translation of medieval Buddhist manuscripts [10]. However, it is perhaps only in the last decade or so that interest in the cardiovascular effects of inorganic nitrite and nitrate ions has grown enormously. Over the past several years, a wealth of investigations, ranging from clinical and animal model studies to in vitro analyses, have examined the cardiovascular effects of nitrite and nitrate. These studies, mostly from animal models, in general indicate that exogenous nitrite and nitrate ingestion may have beneficial effects on cardiovascular health, most notably in the treatment and prevention of myocardial ischemia and hypertension.
Myocardial ischemia
The health of cardiovascular system depends on structural and functional integrity of the heart and blood vessels. Limited supply of the blood to the heart muscle (myocardium) leads to myocardial ischemia (MI). Reperfusion therapy, the procedure that allows the rapid return of blood flow to the ischemic zone of the myocardium, is the most effective strategy for reducing the size of myocardial infract and improving the clinical outcome in these patients. However, reperfusion after extensive ischemia may lead to further damage to the myocardium, referred to as myocardial ischemia–reperfusion (IR) injury. Therefore, strategies that can limit the extent of infarction during ischemia and reperfusion are of great clinical importance in MI patients. Several studies over the past decade have demonstrated that nitrite and nitrate administration can ameliorate myocardial IR injury [17–19]. Effects of exogenous nitrite against myocardial IR injury have now been studied in a variety of in vitro and in vivo models. For example, Webb et al. [19] investigated the protective effects of exogenous nitrite (10 and 100 µM) against the damaging effects of IR injury using a Langendroff isolated heart model. In this study, Webb et al. [19] demonstrated that perfusion of hearts with nitrite before and during the IR insult reduced infract size by >60% with corresponding improvements in recovery of left ventricular function. In another study, using mice subjected to left coronary artery occlusion (model for myocardial ischemia), Duranski et al. [18] reported that nitrite administered at near physiological blood concentrations (2.4–960 nM) exerted dose-dependent protective effects against cellular necrosis and myocardial IR injury. This study also demonstrated that the nitrite-mediated protection of heart from IR injury was dependent on NO generation and independent of eNOS and hemeoxygenase-1 enzyme activities. Moreover, in a recent study, Gonzalez et al. [17] revealed that nitrite dosing during the last 5 min of a 120-min occlusion period reduced myocardial infarction size from 70 to 36% in a canine model. In another study, the eNOS-deficient mouse has been used to investigate the effect of nitrite supplementation (50 mg/L, in drinking water) on steady-state nitrite and NO metabolite levels and on severity of myocardial IR injury [21]. This study found that nitrite supplementation restored impaired NO homeostasis in eNOS-deficient mice and protected against myocardial IR injury. On the other hand, inorganic nitrate has been used to treat digital ischemia for centuries [10]. However, unlike the case with nitrite, the effect of nitrate on IR injury has not been studied extensively. In a recent study, mice fed with nitrite- and nitrate-deficient diet have been used as animal models to investigate the effects of nitrite and nitrate on the severity of myocardial IR injury [22]. In this study, dietary nitrite and nitrate insufficiency led to exacerbated myocardial IR injury and post-myocardial infarction morbidity rates in mice. Collectively, these studies clearly indicate that exogenous administration of nitrite and nitrate could limit the extent of myocardial injury following ischemia and reperfusion.
Hypertension
Hypertension is a chronic medical condition in which the arterial blood pressure is elevated (systolic >140 mm Hg, diastolic >90 mm Hg). It affects health of millions of people worldwide and remains the most common risk factor for cardiovascular morbidity and mortality. Several studies over the past decade have studied the effects of dietary nitrite and nitrate ingestion on blood pressure in animal models and in humans [27, 28, 30, 96, 97]. The results of these studies suggest that ingestion of dietary nitrite and nitrate can reduce blood pressure in both healthy and hypertensive conditions. For example, in Nω-nitro-l-arginine methyl ester (L-NAME)-induced hypertensive rat model, co-administration of nitrite with L-NAME was found to significantly attenuate blood pressure increases [96]. In another study, oral nitrate administration (at a dose of 0.1 mmol per kg of body weight per day for 3 days) has been shown to lower diastolic and mean arterial blood pressures in young normotensive subjects [27]. Indeed, a picture is now emerging showing that nitrite and nitrate contribute significantly to blood pressure reduction associated with fruit- and vegetable-rich diet intake, since these foods are rich in nitrite and nitrate content. For instance, in a recent study, Sobko et al. [98] demonstrated that consumption of vegetable-rich diet (traditional Japanese diet, 18.8 mg of nitrate per kg body weight per day) for 10 days greatly increased plasma nitrite and nitrate levels and lowered diastolic blood pressure by an average of 4.5 mm Hg compared to Western-style diet in healthy volunteers. In another study, Webb and colleagues [30] demonstrated that ingestion of nitrate load (beetroot juice, 500 mL, ~0.3 mmol nitrate per kg) reduced both systolic and diastolic blood pressures in healthy human volunteers, an effect that correlated with peak increases in plasma nitrite concentrations. Collectively, these studies clearly indicate that exogenous administration of nitrite and nitrate could reduce the blood pressure in both healthy and hypertensive conditions.
Other blood vessel-related conditions
Peripheral vascular disease and cerebrovascular ischemia (i.e., stroke) are often characterized by narrowing or blockade of the blood vessels supplying blood from or to the heart, leading to defective vascular perfusion, and, conversely, to decreased oxygen supply to tissues (i.e., ischemia). Emerging evidence supports the view that nitrite therapy could be of clinical utility in the treatment of these conditions. For instance, using left common femoral artery ligation method, Kumar et al. [99] recently demonstrated that sodium nitrite increases the percentage blood flow in ischemic hind limbs by day 3 of the ligation, which progressively increased by day 7. In another study, Jung et al. [100] investigated whether nitrite therapy limits ischemic injury in brain using adult male rats subjected to middle cerebral artery occlusion. The results of the later study demonstrated that early (30 min before ischemic surgery) intravenous infusion of low doses of sodium nitrite (48 and 480 nmol) protects brain against IR injury. An attractive possibility to explain the beneficial effects of nitrite and nitrate in the treatment of the above conditions is their ability to improve the blood flow, coupled at least in part to their vasodilator and/or antiplatelet effects (discussed in Sects. 4.2, 4.3). In addition, inhibition of superoxide anion production (discussed in Sect. 4.1.3), which promotes cellular damage by forming peroxynitrite via its reaction with NO, may also explain the beneficial effects of nitrite and nitrate in the prevention of cerebrovascular ischemia [100].
Toxicity of nitrite and nitrate
Interestingly, nitrite and nitrate have for long been considered as unwanted carcinogenic residues in the diet because several earlier studies (1970s and 1980s) have demonstrated that nitrite-derived reaction products in stomach may nitrosate dietary amines leading to the formation of N-nitrosamines, a class of carcinogenic substances that could cause tumors by reacting with nucleic acids [46, 101, 102]. However, several lines of experimental and epidemiological research failed to show any causative link between nitrite and nitrate intake and development of cancer [103–106]. For instance, a multiyear dose escalation study in rats and mice found no evidence of carcinogenicity by nitrite and nitrate in either species [103]. In addition, in healthy volunteers, high dietary nitrate (< 3.65 mg/kg body weight or ~250 mg nitrate) intake has been demonstrated to have no significant effect on both protein-bound (1.31 ± 0.14 ng/mg dry protein pre-intake vs. 1.39 ± 0.2 ng/mg dry protein postnitrate intake) and free (5.44 ± 1.19 nM pre-nitrate intake vs. 5.26 ± 1.54 nm post-nitrate intake) plasma 3-nitrotyrosine concentrations [49]. On the other hand, there are also reports showing association between nitrite and nitrate in meats with increased risk of bladder, prostate, and colorectal cancer [107, 108]. However, most associations in these studies were relatively weak (relative risk in most cases < 2) and should not be used for public policy recommendations according to the US National Cancer Institute [109] (Figs. 1, 2).
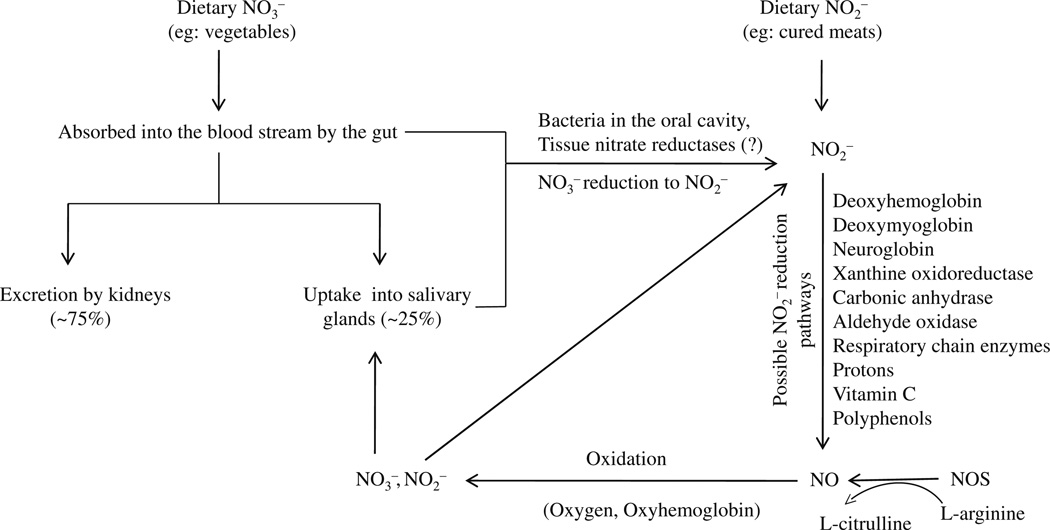
Fig. 1.
Schematic presentation of nitric oxide (NO) generation and metabolism in the body. NO is generated from the classical nitric oxide synthase (NOS)-l-arginine pathway and from the reduction of systemic nitrate () and nitrite (). Systemic originates from the oxidation of NO in biological fluids and from the diet. undergoes reduction to and then to NO. Possible reduction pathways to NO in the body are depicted
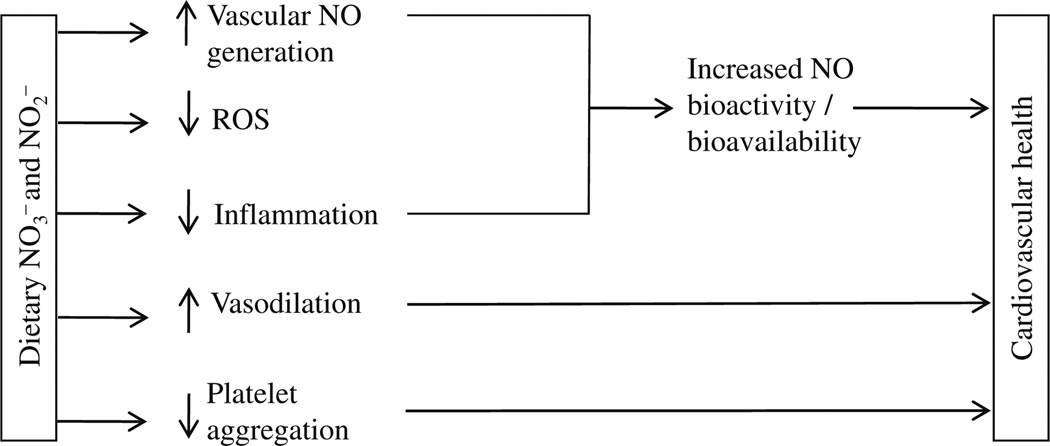
Fig. 2.
Schematic presentation of potential mechanisms by which dietary nitrite and nitrate could modulate cardiovascular health
Conclusion
At present, it seems that dietary nitrite and nitrate have cardiovascular protective effects. As discussed in this review, the effects of nitrite and nitrate to enhance NO bioavailability, to improve endothelial function, to cause vasodilation, and to inhibit platelet aggregation may at least partly mediate their cardiovascular beneficial effects. Considering the fact that reduced bioavailability of endothelium-derived NO (i.e., endothelial dysfunction) is a cause of, or is associated with cardiovascular diseases, a role for nitrite and nitrate in NO biosynthesis and/or in improving NO bioavailability could provide a rationale for using dietary nitrite and nitrate supplementation in the treatment and prevention of cardiovascular diseases. Taking the data presented above together with the failure of recent studies to show significant correlation between nitrite and nitrate exposure and cancer, we suggest that the benefits of dietary nitrite and nitrate will strongly outweigh any potential risks, particularly for cardiovascular disease patients.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at National Institutes of Health (NIH), Bethesda, Maryland, USA. We thank Dr Barbora Piknova (Molecular Medicine Branch, NIDDK) for her valuable inputs.
References
- 1.Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 2.van’t Veer P, Jansen MC, Klerk M, Kok FJ. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr. 2000;3:103–107. doi: 10.1017/s1368980000000136. [DOI] [PubMed] [Google Scholar]
- 3.Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 4.Miller ER, III, Erlinger TP, Appel LJ. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart trials. Curr Atheroscler Rep. 2006;8:460–465. doi: 10.1007/s11883-006-0020-1. [DOI] [PubMed] [Google Scholar]
- 5.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 6.Dauchet L, Amouyel P, Dallongeville J. Fruits, vegetables and coronary heart disease. Nat Rev Cardiol. 2009;6:599–608. doi: 10.1038/nrcardio.2009.131. [DOI] [PubMed] [Google Scholar]
- 7.Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood Cells Mol Dis. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapil V, Webb AJ, Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart. 2010;96:1703–1709. doi: 10.1136/hrt.2009.180372. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg JO, Weitzberg E, Gladwin MT. The nitratenitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 11.Garg HK, Bryan NS. Dietary sources of nitrite as a modulator of ischemia/reperfusion injury. Kidney Int. 2009;75:1140–1144. doi: 10.1038/ki.2009.13. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO, Weitzberg E. NO generation from inorganic nitrate and nitrite: role in physiology, nutrition and therapeutics. Arch Pharm Res. 2009;32:1119–1126. doi: 10.1007/s12272-009-1803-z. [DOI] [PubMed] [Google Scholar]
- 13.Bryan NS. Cardioprotective actions of nitrite therapy and dietary considerations. Front Biosci. 2009;14:4793–4808. doi: 10.2741/3568. [DOI] [PubMed] [Google Scholar]
- 14.Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci U S A. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dejam A, Hunter CJ, Tremonti C, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 16.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–H1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez FM, Shiva S. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J Mol Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–474. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiva S, Gladwin MT. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res Cardiol. 2009;104:113–119. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 24.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter CJ, Dejam A, Blood AB, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 26.Johnson G, III, Tsao PS, Mulloy D, Lefer AM. Cardioprotective effects of acidified sodium nitrite in myocardial ischemia with reperfusion. J Pharmacol Exp Ther. 1990;252:35–41. [PubMed] [Google Scholar]
- 27.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 28.Kapil V, Milsom AB, Okorie M, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 29.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 30.Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiva S, Wang X, Ringwood LA, et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 34.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 35.Godecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Godecke S, Schrader J. Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res. 1998;82:186–194. doi: 10.1161/01.res.82.2.186. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes P, Leone AM, Francis PL, Struthers AD, Moncada S. The l-arginine:nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun. 1995;209:590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 37.Pennington JAT. Dietary exposure models for nitrates and nitrites. Food Control. 1998;9:385–395. [Google Scholar]
- 38.Duncan C, Dougall H, Johnston P, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 39.Dougall HT, Smith L, Duncan C, Benjamin N. The effect of amoxycillin on salivary nitrite concentrations: an important mechanism of adverse reactions? Br J Clin Pharmacol. 1995;39:460–462. doi: 10.1111/j.1365-2125.1995.tb04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Jansson EA, Huang L, Malkey R, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 42.Kelm M, Preik-Steinhoff H, Preik M, Strauer BE. Serum nitrite sensitively reflects endothelial NO formation in human forearm vasculature: evidence for biochemical assessment of the endothelial l-arginine-NO pathway. Cardiovasc Res. 1999;41:765–772. doi: 10.1016/s0008-6363(98)00259-4. [DOI] [PubMed] [Google Scholar]
- 43.Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Radic Biol Med. 2004;36:413–422. doi: 10.1016/j.freeradbiomed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Piknova B, Schechter AN. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol. 2011;704:39–56. doi: 10.1007/978-1-61737-964-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez J, Maloney RE, Rassaf T, Bryan NS, Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc Natl Acad Sci U S A. 2003;100:336–341. doi: 10.1073/pnas.0234600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tannenbaum SR, Weisman M, Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 47.Bartholomew B, Hill MJ. The pharmacology of dietary nitrate and the origin of urinary nitrate. Food Chem Toxicol. 1984;22:789–795. doi: 10.1016/0278-6915(84)90116-9. [DOI] [PubMed] [Google Scholar]
- 48.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576–584. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 50.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 51.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 52.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 53.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Ajay M, Achike FI, Mustafa AM, Mustafa MR. Direct effects of quercetin on impaired reactivity of spontaneously hypertensive rat aortas: comparative study with ascorbic acid. Clin Exp Pharmacol Physiol. 2006;33:345–350. doi: 10.1111/j.1440-1681.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- 55.Hadi HAR, Carr CS, Suwaidi JA. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 56.Hirata Y, Nagata D, Suzuki E, Nishimatsu H, Suzuki J, Nagai R. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J. 2010;51:1–6. doi: 10.1536/ihj.51.1. [DOI] [PubMed] [Google Scholar]
- 57.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 58.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 60.Shiva S, Huang Z, Grubina R, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 61.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 62.Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL. Characterization of the mechanism of cytochrome P450 reductase–cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem. 2006;281:12546–12554. doi: 10.1074/jbc.M511803200. [DOI] [PubMed] [Google Scholar]
- 63.Castello P, David P, McClure T, Crook Z, Poyton R. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signalling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 66.Takahama U, Yamamoto A, Hirota S, Oniki T. Quercetin-dependent reduction of salivary nitrite to nitric oxide under acidic conditions and interaction between quercetin and ascorbic acid during the reduction. J Agric Food Chem. 2003;51:6014–6020. doi: 10.1021/jf021253+. [DOI] [PubMed] [Google Scholar]
- 67.Sibmooh N, Piknova B, Rizzatti F, Schechter AN. Oxidation of iron-nitrosyl-hemoglobin by dehydroascorbic acid releases nitric oxide to form nitrite in human erythrocytes. Biochemistry. 2008;47:2989–2996. doi: 10.1021/bi702158d. [DOI] [PubMed] [Google Scholar]
- 68.van Faassen EE, Bahrami S, Feelisch M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feelisch M, Fernandez BO, Bryan NS, et al. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 71.Gladwin MT, Shelhamer JH, Schechter AN, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blebea J, Bacik B, Strothman G, Myatt L. Decreased nitric oxide production following extremity ischemia and reperfusion. Am J Surg. 1996;172:158–161. doi: 10.1016/S0002-9610(96)00141-9. [DOI] [PubMed] [Google Scholar]
- 73.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 74.Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem Biophys Res Commun. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 75.Vanin A, Bevers L, Slama-Schwok A, van Faassen E. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 78.Graham D, Huynh NN, Hamilton CA, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 79.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 81.Sattar N. Inflammation and endothelial dysfunction: intimate companions in the pathogenesis of vascular disease? Clin Sci (Lond) 2004;106:443–445. doi: 10.1042/CS20040019. [DOI] [PubMed] [Google Scholar]
- 82.Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L, Jialal I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 2009;206:61–68. doi: 10.1016/j.atherosclerosis.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chrysant SG. The role of angiotensin receptor blocker and calcium channel blocker combination therapy in treating hypertension: focus on recent studies. Am J Cardiovasc Drugs. 2010;10:315–320. doi: 10.2165/11538850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 84.Godfraind T. Calcium-channel modulators for cardiovascular disease. Expert Opin Emerg Drugs. 2006;11:49–73. doi: 10.1517/14728214.11.1.49. [DOI] [PubMed] [Google Scholar]
- 85.Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953;108:128–143. [PubMed] [Google Scholar]
- 86.Weiss S, Wilkins RW, Haynes FW. The nature of the collapse induced by sodium nitrite. J Clin Invest. 1937;16:73–84. doi: 10.1172/JCI100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeffers A, Xu X, Huang KT, Cho M, Hogg N, Patel RP, Kim-Shapiro DB. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:130–135. doi: 10.1016/j.cbpb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 88.Laustiola KE, Vuorinen P, Porsti I, Metsa-Ketela T, Manninen V, Vapaatalo H. Exogenous GTP enhances the effects of sodium nitrite on cyclic GMP accumulation, vascular smooth muscle relaxation and platelet aggregation. Pharmacol Toxicol. 1991;68:60–63. doi: 10.1111/j.1600-0773.1991.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 89.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gruetter CA, Kadowitz PJ, Ignarro LJ. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981;59:150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- 91.Pinder AG, Pittaway E, Morris K, James PE. Nitrite directly vasodilates hypoxic vasculature via nitric oxidedependent and -independent pathways. Br J Pharmacol. 2009;157:1523–1530. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matthew E. Vaso-dilators in high blood pressure. Q J Med. 1908;2:261–270. [PMC free article] [PubMed] [Google Scholar]
- 93.Crandall LA, Jr, Leake CD, Loevenhart AS, Muehlberger CW. Acquired tolerance to and cross tolerance between the nitrous and nitric acid esters and sodium nitrite in man. J Pharmacol Exp Ther. 1931;41:103–119. [Google Scholar]
- 94.Richardson G, Hicks SL, O’Byrne S, Frost MT, Moore K, Benjamin N, McKnight GM. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 95.McKnight GM, Duncan CW, Leifert C, Golden MH. Dietary nitrate in man: friend or foe? Br J Nutr. 1999;81:349–358. doi: 10.1017/s000711459900063x. [DOI] [PubMed] [Google Scholar]
- 96.Tsuchiya K, Kanematsu Y, Yoshizumi M, et al. Nitrite is an alternative source of NO in vivo. Am J Physiol Heart Circ Physiol. 2005;288:H2163–H2170. doi: 10.1152/ajpheart.00525.2004. [DOI] [PubMed] [Google Scholar]
- 97.Petersson J, Carlstrom M, Schreiber O, et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–140. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Kumar D, Branch BG, Pattillo CB, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung KH, Chu K, Ko SY, et al. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 101.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 102.Lin JK. Nitrosamines as potential environmental carcinogens in man. Clin Biochem. 1990;23:67–71. doi: 10.1016/0009-9120(90)90489-h. [DOI] [PubMed] [Google Scholar]
- 103.National Toxicology Program Technical Report on the toxicology and carcinogenesis studies of sodium nitrite (CAS No. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies) (2001). NIH publication no 01-3954;7-273. [PubMed]
- 104.Knight TM, Forman D, Pirastu R, et al. Nitrate and nitrite exposure in Italian populations with different gastric cancer rates. Int J Epidemiol. 1990;19:510–515. doi: 10.1093/ije/19.3.510. [DOI] [PubMed] [Google Scholar]
- 105.Eichholzer M, Gutzwiller F. Dietary nitrates, nitrites, and N-nitroso compounds and cancer risk: a review of the epidemiologic evidence. Nutr Rev. 1998;56:95–105. doi: 10.1111/j.1753-4887.1998.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 106.Tang Y, Jiang H, Bryan NS. Nitrite and nitrate: cardiovascular risk-benefit and metabolic effect. Curr Opin Lipidol. 2011;22:11–15. doi: 10.1097/MOL.0b013e328341942c. [DOI] [PubMed] [Google Scholar]
- 107.Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferrucci LM, Sinha R, Ward MH, et al. Meat and components of meat and the risk of bladder cancer in the NIH-AARP diet and health study. Cancer. 2010;116:4345–4353. doi: 10.1002/cncr.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson L. Abortion and possible risk for breast cancer: analysis and inconsistencies. Bethesda: National Cancer Institute; 1994. [Google Scholar]