Abstract
The ability of T cells that have been genetically engineered to express T cell receptors (TCRs) directed against tumor antigens to mediate tumor regression has been demonstrated in several clinical trials. These TCRs have primarily targeted HLA-A*0201 restricted TCRs, as approximately 50% of Caucasians, who represent the predominant population of patients who develop melanomas, expresses this HLA class I allele. These therapies could be extended to additional patients through the use of TCRs that target epitopes that are presented by additional class I alleles that are prevalent in this population such as HLA-C*07 and HLA-A*01, which are expressed by approximately 50% and 30% of patients, respectively. Therefore, two TCRs that recognize an epitope of MAGE-A12 in the context of HLA-C*07, as well as two TCRs that recognize an epitope of MAGE-A3 in the context of HLA-A*01 were isolated from tumor reactive T cell clones and cloned in a recombinant retroviral expression vector. Comparative studies indicated that one of the two MAGE-A3 reactive TCRs and one of the two MAGE-A12 reactive TCRs were superior to the additional TCRs in conferring transduced PBMC with the capacity to recognize a broad array of antigen and MHC positive target cells. These results provide support the use of these TCRs in cancer adoptive immunotherapy trials.
Keywords: T cell receptors, cancer/germline antigens, T cell epitopes, cancer immunotherapy
Introduction
Patients with metastatic melanoma have a poor prognosis, as their five-year survival rate is only 5% 1. Although IL-2 can mediate complete regressions in 5–10% of patients, complete regressions are rare when using the anti-CTLA4 antibody ipilimumab 2 or PLX4032, an inhibitor of mutated BRAF gene products 3. The adoptive transfer of human tumor infiltrating lymphocytes (TIL) that were selected for specific tumor reactivity lead to objective clinical responses in 50–70% of patients with metastatic melanoma, including complete regressions in approximately 10–40% of patients who were pre-treated with lymphodepleting regimens 4. Therapies that can be more readily applied to a wider patient population, such as the use of non-selected TIL, are also currently being evaluated 5,6; however, the clinical efficacy of TIL generated from histologies other than melanoma has not been demonstrated.
Attempts to develop more broadly applicable cancer therapies have focused on genetic modifications that confer autologous peripheral blood mononuclear cells (PBMC) with the ability to recognize antigens specifically expressed on tumor cells. The first clinical cancer trial to evaluate the efficacy of T cells whose target specificity has been re-directed towards tumor cells was carried out using cells that were genetically modified to express a TCR directed against an HLA-A*0201 restricted epitope of the MART-1 antigen 7. This molecule is a member of the melanocyte differentiation antigen (MDA) family of antigens that are expressed on 80–90% of melanoma but not other cancer types, and are limited in their expression in normal tissues to melanocytes. In this trial, complete regressions were observed in two out of 17 melanoma patients who received autologous PBMC that were retrovirally transduced with a MART-1-reactive TCR 7. In a subsequent trial, treatment of patients with autologous PBMC that were transduced either with a second MART-1 reactive TCR or a TCR directed against an HLA-A*0201-restricted epitope of the MDA gp100 lead to objective clinical response rates of 30% and 19%, respectively 8. The significant skin, eye and ear toxicities observed in this trial, which presumably resulted from responses to the normal melanocytes resident in these tissues, may have been a consequence of the relatively high avidities of these TCRs.
A recent report detailed the results of a clinical trial carried out with T cells that were transduced with a TCR that recognized NY-ESO-1, a protein encoded by a member of the cancer/germline family of genes 9. These genes are expressed in approximately one third of a variety of tumor types that include metastatic melanomas, lung, breast, prostate, bladder, and head and neck cancers, as well as 80% of synovial cell sarcoma, but are limited in their expression in normal adult tissues to the testis 10, 11. Objective clinical responses were observed in five out of 11 melanoma patients and four out of six synovial cell sarcoma patients treated with a high-avidity TCR directed against an HLA-A*0201-restricted NY-ESO-1 epitope 9, supporting the efficacy of adoptive immunotherapy for treatment of patients other than those with melanoma.
Chimeric antigen receptors (CARs), molecules in which antibody combining sites have been genetically linked to TCR signaling domains, have also been used to target T cells to cell surface antigens that are over-expressed on tumors or that are expressed in a highly tissue-specific manner on the tumor cell surface 12–14. In contrast to TCRs, CARs are not MHC restricted, broadening the application of cancer adoptive immunotherapies to a wider patient population. Recent reports have demonstrated the efficacy of treatment with CARs that recognize the CD19, a cell surface molecule that is expressed in B cell malignancies and limited in its expression in non-malignant tissues to normal B cells 15,16. In an initial case report, one patient with CLL demonstrated a partial remission that lasted for 32 weeks 17, and subsequent reports have further demonstrated the efficacy of this approach in treating patients with CD19+ tumors 18,19. While these reports are promising, there is a paucity of cell surface antigens with limited or no expression in normal tissues.
This report details the activity of T cells transduced with tumor-reactive TCRs that target an epitope of protein MAGE-A12 recognized in the context of HLA-C*07, as well as TCRs that target an epitope of MAGE-A3 in the context of HLA-A*01. The HLA-C*07 and A*01 class I alleles represent prevalent HLA class I alleles that are expressed by approximately 50% and 30% of Caucasians, respectively 20. Comparative analysis provided evidence for the superiority of two of the TCRs in mediating potent recognition of a broad panel of tumor targets by TCR transduced T cells. The adoptive transfer of autologous PBMC transduced with these TCRs in future clinical trials should facilitate further evaluation of the efficacy of cancer therapies targeting antigens encoded by cancer/germline genes.
Materials and methods
Cloning of TCR genes from T cell clones
The α and β chains encoding functional TCRs were isolated from two MAGE-A12 reactive, HLA-C*07 reactive T cell clones, PHIN LB831–501D/19, referred to below as 502 21 and FM8 22, as well as two MAGE-A3 reactive, HLA-A*01 restricted T cell clones, LAU147 CTL1 or 810/A10, referred to below as A10 23 and NW1000 AVP-1 13–18, referred to below as 13–18. Briefly, oligo-dT was used to reverse transcribe total RNA isolated from the T cell clones into cDNA using the SMART RACE cDNA amplification kit (Clontech, Mountain View, CA). The TCR α and β chains expressed by the T cell clones were identified by carrying out 5’-RACE reactions using a primer 5’-CACTGTTGCTCTTGAA GTCC-3’ that is complementary to the TCR α chain constant region and 5’-CAGGCAGTAT CTGGAGTCATTGAG-3’ that is complementary to the TCR β chain constant region in combination with adaptor primers from the SMART RNA synthesis kit. After sequencing of the 5’-RACE products, full length gene products were amplified using specific primers designed to amplify the appropriate full length TCR α and β chains. The A10 TCR expresses AV12-1/BV24-1, 13–18 expresses AV12-3/BV15, 502 TCR expresses AV13-1/BV25-1, and FM8 expresses AV38-2/BV4-3.
Generation of TCR constructs and retroviral transduction
Constructs were generated to encode the appropriate TCR α chain followed by a furin cleavage site, an SGSG spacer, the P2A “self-cleaving peptide” 24 and the appropriate TCR β chain, as previous studies comparing TCR constructs generated using 2A linkers with retroviral construct containing internal promoters as well as constructs that included an internal ribosomal entry site provided evidence for the superiority of 2A constructs 25. Amplified PCR products were cloned into the MSGV1 retroviral expression vector 26. Two TCRs recognizing the MAGE-A3 restricted HLA-A*01 restricted epitope were designated A10 and 13–18, and two TCRs that recognized the MAGE-A12 HLA-C*07 restricted epitope were designated 502 and FM8. Transient recombinant retroviral supernatants were generated and used to transduce PBMC that were stimulated using the anti-CD3 antibody OKT3 as previously described 25.
Isolation of purified CD8+ and CD4+ T cells
Purified CD8+ and CD4+ T cells were isolated by negative selection using CD8 and CD4 T lymphocyte enrichment kits (Becton/Dickinson, Franklin Lakes, N.J.), followed by positive selection using CD8 and CD4 magnetic beads (Becton/Dickinson). The isolated CD8+ and CD4+ cells were estimated by FACS analysis to contain less than 1% contaminating CD4+ and CD8+ T cells, respectively.
Real time PCR for MAGE A family antigens and retrovirus vector
Expression of the MAGE-A12 gene product was evaluated by Q-PCR using two primers (5’- TCCGTGAGGAGGCAAGGTTC-3’ and 5’- GAGCCTGCGCACCCACCAA-3’) designed to specifically amplify the MAGE-A12 gene product but not other members of the MAGE-A gene family 27 as well as a MAGE-A12 specific probe (5’- AGTGTGGGCAGGAGCTAGT GCTGCTCCG-3’). Antigen expression was determined using plasmid controls as standards for estimating copy numbers and using GAPDH for normalization. Tumor cell lines and fresh tumors expressing greater than 1,000 copies of MAGE-A12 per 106 copies of GAPDH were denoted as positive for MAGE-A12 expression.
The levels of transduction of the four TCRs were evaluated in some experiments using a quantitative PCR assay carried out using genomic DNA with forward (5’- TGCAAGGCATGGAAAATAC ATAACTGA-3’) and reverse (5’- CACAGATATCCTGTTTGGCCCATAT-3’) primers and a probe (5’- TCTCTCTGTTCCTAACCTTG-3’) designed to specifically detect the MSGV1 retroviral LTR but not human endogenous retroviral sequences. Levels of the amplified products were normalized to a positive control sample of PBMC that had been transduced with a TCR directed against the NY-ESO-1:157–165 epitope that was estimated by staining with an NY-ESO-1 tetramer to contain approximately 80% transduced T cells (data not shown).
Chromium release and IFN-γ assays
Lysis of target cells by TCR-transduced T cells was evaluated using standard 51Cr-release assays that were carried out as previously described 28, and the release of soluble IFN-γ from T cells that were incubated with target cells for 18 hours was evaluated as previously described 29.
Results
Cloning and characterization of genes encoding TCRs directed against epitopes of MAGE-A3 and MAGE-A12
Four T cell clones were initially identified that recognized epitopes of the MAGE-A gene family in the context of the dominant class I alleles HLA-A*01 and C*07. Approximately 30% of the melanoma patient population expresses HLA-A*01, and more than 95% of HLA-A*01+ individuals express the HLA-A*0101 sub-type, while more than 50% of melanoma patients express one of the two dominant HLA-C*07 sub-types, C*07:01 and C* 07:02. The expressed TCR α and β chains were isolated from two clones, A10 and 13–18, that recognized residues 168–176 of protein MAGE-A3 (MAGE-A3:168–176) in the context of HLA-A*01. In addition, HLA-C*07 restricted TCRs recognizing a peptide corresponding to residues 170–178 of the MAGE-A12 protein (MAGE-A12:170–178) were isolated from clones 502 and FM8 T, and transcripts encoding the paired α and β chains from the four T cell clones were inserted into the MSGV1 retroviral expression vector.
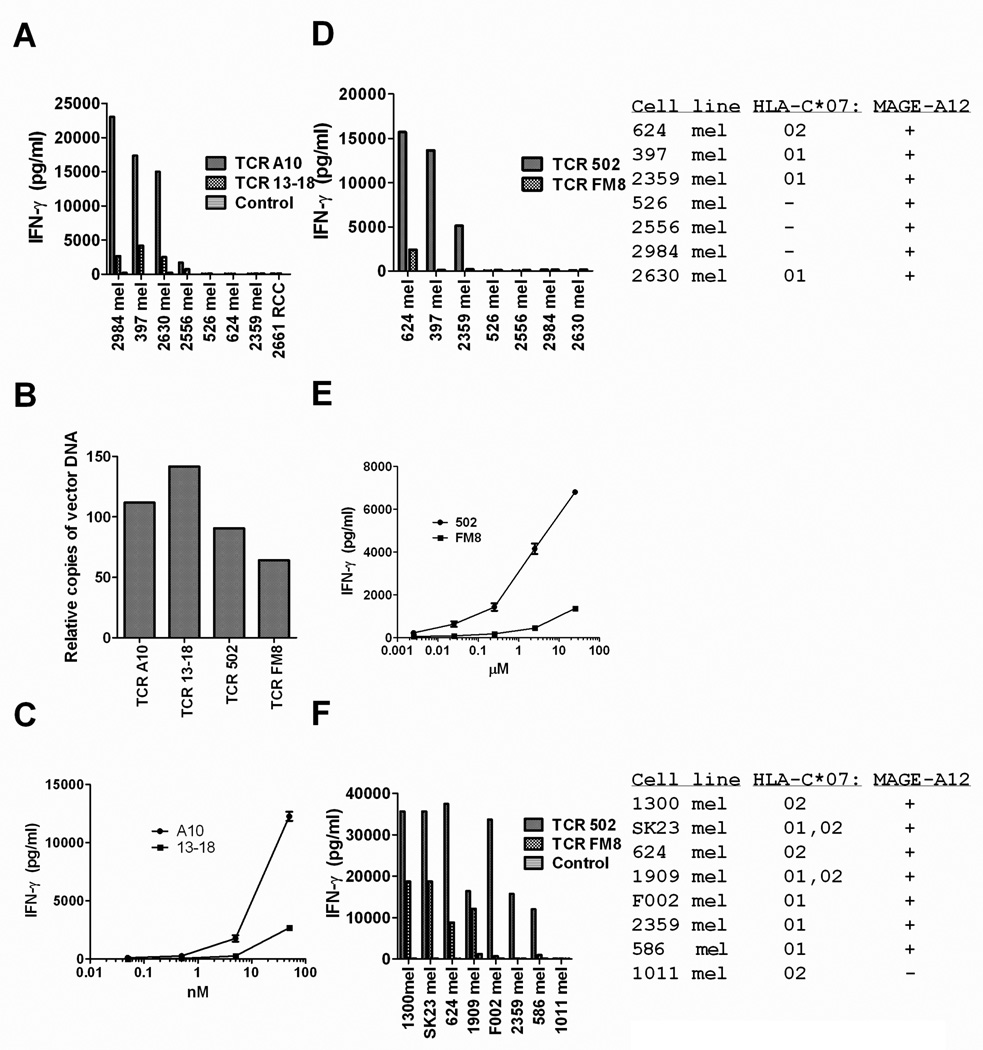
The results of co-culture assays carried out with transduced PBMC demonstrated that TCR A10-transduced T cells generated high levels of IFN-γ in response to the HLA-A*01+/MAGE-A3+ tumor cell lines 397 mel, 2984 mel, and 2556 mel, cytokine levels that were between five and ten times those generated from TCR 13–18 transduced T cells (Fig. 1A). The MAGE-A3+ but HLA-A*01 negative cell lines 562, 624 and 2359 mel, as well as the MAGE-A3 negative but HLA-A*01+ renal cancer cell line 2661 RCC failed to stimulate significant levels of cytokine from either TCR A10 or 13–18 transduced T cells (Fig. 1A). The levels of cell surface expression of the transduced A10 and 13–18 TCRs could not be quantitated due to a lack of detectable staining with a specific MAGE-A3:168–176 HLA-A*01 tetramer (data not shown) as well as a lack of antibodies against the AV12-1/BV24-1 and AV12-3/BV15 TCR α and β chains that comprised the A10 and 13–18 TCRs, respectively. Nevertheless, the differences in activity of T cell transduced with the A10 and 13–18 TCR did not appear to be due to differences in the frequency of transduction with the two TCRs, as they appeared to be equivalent (Fig. 1B). In addition, T cells transduced with the A10 TCR recognized target cells incubated with a minimum concentration of 0.5 nM MAGE-A3 168–176 peptides, a 10-fold lower concentration than required for recognition by cells transduced with the 13–18 TCR (Fig. 1 C), indicating that the A10 TCR possessed a higher functional avidity than the 13–18 TCR.
Figure 1.
Responses of TCR transduced T cells to tumor cell lines. Anti-CD3 stimulated PBMC were transduced with TCR A10 or 13–18, which recognized the MAGE-A3:168–176 HLA-A*01 restricted epitope (A–C), or with TCR 502 or FM8, which recognized the MAGE-A12:170–178 HLA-C*07 restricted epitope (B, D–F). The TCR-transduced T cells, as well as un-transduced control T cells, were evaluated for their ability to release IFN-γ in response to a panel of tumor cell lines in an overnight co-culture assay. Estimates of relative transduction frequencies of PBMC used for the experiments shown in (A) and (D) are shown in (B). Representative results from two of three independent experiments assessing responses of T cells transduced with these TCRs are presented.
Tumor cell lines that expressed either the HLA-C*07:01 or 07:02 sub-types were then evaluated for their ability to be recognized by either TCR 502 or FM8-transduced T cells. The TCR 502 transduced T cells recognized the HLA-C*0702+, MAGE-A12+ tumor 624 mel as well as two HLA-C*07:01+, MAGE-A12+ tumors, 397 and 2359 mel, whereas FM8 transduced T cells recognized the HLA-C*07:02+ tumor cell line 624 mel but failed to recognize 397 and 2359 mel (Fig. 1D). Neither population of transduced T cells recognized 526, 2556, or 2984 mel, MAGE-A3+ melanoma cell lines that lacked expression of HLA-C*07, or 2630 mel, an HLA-C*07:01+ tumor cell line that lacked expression of MAGE-A12 (Fig. 1D). A direct evaluation of cell surface expression of the 502 and FM8 TCRs could not be carried out due to lack of availability of a MAGE-A12:170–178 HLA-C*07 tetramer, as well as the lack of antibodies directed against the AV13-1 and AV38-2 α chains present in the 502 and FM8 TCRs, respectively. As shown above for the A10 and 13–18 TCRs, however, these differences did not appear to be due to differences in transduction frequencies of the two TCRs, which appeared to be similar in cells transduced with either TCR (Fig. 1B). In addition, cells transduced with the 502 TCR recognized target cells incubated with a minimum concentration of 2.5 nM MAGE-A12:170–178, a 100-fold lower concentration than that required for recognition by cells transduced with the FM8 TCR (Fig. 1E), indicating that the 502 TCR possessed a higher functional avidity than the FM8 TCR. Seven out of the seven MAGE-A12+ melanoma cell lines that expressed either HLA-C*0701 or 0702 were recognized by TCR 502 transduced T cells (Fig. 1F). The T cells transduced with TCR FM8 recognized 1300 and 624 mel, two MAGE-A12+ HLA-C*07:02+ melanomas, as well as SK23 and 1909 mel, two MAGE-A12+ melanomas that expressed both HLA-C*07:01 and 07:02, but demonstrated little or no recognition of F002, 2369 or 586 mel, three MAGE-A12+ tumor cell lines that expressed HLA-C*07:01 alone (Fig. 1F). Neither TCR 502 nor FM8-transduced T cells recognized 1011 mel, an HLA-C*0702+ tumor cell line that lacked expression of MAGE-A12 (Fig. 1F). These results are consistent with previous observations indicating that clone 502, which was isolated from a patient who expressed the HLA-C*07:01 sub-type, recognized MAGE-A3+ target cells expressing either HLA-C*07:01 or 07:02 21. In contrast, clone FM8, which was isolated from a patient who expressed the HLA-C*0702 sub-type, failed to recognize MAGE-A3+ targets that expressed the HLA-C*0701 sub-type 22. The levels of IFN-γ released by TCR 502-transduced T cells in response to the HLA-C*07:02+ tumor cell lines 624 mel, 1300 mel and SK23 mel were also consistently higher than those released by FM8 TCR-transduced T cells (Fig. 1D and F), corroborating the results of the peptide titration assay.
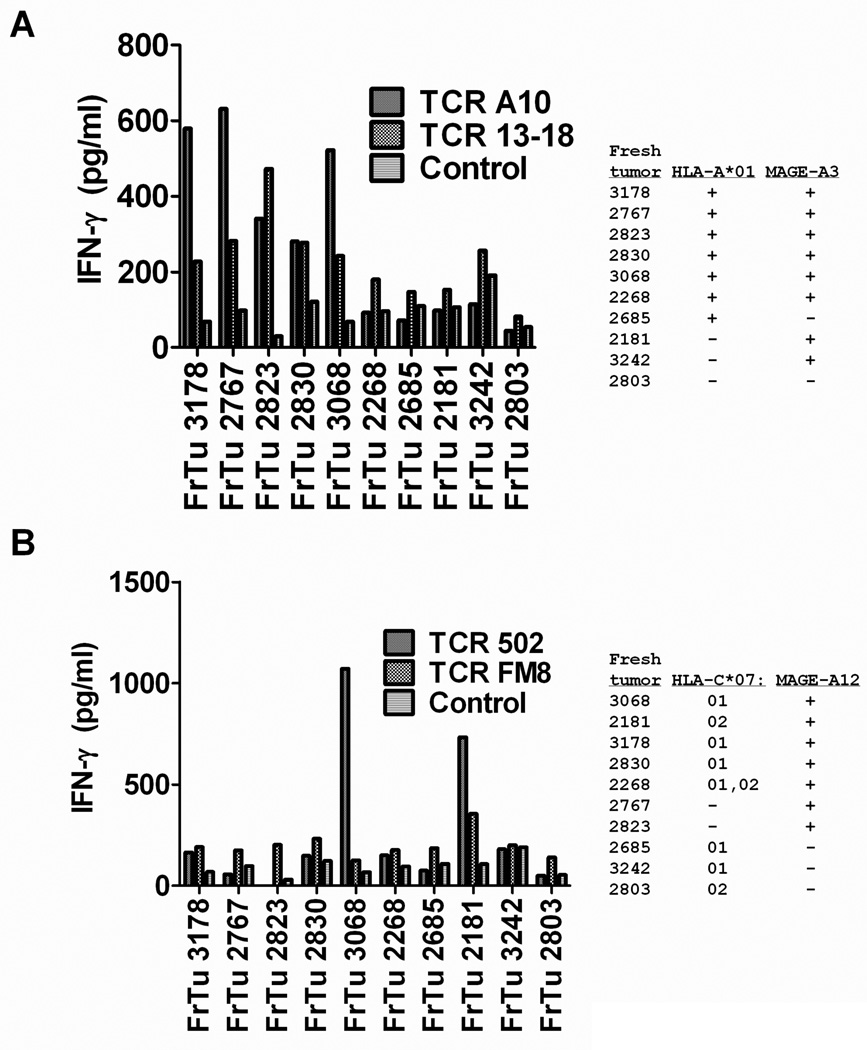
Recognition of fresh un-cultured melanomas by T cells transduced with MAGE-A3 or MAGE-A12 TCRs
The T cells that were transduced with MAGE-A3 or MAGE-A12 reactive TCRs were then evaluated for their responses to enzymatic digests of fresh, un-cultured tumor cells. T cells that were transduced with TCR A10 and 13–18 recognized five of the six MAGE-A3+ and HLA-A*01+ fresh tumors (FrTu), FrTu 3178, 2767, 2823, 2830 and 3068, but did not recognize either FrTu 2685, an HLA-A*01+ fresh tumor that lacked expression of MAGE-A3 or the three MAGE-A3+ fresh tumors, FrTu 2181, 3242 and 2803, that lacked expression of HLA-A*01 (Fig. 2A). The T cells transduced with TCR 502 recognized one of the four MAGE-A12+ fresh tumors that expressed HLA-C*0701, FrTu 3068, and TCR 502 as well as FM8 transduced T cells recognized one of the two MAGE-A12+ tumors that expressed HLA-C*07:02, FrTu 2181 (Fig. 2B). Neither population of TCR transduced T cells recognized the HLA-C*07:01− and 07:02− fresh tumors 2767 or 2823, or the MAGE-A12− tumors 2685, 3242 and 2803 that lacked expression of MAGE-A12. The responses to the appropriate fresh tumors were relatively low, which may in part have been due to the reduced viability of many of the these cells; however, the patterns of tumor recognition indicated that T cells transduced with these TCRs could mediate specific recognition of these targets.
Figure 2.
Responses of TCR transduced T cells to fresh uncultured melanomas. Patient PBMC that were transduced with either TCR A10, 13–18, 502 or FM8 were assayed for their ability to release IFN-γ in response to a panel of fresh un-cultured melanomas that either did or did not express HLA-A*01 and MAGE-A3 (A) or did or did not express HLA-C*07:01 or HLA-C*07:02 and MAGE-A12 (B) in an overnight co-culture assay. In this experiment, the HLA-A*01+/MAGE-A3+ tumor cell line 397 mel stimulated the release of 17,400 and 4,200 pg/ml of IFN-γ from T cells transduced with TCR A10 and 13–18, respectively, while T cells transduced with TCR 502 and FM8 released 15,700 and 2,400 pg/ml of IFN-γ , respectively, in response to the MAGE-A12+/HLA- C*07:02+ tumor cell line 624. Representative results from one of three independent experiments assessing responses of T cells transduced with these TCRs are presented.
Evaluation of responses to additional MAGE-A family members
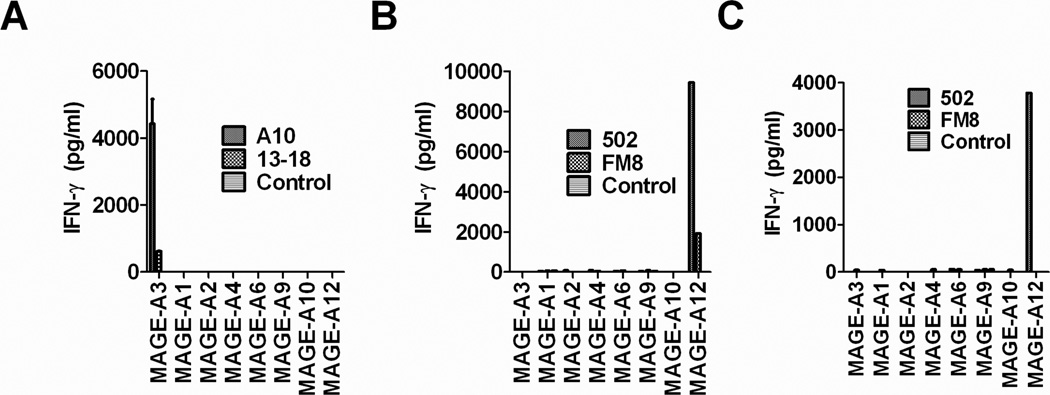
Although members of the MAGE-A family possess very similar amino acid sequences, previous studies have indicated that T cells recognizing the HLA-A*01 restricted MAGE-A3:168–176 30 and MAGE-A12:170–178 epitopes 22,21 failed to recognize similar epitopes expressed by additional MAGE-A family members. In agreement with previous results, T cells transduced with the MAGE-A3-reactive TCR A10 recognize HLA-A1+ target cells transfected with MAGE-A3 but failed to recognize targets transfected with MAGE-A1, A2, A4, A6, A9, A10 or A12 constructs (Fig. 3A) that encoded peptides that differed at between one and three positions from the MAGE-A3:170–178 epitope (Table I). The T cells that were transduced with either TCR 502 or FM8 recognized HLA-C*07:02+ targets transfected with MAGE-A12 but not MAGE-A3, A1, A2, A4,A6, A9, A10 (Fig. 3B), while T cells transduced with TCR 502 but not FM8 recognized HLA-C*07:01+ targets transfected with MAGE-A12 but not the additional MAGE-A family members tested (Fig. 3C).
Figure 3.
Ability of TCR transduced T cells to recognize target cells transfected with MAGE-A gene family members. The monkey kidney cell line COS-7 was transiently transfected with either HLA-A*01 (A), C*07:01 (B) or C*07:02 (C) plus either MAGE-A3, A1, A2, A4, A6, A9, A10 or A12 overnight. The following day T cells transduced with either TCR A10, 13–18 or un-transduced control cells (A) or TCR 502, FM8 or un-transduced control cells (B and C) were added and the release of soluble IFN-γ was evaluated following an overnight co-culture by ELISA.
Table 1.
Comparison of sequences of MAGE-A family members
| MAGE family member | HLA restriction element | Epitope region |
|---|---|---|
| MAGE-A3 | A*01 | EVDPIGHLYIF |
| MAGE-A12 | C*07 | EVVRIGHLYIL |
| MAGE-A1 | - | EADPTGHSYVL |
| MAGE-A2 | - | EVVPISHLYIL |
| MAGE-A4 | - | EVDPTSNTYTL |
| MAGE-A6 | - | EVDPIGHVYIF |
| MAGE-A9 | - | EVDPAGHSYIL |
| MAGE-A10 | - | EVDPTGHSFVL |
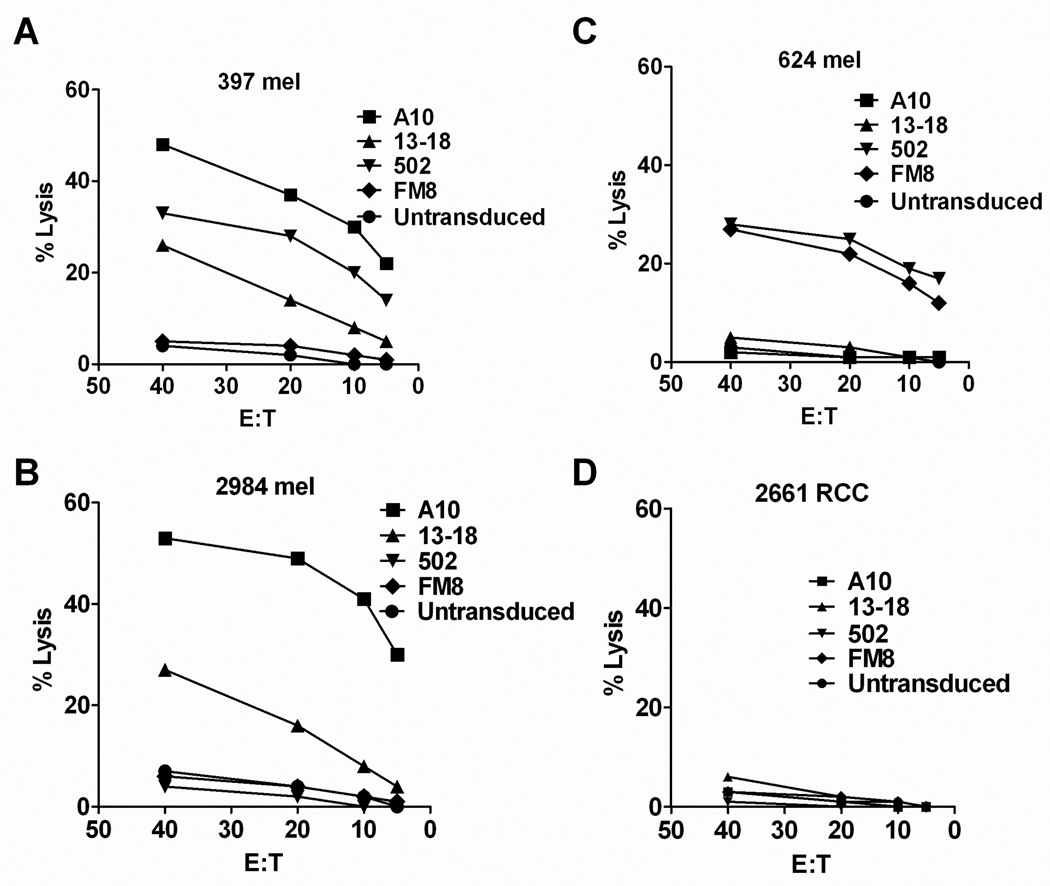
Tumor cell lysis mediated by TCR transduced T cells
The T cells transduced with the MAGE-A3 and A12 reactive TCRs were then evaluated for their ability to lyse tumor targets. The TCR A10 transduced T cells efficiently lysed the MAGE-A3+, HLA-A*01+ tumor targets 397 and 2984 mel, whereas lower levels of lysis were observed with TCR 13–18 transduced T cells (Fig. 4A,B). The TCR A10 and 13–18 transduced T cells failed to lyse either the HLA-A*01 negative MAGE-A3+ target 624 mel or 2661 RCC (Fig. 4C,D), an HLA-A*01+ renal cancer cell line that lacked expression of MAGE-A3. T cells transduced with TCR 502 lysed the MAGE-A12+ tumor expressing either HLA-C*07:01 (397 mel) or C*07:02 (624 mel) (Fig. 4 A,C), whereas FM8 transduced T cells only recognized the MAGE-A3+ HLA-C*07:02+ tumor target 624 mel (Fig. 4C).
Figure 4.
Cytolytic activity of TCR transduced T cells. Following stimulation with anti-CD3 antibody, PMBC from a single donor were transduced with TCRs that recognize the MAGE-3 and MAGE-A12 tumor antigens. Thirteen days after stimulation, transduced T cells were incubated with the indicated tumor targets in a standard 4 hour 51Cr release assay. Representative results from one of two independent experiments are presented.
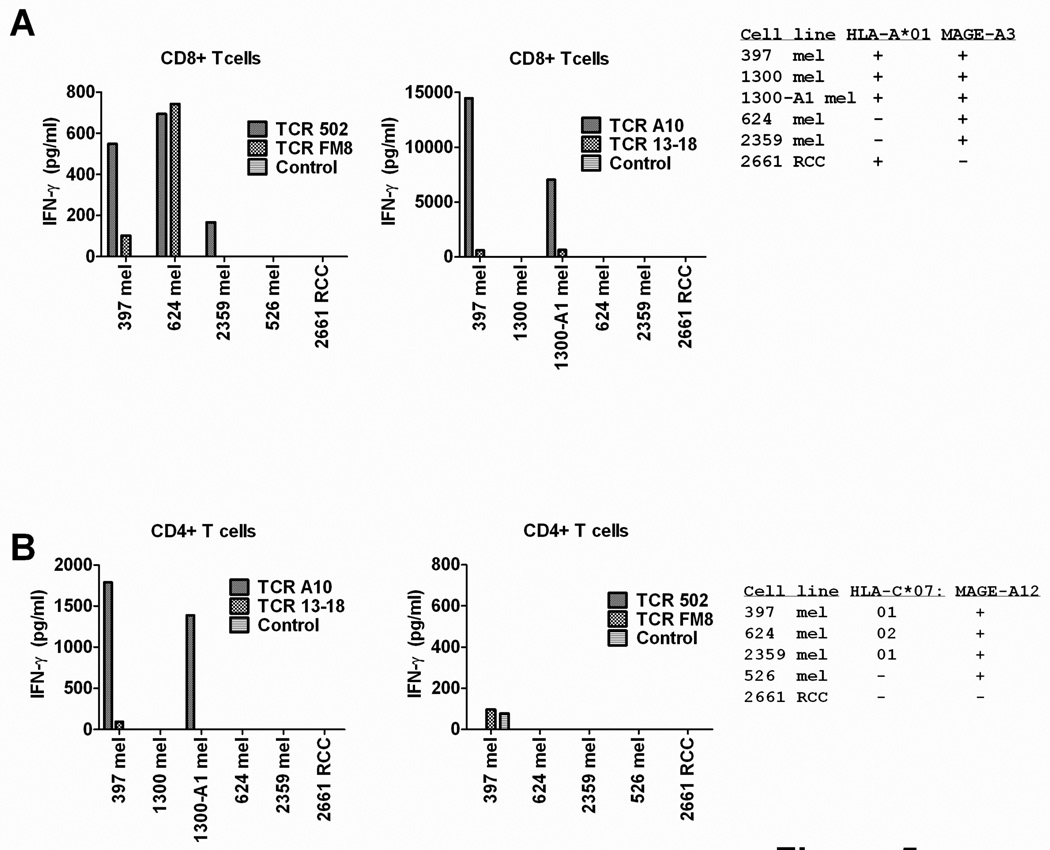
Reactivity of transduced CD8+ and CD4+ T cells
The responses of separated populations of CD8+ and CD4+ T cells transduced with the four TCRs to tumor cell targets was then evaluated, as previous results have demonstrated that high affinity class I restricted TCRs possessed significant activity in CD4+ T cells 31,32,33. Highly purified CD4+ T cells transduced with TCR A10 containing fewer than 1% contaminating CD8+ T cells released low but significant levels of IFN-γ in response to MAGE-A3+ tumor cell line 397 mel as well as the MAGE-A3+ tumor cell line 1300A1 mel that was stably transfected with HLA-A*01, whereas CD4+ T cells transduced with TCR 13–18 failed to recognize antigen positive targets (Fig. 5A). Transduced CD4+ T cells expressing either the 502 or FM8 TCR failed to recognize the MAGE-A12+ and HLA-C*07+ tumor cell lines that were tested (Fig. 5B).
Figure 5.
Response of TCR transduced CD8+ and CD4+ T cells to tumor targets. Populations of purified CD8+ and CD4+ T cells were incubated with tumor cells in an overnight co-culture and the release of IFN-γ was measured by ELISA. Representative results from one of two independent experiments are presented.
DISCUSSION
The development of efficient methods for transducing autologous PBMC with recombinant retroviral and lentiviral constructs that encode tumor-reactive TCRs has facilitated the extension of adoptive T cell immunotherapies to a broad patient population. Objective response rates of 30% and 19% were observed in melanoma patients who received autologous PBMC transduced with TCRs targeting HLA-A*0201 restricted epitopes of the MART-1 and gp100 antigens, respectively 8; however, many of the patients treated in this trial developed severe skin, eye and ear toxicity due to the expression of MART-1 and gp100 on normal melanocytes. In a recent trial targeting an HLA-A*02:01 restricted epitope of NY-ESO-1, melanoma and synovial sarcoma patients who received autologous TCR transduced T cells demonstrated objective response rates of 45% and 67%, respectively 9. Normal tissue toxicity was not evident in patients enrolled in this trial, presumably due to the fact that NY-ESO-1 expression is limited to cells in the normal testis that lack HLA expression.
In the current study, TCRs were characterized that recognize epitopes of MAGE-A3 and MAGE-A12, which have been shown to be expressed in between 30 and 60% of a variety of tumors that include melanoma, bladder, esophageal and prostate cancer. The lack of tetramer staining observed in cells transduced with the MAGE-A3:168–176-reactive TCRs and the unavailability of a MAGE-A12:170–178 tetramer hampered direct evaluation of cell surface expression of the transduced TCR. Tetramer staining, however, is influenced by TCR avidity for the peptide/MHC complex affinity, and thus does not provide a direct readout of cell surface TCR expression 34. Therefore, a direct comparison of the functional avidity of the T cells was carried out be evaluating their ability to recognize tumor targets, as well as cells pulsed with titrated doses of peptide. Results of comparisons between two TCRs that recognized an HLA-A*01-restricted epitope derived from MAGE-A3 but not from any additional MAGE family members revealed that cells transduced with the A10 TCR efficiently lysed and secrete high levels of IFN-γ in response to appropriate tumor target cells, while cells transduced with the 13–18 TCR demonstrated significantly weaker responses to these targets. In addition, cells transduced with the A10 TCR recognized target cells incubated with a 10-fold lower concentration of the MAGE-A3:168–176 peptide than those transduced with the 13–18 TCR. A similar comparison was carried out between two TCRs, 502 and FM8, directed against an HLA-C*07 restricted epitope derived from MAGE-A12 but not from any additional MAGE family members. The T cells that were transduced with TCR 502 lysed and secreted IFN-γ in response to MAGE-A12 targets expressing either HLA-C*07:01 or 07:02, two highly prevalent HLA-C*07 subtypes that are both expressed by approximately 25% of the patient population. In contrast, T cells transduced with TCR FM8 only recognized MAGE-A12+/HLA-C*0702+ target cells. Cells transduced with the 502 TCR also recognized HLA-C*0702+ target cells incubated with a 100-fold lower concentration of the MAGE-A12:170–178 peptide than those transduced with the FM8 TCR, indicating that the 502 TCR possessed a higher functional avidity than the FM8 TCR. The levels of cytokines secreted in response to fresh, un-cultured tumor cells by T cells transduced with the MAGE-A3 and MAGE-A12 reactive TCRs were lower than those produced in response to tumor cell lines but were consistent with the patterns of HLA and antigen expression in these cells. Further evaluation indicated that TCR A10 transduced CD4+ T cells generated low but significant levels of IFN-γ in response to HLA-A1 and MAGE-A3+ tumor cells, indicating that this TCR possesses a relatively high avidity.
Mis-pairing of transduced TCR α or β chains with endogenous receptor chains poses potential risks to patients, as studies carried out in murine model systems have demonstrated that adoptively transferred autologous TCR-transduced T cells can lead to lethal graft versus host disease (GVHD) 35. The observed toxicities were dependent on the particular TCR used for transduction, however, and off-target toxicity has not been observed in the nearly 150 patients who received autologous T cells transduced with TCR directed against a variety of target antigens (summarized in reference 36). In recent clinical TCR trials 4,8, TCR α and β transcripts have also been linked with a porcine teschovirus-derived P2A sequence rather than an internal ribosomal entry site, which enhanced pairing of the transduced α and β chain products 37 and reduced the GVHD observed in the murine TCR study 35. While these observations suggest that caution may be warranted in clinical trials utilizing TCRs that have not yet been administered to patients, it should be weighed against the urgent need to develop effective treatments for patients bearing lethal cancers.
Currently available antibodies cross-react with multiple MAGE-A family members and thus will not allow tumors that specifically express MAGE-A3 or MAGE-A12 to be distinguished from those expressing other family members 38. The MAGE-A2, A3, A6 and A12 genes are tightly clustered in a region on the long arm of the X chromosome and are coordinately expressed in melanomas as well as additional tumor types 39. Genes present in this cluster also appear to be expressed in a higher percentages of tumors than other MAGE-A family members that are present on different regions of the X chromosome 39, and as a result, nearly all of the tumors that stain positively with these antibodies will express MAGE-A3 and MAGE-A12. Quantitative RT-PCR assays of fresh tumor samples using primers and probes that are specific for MAGE-A3 and MAGE-A12 transcripts can also be used to confirm expression of these gene products.
The identification of TCRs that target a variety of antigens encoded by cancer/germline genes will allow the treatment of a significantly larger population of cancer patients with TCR-based therapies. An HLA-A*02:01 restricted TCR that recognizes an epitope shared by MAGE-A3 and MAGE-A9, as well as a similar epitope expressed by MAGE-A12 has recently been characterized 40 and can be used to treat approximately 20% of patients with a variety of cancer types that include melanoma, prostate, lung, bladder and head and neck cancer. Between 15 and 20% of patients with these cancers could potentially be treated with a TCR that target the HLA-C*07 restricted MAGE-A12 epitope, and approximately 10% of patients could potentially be treated with a TCR that recognizing the HLA-A*01 restricted MAGE-A3 epitope, taking into account the frequency of these class I alleles in the patient population as well as the frequency of expression of the MAGE-A3 and MAGE-A12 antigens in tumors. The identification and characterization of potent TCRs that target epitopes derived from antigens encoded by cancer/germline gene in the context of additional HLA alleles such as HLA-A*24, B*35 or B44, class I alleles that are expressed by between 10 and 20% of patients, may lead to the development of a set of reagents that could be used to treat the majority of patients bearing these common malignancies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: All authors have declared there are no conflicts of interest in regards to this work.
REFERENCES
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2010;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 12.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleumer I, Knuth A, Oosterwijk E, et al. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer. 2004;90:985–990. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadler LM, Anderson KC, Marti G, et al. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983;131:244–250. [PubMed] [Google Scholar]
- 16.Uckun FM, Jaszcz W, Ambrus JL, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71:13–29. [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–D919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidecker L, Brasseur F, Probst-Kepper M, Gueguen M, Boon T, Van den Eynde BJ. Cytolytic T lymphocytes raised against a human bladder carcinoma recognize an antigen encoded by gene MAGE-A12. J Immunol. 2000;164:6041–6045. doi: 10.4049/jimmunol.164.11.6041. [DOI] [PubMed] [Google Scholar]
- 22.Panelli MC, Bettinotti MP, Lally K, et al. A tumor-infiltrating lymphocyte from a melanoma metastasis with decreased expression of melanoma differentiation antigens recognizes MAGE-12. J Immunol. 2000;164:4382–4392. doi: 10.4049/jimmunol.164.8.4382. [DOI] [PubMed] [Google Scholar]
- 23.Parmentier N, Stroobant V, Colau D, et al. Production of an antigenic peptide by insulin-degrading enzyme. Nat Immunol. 2010;11:449–454. doi: 10.1038/ni.1862. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly ML, Hughes LE, Luke G, et al. The 'cleavage' activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring '2A-like' sequences. J Gen Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- 25.Wargo JA, Robbins PF, Li Y, et al. Recognition of NY-ESO1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother. 2009;58:383–394. doi: 10.1007/s00262-008-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mecklenburg I, Weckermann D, Zippelius A, et al. A multimarker real-time RT-PCR for MAGE-A gene expression allows sensitive detection and quantification of the minimal systemic tumor load in patients with localized cancer. J Immunol Methods. 2007;323:180–193. doi: 10.1016/j.jim.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. JImmunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 29.Robbins PF, El-Gamil M, Kawakami Y, Stevens E, Yannelli JR, Rosenberg SA. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 30.Gaugler B, Van den Eynde B, van der Bruggen P, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuball J, Schmitz FW, Voss RH, et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–129. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankel TL, Burns WR, Peng PD, et al. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J Immunol. 2010;184:5988–5998. doi: 10.4049/jimmunol.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi EM, Chen JL, Wooldridge L, et al. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J Immunol. 2003;171:5116–5123. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 35.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570. doi: 10.1038/nm.2128. 561p following 570. [DOI] [PubMed] [Google Scholar]
- 36.Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29:550–557. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uckert W, Schumacher TN. TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol Immunother. 2009;58:809–822. doi: 10.1007/s00262-008-0649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimoldi D, Salvi S, Schultz-Thater E, Spagnoli GC, Cerottini JC. Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer. 2000;86:749–751. doi: 10.1002/(sici)1097-0215(20000601)86:5<749::aid-ijc24>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Bredenbeck A, Hollstein VM, Trefzer U, Sterry W, Walden P, Losch FO. Coordinated expression of clustered cancer/testis genes encoded in a large inverted repeat DNA structure. Gene. 2008;415:68–73. doi: 10.1016/j.gene.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Chinnasamy N, Wargo JA, Yu Z, et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2010;186:685–696. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]