Abstract
Second hand tobacco smoke (SHS) is an environmental toxin and an established cause of cardiovascular disease in nonsmokers. Smoke free laws reduce SHS and its downstream cardiovascular disease, but until recently evidence to support smoke free law implementation in low and middle income country settings was limited. In 14 low and middle income nations surveyed by the Global Adult Tobacco Survey active smoking prevalence in adults (≥15 years old) was universally higher in males (range 21.6–60.2%) compared with females (0.5–24.4%), and the highest burden of SHS exposure was in women (strong positive association between male/female active smoking ratio and female SHS exposure prevalence). A systematic review was conducted of MEDLINE-indexed studies of self-reported SHS exposure and cardiovascular harms in low or middle income nations. Eight papers reported the association of SHS with ischemic heart disease and four reported the association of SHS with stroke. For all the studies, and almost all sources of SHS surveyed, a strong positive association between SHS and ischemic heart disease (main relative odds ratio range 1.17–2.36) and SHS and stroke (odds ratio or hazard ratio 1.41–1.49). Prevalence of SHS exposure is high in low and middle income nations, especially among women. Epidemiologic evidence supports the conclusion that SHS harms are the same across low, middle and high income nations. Governments have an obligation to protect citizens from SHS exposure, enforcing smoke-free legislation and providing public education about SHS harms.
Keywords: second hand smoke, environmental tobacco smoke, cardiovascular disease, tobacco control
Introduction
Involuntary exposure to second hand tobacco smoke (SHS) increases the risk of ischemic heart disease (IHD) and lung cancer in non-smoking adults and likely increases stroke risk.[1–2] Reduced myocardial infarction rates after implementation of smoke-free policies in specific jurisdictions support the hypothesis that IHD risk declines rapidly when SHS exposures are reduced.[3] SHS is therefore a cause of cardiovascular disease preventable by public health policy.
Most past studies of the public health burden attributable to tobacco smoking have underestimated tobacco’s contribution to global morbidity and mortality by neglecting SHS.[4–5] Likewise, past tobacco control policy analyses have often underestimated the policy impact of work place and public space smoking bans by projecting reductions in active smoking exposures alone. [6–9]
One in ten cardiovascular disease deaths are attributable to active smoking, and approximately 40% of the global tobacco-related cardiovascular disease burden is borne by low and middle income nations. [4] Furthermore, many of the low and middle income nations have high male smoking rates, but substantially lower female rates so that non-smoking females may be more likely to be exposed in the home. A 2010 study of the burden of disease attributable to SHS in 192 countries estimated that 603,000 deaths, including 379,000 ischemic heart disease deaths and 1.0% of global mortality were due to SHS exposures in 2004.[10] That study relied on incomplete exposure data in adults and SHS effects on ischemic heart disease estimated mostly from high income nation studies, gaps filled in part by recent publication exposure estimates from the Global Adult Tobacco Survey (GATS)[11] and recent epidemiologic studies of the association between SHS and ischemic heart disease and stroke in low and middle income nations.[12–18]
Smoke-free laws are a cornerstone policy arm of global tobacco control policy, incorporated in the World Health Organization Framework Convention for Tobacco Control (FCTC).[19] In this paper, we review adult SHS prevalence data from the GATS and studies of SHS and CVD in low and middle income nations in order to present the case for preventing CVD by implementing smoke free laws worldwide. We present evidence that SHS is an important public health problem in low and middle income nations, where women are often the main “innocent victims” of SHS.
Prevalence of second hand smoke exposures in low and middle income nations: the Global Adult Tobacco Survey
In the United States, in 2010 19.3% of adults ≥18 years were active smokers.[20] When a sensitive biomarker like serum cotinine ≥5 ng/mL was used to define SHS exposure, approximately 37% of U.S. adults were estimated to be exposed to SHS (prevalence was higher in ages <20 years, the poor, and African Americans).[21] However, SHS exposure has declined along with active smoking in high income nations.[21]
Active smoking declines have not been replicated in many low and middle income nations, and until recently SHS prevalence was unknown in these nations. In 2007, the World Health Organization (WHO) sponsored the nationally-representative GATS in 16 low and middle income nations (Bangladesh, Brazil, China, Egypt, India, Indonesia, Mexico, Pakistan, Philippines, Poland, Russian Federation, Thailand, Turkey, Ukraine, Uruguay and Viet Nam). Data from only 14 sites were available for this review (Pakistan and Indonesia data not available).[11] The GATS were multi-stage stratified samples of urban and rural men and women ≥15 years of age in each country. Prevalence of self-reported active smoking and SHS exposures were weighted in order to provide nationally-representative estimates (Table 1, Appendix Table 1). Most countries elicited self-reported public place SHS exposure among persons visiting those places within the prior 30 days.
Table 1.
Active smoking and prevalence of selected SHS exposures (%) in 14 low and middle income nations, the Global Adult Tobacco Survey, 2007–2009.[11] Smoke free work place or public space laws status obtained from the 2011 World Health Organization Tobacco Control Country Profiles report (reporting on laws as of 31 December, 2010).[22]
| Active smoking | Second hand smoke exposures among those visiting these places
|
|||
|---|---|---|---|---|
| Home | Workplace | Restaurants | ||
| Bangladesh | ||||
| smoke free laws | no | no | ||
| males | 44.7 | n/a | 67.8 | 53.4 |
| females | 1.5 | n/a | 30.4 | 2.2 |
|
| ||||
| Brazil | ||||
| smoke free laws | yes† | yes† | ||
| males | 21.6 | 28.9 | 28.5 | * |
| females | 13.1 | 27 | 20.4 | * |
|
| ||||
| China | ||||
| smoke free law§ | no | no | ||
| males | 52.9 | 70.5 | 71.1 | 91.8 |
| females | 2.4 | 63.9 | 53.2 | 83.3 |
|
| ||||
| Egypt | ||||
| smoke free laws | yes | no | ||
| males | 37.7 | 82.5 | 62.4 | 75.6 |
| females | 0.5 | 80.5 | 54 | 62.1 |
|
| ||||
| India | ||||
| smoke free laws | yes | no | ||
| males | 24.3 | 52.2 | 32.2 | 19.2 |
| females | 2.9 | 52.5 | 19.4 | 2.8 |
|
| ||||
| Mexico | ||||
| smoke free laws‡ | yes | yes | ||
| males | 24.8 | 17.2 | 23.3 | 30.9 |
| females | 7.8 | 17.4 | 13.9 | 28.1 |
|
| ||||
| Philippines | ||||
| smoke free laws | no | no | ||
| males | 47.7 | 58.1 | 43.3 | 38.3 |
| females | 9.0 | 50.6 | 28.8 | 28.6 |
|
| ||||
| Poland | ||||
| smoke free laws | no | no | ||
| males | 36.9 | 44.9 | 41.3 | 53.4 |
| females | 24.4 | 43.6 | 24.9 | 54.3 |
|
| ||||
| Russian Federation | ||||
| smoke free laws | no | no | ||
| males | 60.2 | 36.7 | 45.7 | 78.3 |
| females | 21.7 | 33.0 | 25.7 | 78.8 |
|
| ||||
| Thailand | ||||
| smoke free laws | yes | yes | ||
| males | 45.6 | 43.4¶ | 34.9 | * |
| females | 3.1 | 35.1¶ | 18.9 | * |
|
| ||||
| Turkey | ||||
| smoke free laws | yes | yes | ||
| males | 47.9 | 56.1 | 41.5 | 57.7 |
| females | 15.2 | 56.5 | 28.3 | 52.3 |
|
| ||||
| Ukraine | ||||
| smoke free laws | no | no | ||
| males | 50 | 25.4 | 44 | 65.7 |
| females | 11.2 | 21.9 | 22.9 | 62.3 |
|
| ||||
| Uruguay | ||||
| smoke free laws | yes | yes | ||
| males | 30.7 | 32.0¶ | 21.4 | n/a |
| females | 19.8 | 26.7¶ | 11.8 | n/a |
|
| ||||
| Vietnam | ||||
| smoke free laws | yes | no | ||
| males | 47.4 | 77.2 | 68.7 | 90.9 |
| females | 1.4 | 69.2 | 41.4 | 75.2 |
Brazil and Thailand reported public place SHS exposures using total adult population as the denominator (unlike the other countries in this table, who used population visiting the selected public place in the prior 30 days as the denominator). See Appendix Table 3 for the Brazil and Thailand data using total population as the denominator.
Brazil did not have a national smoke-free policy, but does have complete smoke-free legislation in seven jurisdictions that govern almost 40% of the national population: Amazonas, Paraiba, Parana, Rio de Janeiro, Rondonia, Roraima, and Sao Paolo).
Exception for China is that the Hong Kong Administrative Region was comprehensive smoke-free laws.
Mexico City and Tabasco had comprehensive smoke-free laws (covering 4% of Mexico’s population).
Thailand and Uruguay did not use the same monthly threshold for measuring exposure in the home as the other countries. Thailand included exposure that occurs less than monthly, and Uruguay reported exposure that occurs at least weekly.
Active smoking prevalence was higher in males than in females in all the GATS nations, but home SHS exposures were common in males and females alike (Table 1, Appendix Table 1). Though the exposure denominator in Table 1 was smokers and nonsmokers, a similar prevalence was found in nonsmokers (Appendix Table 2). All of the GATS nations with the lowest levels of SHS have the most active tobacco control policies (Brazil, Mexico, Thailand, and Uruguay). All of the nations listed as having smoke-free legislation have expanded the scope of their laws to cover more public spaces since the GATS was conducted.[22]
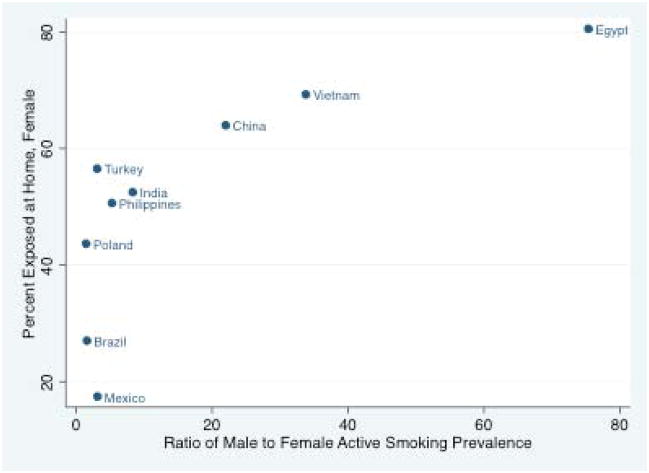
Male active smoking was the driver of female SHS exposures at home or at work, especially in countries with high male and low female active smoking prevalence (high male/female active smoking ratios, Figures 1 and 2). The same strong association between male/female active smoking ratio and SHS among female nonsmokers was observed in the nations reporting SHS exposures in nonsmokers only (Appendix Figure 1). Variability in female SHS among nations with similar male/female ratios--e.g., Mexico and Turkey--could be explained by different male smoking patterns, relatively higher rates of both male and female smoking in some nations (e.g. Turkey), or SHS under- or over-reporting.
Figure 1.
Ratio of male/female active smoking and home second hand smoke exposure in females, in nine nations surveyed for the Global Adult Tobacco Survey, 2007–2009.*
*Reasons for excluding five GATS countries:
--Thailand was excluded because it included smoking that occurs in the home less than monthly, which was a lower threshold than the other countries have
--Uruguay reported smoking that occurs in the home at least weekly, which was a higher threshold than the other countries
--Bangladesh didn’t report home exposure
--Russia and Ukraine were excluded because they appeared to be outliers
Figure 2.
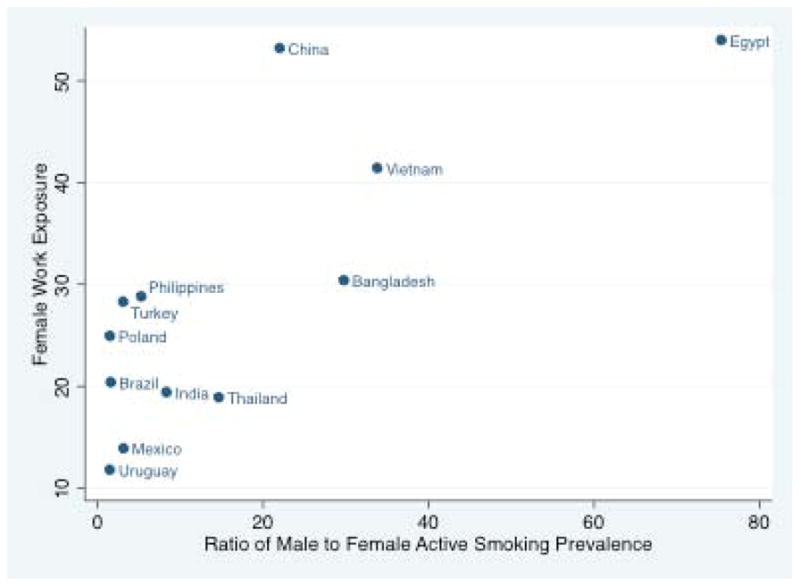
Ratio of male/female active smoking and indoor work place second hand smoke exposure in females, in 12 nations surveyed in the Global Adult Tobacco Survey, 2007–2009.*
*Reasons for excluding two GATS countries:
--Russia and Ukraine appeared to be outliers
How does SHS cause cardiovascular disease?
SHS is also known as environmental tobacco smoke or passive smoking. SHS is a combination of mainstream smoke exhaled by active smokers and sidestream smoke given off by smoldering cigarettes or other smoked tobacco sources. Human subjects studies have documented that SHS exposure leads to both acute and chronic damage to the cardiovascular system. Acutely, SHS causes platelet activation, causes coronary artery endothelial dysfunction[23–24] and impairs heart rate variability[25] similar to the effects of active smoking exposure. Chronically, SHS leads to increased arterial stiffness[26–27] and atherosclerosis progression,[28] perhaps mediated by raised and oxidized low density lipoprotein (LDL) cholesterol[29], lowered high density lipoprotein (HDL) cholesterol,[30] and increased inflammatory response.[31] The pathological effects of SHS occur abruptly at low exposure levels—there is no “safe” level of passive smoke exposure.[1, 23, 32]
SHS causes cardiovascular disease in nonsmokers: evidence from observational studies
Similar to active smoking, health hazards from second hand smoking may occur decades after exposure. Therefore, the epidemiologic evidence comes from case-control studies and observational cohort studies with long term follow up. A problem specific to SHS is misclassification bias: most epidemiologic studies are based on self-reported SHS exposure, which could lead to under- or over-reporting exposure duration or intensity. However, under-reporting has likely been more common, since background involuntary second hand exposure has been common in the past. This leads to misclassifying SHS exposed individuals as “non-exposed”, and biasing hazard estimates toward the null (or “no harm”).
The 2006 United States Surgeon General’s report summarized the evidence of SHS effect on cardiovascular disease by reviewing prior meta-analyses and pooling estimates from original observational studies. Of the 19 studies pooled, all were based on self-reported SHS exposure. The overall relative risk of CHD in nonsmokers exposed to SHS was 1.26 (95% confidence interval 1.19—1.36) compared with unexposed nonsmokers. The relative hazard estimate did not vary substantially according to sex, exposure location, or study type (case control versus cohort). The pooled relative risk estimate for studies that adjusted for other IHD risk factors was higher than the estimate for unadjusted studies. Analysis restricted to studies quantifying exposure intensity (number of actively smoked cigarettes exposed to daily) suggested a dose-response relationship between SHS and IHD risk. Only six studies of second hand smoking and stroke were reviewed by the Surgeon General’s report. Exposure measurement, adjustment for confounders, and definitions of stroke varied among the studies. Two studies showed a statistically significant association between second hand smoking and stroke; other studies produced estimates with 95% confidence intervals that crossed the null (i.e., included the possibility of no harm). The 2006 report concluded that causal evidence for the link between SHS and IHD was “sufficient” but evidence of a causal link between SHS and stroke was “suggestive, but not sufficient”. A number of stroke and SHS epidemiology studies have been completed since the Surgeon General’s report review. Oono et al. reviewed 20 stroke studies in 2011 and found a pooled relative risk of 1.25 (1.12—1.38) and evidence of a dose-response relationship for the association of stroke with SHS.[33]
How might misclassification bias have impacted the IHD relative risk estimate from the Surgeon General’s report and other estimates based on self reported SHS exposure? One solution to the problem of misclassification resulting from self-reporting of SHS exposure has been to measure exposure using serum or salivary cotinine levels obtained from nonsmokers. Cotinine is the principal metabolite of nicotine and is a sensitive and specific biomarker of SHS exposure in nonsmokers.[34] Whincup et al. studied the association of SHS exposure, defined as a baseline serum cotinine of 0.8–14.0 ng/ml categorized as exposed and ≤7 ng/ml categorized as unexposed.[35] Defined as having elevated baseline cotinine, SHS had a relative risk of IHD of 3.73 (1.32—10.58) during the first 4 years of follow up, a relative risk of 1.95 (1.09—3.48) in years five to nine, and the risk continued to decline toward the null after 20 years of follow up (because baseline exposure measurement represented future exposure less and less over time or because of a secular trend toward less active smoking in Britain). The effect remained significant after adjustment for other IHD risk factors and showed a (cotinine) dose-response relationship. No significant association was found between stroke and SHS. The Whincup study demonstrates an IHD relative risk effect size related to SHS that is substantially higher when misclassification bias is eliminated.
Little is known about cardiovascular disease risk associated with SHS in low and middle income countries (for example, the U.S. Surgeon General’s 2006 meta-analysis included one IHD study from Argentina[36] and another from China[37]), and whether the association differs in these populations. Though biological effects of SHS are likely the same in all people, quality of building ventilation and use of air conditioning may differ. Also, levels of indoor air pollution from solid fuel combustion (e.g. indoor cooking or heating fires) are high in some low and middle income nations. In order to evaluate epidemiologic evidence related to SHS and cardiovascular disease in low and middle income nations, we conducted a systematic review of epidemiologic studies of SHS and risk for cardiovascular disease using MEDLINE (via PubMed), and restricting the search to low and middle income country studies that gathered SHS exposure information and reported SHS association with a cardiovascular outcome ascertained using standard diagnostic criteria (Appendix Methods). Across all ten included studies, self-reported spousal or total household home SHS exposure was most commonly reported and were most comparable to past reviews,[1–2] so those exposure types were compared (Table 2). We found no low or middle income studies that used cotinine or other biomarkers to assess SHS exposure.
Table 2.
Epidemiologic studies of the association of second hand smoke exposure with cardiovascular disease in low or middle income nations.
| CVD type and study | Nation | Study type | Observation years
|
Population
|
Exposure | Outcome | Effect measure | Effect size
|
controlled for CVD risk factors | dose- response pattern | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| start | end | cohort or cases (if case control) | controls | main | lower | upper | ||||||||
| IHD | ||||||||||||||
|
| ||||||||||||||
| He et al. 1989[46] | China | case control | unreported | 34 female non-smokers, hospital based | 68 female non-smokers: 34 hospital based, 34 population based | Spouse smoked | nonfatal IHD | odds ratio | 1.50 | 1.28 | 1.77 | yes* | yes | |
|
| ||||||||||||||
| He et al. 1994[37] | China | case control | 1989 | 1992 | 59 female never smokers diagnosed with IHD, employed full-time, hospital based | 126 female never smokers, employed full-time, hospital and community based | Spousal or workplace exposure | nonfatal IHD | odds ratio | 2.36 | 1.01 | 5.55 | yes† | yes |
|
| ||||||||||||||
| McGhee et al. 2005[12] | China (Hong Kong) | case control | 1988 | 1998 | 584 IHD deaths in persons age ≥60 years from death registries | 763 living contemporaries age ≥60 years giving information at the same time | Smoker in the household | IHD death | odds ratio | 1.35 | 1.03 | 1.76 | no | yes |
|
| ||||||||||||||
| He et al. 2008[13] | China | case control | 2001 | 2002 | 431 female never smokers aged 60 or older, population based | 778 female never smokers aged 60 or older, population based | Home or workplace | nonfatal IHD | odds ratio | 1.69 | 1.31 | 2.18 | yes‡ | yes |
|
| ||||||||||||||
| Ding et al. 2009[14] | China (Hong Kong) | case control | 2004 | 2007 | 314 female never smokers with IHD, hospital based | 319 female never smokers, hospital based | Household | nonfatal IHD | odds ratio | 1.52 | 1.01 | 2.27 | yes¶ | yes |
|
| ||||||||||||||
| Ciruzzi et al. 1998[36] | Argentina | case control | 1991 | 1994 | 336 never-smokers with first episode of acute MI, hospital based, median age 66 years | 446 never-smokers, hospital base, median age 65 years | Spouse and children smoked | nonfatal MI | odds ratio | 1.68 | 1.20 | 2.37 | yes§ | yes |
|
| ||||||||||||||
| Sulo et al. 2008[15] | Albania | case control | 2003 | 2006 | 169 married never smokers with acute coronary syndrome, aged 35–74 years, hospital based | 323 married never smokers, aged 35–74, population based | Spouse smoked | nonfatal acute coronary syndrome | odds ratio | 1.60 | 0.95 | 2.70 | yes** | no |
|
| ||||||||||||||
| Rossi et al. 2010[16] | Costa Rica | case control | 1994 | 2004 | 2,094 cases with first acute MI, 1543 men and 551 women, hospital based | 2,094 controls matched by age, sex, area of residence, population-based | Smoker in the household | acute non-fatal MI | odds ratio | 1.17 | 1.00 | 1.37 | no | no |
|
| ||||||||||||||
| Stroke | ||||||||||||||
|
| ||||||||||||||
| McGhee et al. 2005[12] | Hong Kong | case control | 1988 | 1998 | 597 stroke deaths in persons age ≥60 yearsfrom death registries | 763 living contemporaries age ≥60 years giving information at the same time | Smoker in the household | stroke death | odds ratio | 1.49 | 1.15 | 1.94 | no | yes |
|
| ||||||||||||||
| Zhang et al. 2005[17] | China | case control | 1997 | 2000 | 526 female married never smokers reporting stroke history, aged 40–70 years | 59,851 female married never smokers without stroke history, aged 40–70 years | Spouse smoked | nonfatal stroke | odds ratio | 1.41 | 1.16 | 1.72 | yes†† | yes |
|
| ||||||||||||||
| Wen et al. 2006[18] | China | cohort | 1997 | 2004 | 65,180 female never smokers aged 40–70 years at baseline, population-based | Spouse smoking at baseline | stroke death | hazard ratio | 1.52 | 1.08 | 2.15 | no | no | |
|
| ||||||||||||||
| He et al. 2008[13] | China | case control | 2001 | 2002 | 172 female never smokers aged 60 or older, population based | 1,037 female never smokers aged 60 or older, population based | Home or workplace | nonfatal stroke | odds ratio | 1.65 | 1.17 | 2.32 | yes‡ | yes |
Alcohol consumption, exercise, personal and family history of CHD, hypertension, hyperlipidemia
Age, hypertension, personality type, total cholesterol, high-density lipoprotein cholesterol
Age, marital status, education, exercise, alcohol consumption, BMI, systolic blood pressure, total cholesterol, triglyceride levels, history of hypertension and diabetes, family history of CHD/stroke
Age, education, hypertension, diabetes, hypercholesterolaemia, gout, history of stroke, family history of IHD, physical inactivity, alcohol intake, estrogen use
Age, cholesterolemia, diabetes, hypertension, BMI, education, social status, exercise, family history of MI
Age, sex, exercise, hypertension, diabetes, family history of CHD, BMI, waist-to-hip ratio
Age, education, occupation, family income, alcohol consumption, exercise, BMI, menopausal status, hormone therapy, oral contraceptive use, history of hypertension, diabetes, use of antihypertensive medication or aspirin
All eight of the studies from low or middle income nations reporting on IHD were case-control, and for most the outcome was non-fatal IHD (since cases were expected to report on SHS exposure history). All main odds ratios were above 1.00, only two estimates included 1.00 in the lower 95% confidence interval bound,[15–16] and no lower bound exceeded the upper bound of the Surgeon General report’s meta-analysis 95% confidence interval (upper limit of relative risk 1.36). The InterHEART study was an international case-control study of myocardial infarction in which approximately 80% of participants were from low and middle income countries. The increased odds of myocardial infarction associated with spousal active smoking in InterHEART overall was 1.28 (odds ratio; 95% confidence interval 1.12—1.47).[38]
Four studies reporting on SHS and stroke in low or middle income nations have been published since the Surgeon General’s 2006 report. One cohort—the Shanghai Women’s Health Study—provided data for both a case-control study[17] and a cohort study[18](Table 2). A strong positive association between household SHS exposure and total stroke was found in all studies. Of note, follow up of the Shanghai Women’s Health Study cohort found no significant association between workplace exposure and stroke [hazard ratio 0.73 (0.44—1.02)],[18] though this estimate may have been affected by insufficient follow up time or exposure misclassification bias.
Smoke free laws reduce IHD rates in nonsmokers: evidence from policy “natural experiments”
Without a policy intervention, SHS exposures may track downward if active smoking declines and societal attitudes toward indoor smoking change, but these mechanisms for an SHS decline are slow and by no means guaranteed. Smoke free laws reduce SHS exposure quickly. Smoke free laws also reduce active smoking prevalence approximately 15% among active smokers and reduce the number of cigarettes smoked daily.[39–40] After a smoke-free workplace legislation in Finland (1995), California (1998), New York (2002), the Republic of Ireland (2004), Scotland (2004), France (2007–2008), and smaller jurisdictions in Colorado, Montana, Canada and Italy, SHS exposures were reduced dramatically within five year periods in workers surveyed before and after the bans.[3, 41] A carbon dioxide measurement study demonstrated the effectiveness of a restaurant, bar and night club smoking ban in reducing SHS levels in Sao Paulo, Brazil.[42] Because of the difficulty of accurately measuring SHS exposure, and the ethical conflict involved with randomizing participants to SHS exposure, the population health benefits of smoke free policies have been estimated primarily based on the “natural experiments” comparing disease rates in the population before and after smoke free policy implementation. Because SHS affects acute myocardial infarction risk immediately, myocardial infarction rates have been the outcome measure of most smoke free policy natural experiments. A dramatic example occurred in the relatively isolated city of Helena, Montana in which acute MI hospitalization rates dropped by approximately 40% during six months when a public smoking ban was in effect, and returned to prior levels when the law was repealed.[43] Lightwood and Glantz pooled and time-standardized 12 before-and-after studies of 100% smoke-free public places laws and found a 0.83 (0.80—0.87) relative MI rate after the bans, and evidence that the benefit appeared to grow for up to 36 months.[3]
Discussion
SHS increases risk for CVD and non-CVD deaths and disability, and may be responsible for 1.0% of global mortality, with at least two-thirds of these deaths due to CVD.[10] The WHO GATS and Global Youth Tobacco Survey (GYTS)[44] showed that SHS is common in homes, workplaces, and most public places in a geographically and culturally diverse set of low and middle income nations. Active smoking is much more frequent in men than in women in many low and middle income nations, and the result is that most SHS exposures occur in female nonsmokers in these countries. We found that SHS-associated cardiovascular disease risks in the few available low and middle income country epidemiologic studies are similar to those observed in studies from higher income nations. Though not reviewed here, other evidence supports associations between SHS and increased risk for lung cancer and a number of other non-cardiovascular diseases.[1] Smoke free laws have been highly effective public health interventions in high income nations, and should also be effective in low and middle income nations if enforced effectively. In making the business and policy case for implementation of FCTC smoke free policies, the health and economic development gains from SHS reductions must be weighed along with effects on active smoking.
The GATS suggests SHS exposure is ubiquitous in low and middle income nations, but inconsistencies among the countries beg several questions that are important to consider in future surveillance that is important for measuring the effectiveness of smoke free policies. How accurate was self-reported SHS in the GATS surveys? Exposure self-report questionnaires developed in the high income West may not be appropriately tailored to specific tobacco use patterns and other contributing social behaviors in LMIC. Also, self-report may be biased by low levels of SHS health risks awareness. In the Chinese GATS, only 38.7% of respondents were aware that SHS causes IHD and 27.2% were aware SHS causes stroke.[45] Given these multiple uncertainties surrounding the GATS SHS estimates, we advocate that future surveillance surveys and cohort studies employ serum, urine, or salivary cotinine as a more sensitive and accurate measure of SHS exposures.
Study questionnaire questions and biomarkers related to other sources of indoor air pollution (e.g. indoor solid fuel combustion fumes) should be added to future studies. The low and middle income nation studies reviewed here mostly sampled urban populations, so more studies of the interaction between SHS and indoor cooking fire fumes are needed in rural areas, where solid fuel combustion is more common.
Smoke free laws are a cornerstone of the WHO Framework Convention on Tobacco Control policy agenda, and have been shown to be very effective, both in limited (e.g. workplace) scope[41] or as 100% public space bans.[3] Argentina, Brazil, Mexico, Thailand, Turkey and Uruguay are among the low and middle income nations currently pursuing aggressive smoke free legislation.[22] Smoke free laws reduce tobacco harms from SHS in those unintentionally exposed—in low and middle income nations predominantly innocent women and children. Smoke free policy is not individual primary prevention as an intervention on one patient’s risk factor, but more akin to an infectious disease public health intervention: as in treatment for the cure of tuberculosis, smoke free laws are likely to improve the health of the smoker and those around him “contaminated” with SHS. Smoke-free laws are an expression of a government’s determination to protect its people’s health and change society’s expectations so that public smoking is viewed as socially unacceptable.
Conclusions
Most past estimates of tobacco’s impact on health underestimated its burden due to lack of consideration of the SHS harms to the public’s health. Governments have an obligation to protect citizens from preventable environmental toxins like SHS. Governments should not wait for perfect data from low and middle income nations, because the evidence is sufficient to justify implementation of smoke free laws now. Along with smoke free laws, public education about SHS harms is needed, or there may be resistance to the laws from smokers and nonsmokers alike.
Abbreviations
- SHS
second hand smoke
- IHD
ischemic heart disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 2.California Environmental Protection Agency, Office of Environmental Hazard Assessment. Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant. Part B: Health Effects. Jun 24, 2005. [Google Scholar]
- 3.Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation. 2009;120:1373–1379. doi: 10.1161/CIRCULATIONAHA.109.870691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–497. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 5.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 7.Zaloshnja E, Ross H, Levy DT. The impact of tobacco control policies in Albania. Tob Control. 2010;19:463–468. doi: 10.1136/tc.2009.034652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DT, Benjakul S, Ross H, Ritthiphakdee B. The role of tobacco control policies in reducing smoking and deaths in a middle income nation: results from the Thailand SimSmoke simulation model. Tob Control. 2008;17:53–59. doi: 10.1136/tc.2007.022319. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante D, Levy D, Peruga A, Compton C, Romano E. The role of public policies in reducing smoking prevalence and deaths: the Argentina Tobacco Policy Simulation Model. Rev Panam Salud Publica. 2007;21:37–49. doi: 10.1590/s1020-49892007000100005. [DOI] [PubMed] [Google Scholar]
- 10.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Global Adult Tobacco Survey (GATS) [ http://www.who.int/tobacco/surveillance/survey/gats/en/]
- 12.McGhee SM, Ho SY, Schooling M, Ho LM, Thomas GN, Hedley AJ, Mak KH, Peto R, Lam TH. BMJ. Vol. 330. England: 2005. Mortality associated with passive smoking in Hong Kong; pp. 287–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y, Lam TH, Jiang B, Wang J, Sai X, Fan L, Li X, Qin Y, Hu FB. Circulation. Vol. 118. United States: 2008. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked; pp. 1535–1540. [DOI] [PubMed] [Google Scholar]
- 14.Ding D, Wing-Hong Fung J, Zhang Q, Wai-Kwok Yip G, Chan CK, Yu CM. Tob Control. Vol. 18. England: 2009. Effect of household passive smoking exposure on the risk of ischaemic heart disease in never-smoke female patients in Hong Kong; pp. 354–357. [DOI] [PubMed] [Google Scholar]
- 15.Sulo G, Burazeri G, Dehghan A, Kark JD. Partner’s smoking status and acute coronary syndrome: population-based case-control study in Tirana, Albania. Croat Med J. 2008;49:751–756. doi: 10.3325/cmj.2008.49.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi M, Negri E, La Vecchia C, Campos H. Eur J Cardiovasc Prev Rehabil. Vol. 18. England: 2011. Smoking habits and the risk of non-fatal acute myocardial infarction in Costa Rica; pp. 467–474. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Shu XO, Yang G, Li HL, Xiang YB, Gao YT, Li Q, Zheng W. Am J Epidemiol. Vol. 161. United States: 2005. Association of passive smoking by husbands with prevalence of stroke among Chinese women nonsmokers; pp. 213–218. [DOI] [PubMed] [Google Scholar]
- 18.Wen W, Shu XO, Gao YT, Yang G, Li Q, Li H, Zheng W. BMJ. Vol. 333. England: 2006. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study; p. 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization Framework Convention on Tobacco Control. [ http://www.who.int/fctc/en/]
- 20.United States Centers for Disease Control and Prevention. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥18 Years --- United States, 2005–2010. Morbidity and Mortality Weekly Report. 2011 Sep 9;60(35):1207–1212. [ http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6035a5.htm?s_cid=mm6035a5_w ] [PubMed] [Google Scholar]
- 21.United States Centers for Disease Control and Prevention. Nonsmokers’ Exposure to Secondhand Smoke --- United States, 1999–2008. Morbidity and Mortality Weekly Report. 2010 Sep 10;59(35):1141–1146. [ http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5935a4.htm] [PubMed] [Google Scholar]
- 22.World Health Organization Tobacco Free Initiative. Tobacco Control Country Profiles. 2011 [ http://www.who.int/tobacco/surveillance/policy/country_profile/en/index.html]
- 23.Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991;83:1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T, Yoshiyama M, Takeuchi K, Yoshikawa J. Acute effects of passive smoking on the coronary circulation in healthy young adults. JAMA. 2001;286:436–441. doi: 10.1001/jama.286.4.436. [DOI] [PubMed] [Google Scholar]
- 25.Pope CA, 3rd, Eatough DJ, Gold DR, Pang Y, Nielsen KR, Nath P, Verrier RL, Kanner RE. Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Perspect. 2001;109:711–716. doi: 10.1289/ehp.01109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanadis C, Vlachopoulos C, Tsiamis E, Diamantopoulos L, Toutouzas K, Giatrakos N, Vaina S, Tsekoura D, Toutouzas P. Unfavorable effects of passive smoking on aortic function in men. Ann Intern Med. 1998;128:426–434. doi: 10.7326/0003-4819-128-6-199803150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Mack WJ, Islam T, Lee Z, Selzer RH, Hodis HN. Environmental tobacco smoke and carotid arterial stiffness. Prev Med. 2003;37:148–154. doi: 10.1016/s0091-7435(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 28.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 29.Valkonen M, Kuusi T. Passive smoking induces atherogenic changes in low-density lipoprotein. Circulation. 1998;97:2012–2016. doi: 10.1161/01.cir.97.20.2012. [DOI] [PubMed] [Google Scholar]
- 30.Moffatt RJ, Chelland SA, Pecott DL, Stamford BA. Acute exposure to environmental tobacco smoke reduces HDL-C and HDL2-C. Prev Med. 2004;38:637–641. doi: 10.1016/j.ypmed.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Iso H, Shimamoto T, Sato S, Koike K, Iida M, Komachi Y. Passive smoking and plasma fibrinogen concentrations. Am J Epidemiol. 1996;144:1151–1154. doi: 10.1093/oxfordjournals.aje.a008893. [DOI] [PubMed] [Google Scholar]
- 32.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315:973–980. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf) 2011 doi: 10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 34.Jarvis M, Tunstall-Pedoe H, Feyerabend C, Vesey C, Salloojee Y. Biochemical markers of smoke absorption and self reported exposure to passive smoking. J Epidemiol Community Health. 1984;38:335–339. doi: 10.1136/jech.38.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whincup PH, Gilg JA, Emberson JR, Jarvis MJ, Feyerabend C, Bryant A, Walker M, Cook DG. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ. 2004;329:200–205. doi: 10.1136/bmj.38146.427188.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciruzzi M, Pramparo P, Esteban O, Rozlosnik J, Tartaglione J, Abecasis B, Cesar J, De Rosa J, Paterno C, Schargrodsky H. J Am Coll Cardiol. Vol. 31. United States: 1998. Case-control study of passive smoking at home and risk of acute myocardial infarction. Argentine FRICAS Investigators. Factores de Riesgo Coronario en America del Sur; pp. 797–803. [DOI] [PubMed] [Google Scholar]
- 37.He Y, Lam TH, Li LS, Du RY, Jia GL, Huang JY, Zheng JS. Passive smoking at work as a risk factor for coronary heart disease in Chinese women who have never smoked. BMJ. 1994;308:380–384. doi: 10.1136/bmj.308.6925.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, et al. Lancet. Vol. 368. England: 2006. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study; pp. 647–658. [DOI] [PubMed] [Google Scholar]
- 39.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ. 2002;325:188. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong MK, Glantz SA. Cardiovascular health and economic effects of smoke-free workplaces. Am J Med. 2004;117:32–38. doi: 10.1016/j.amjmed.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Jaakkola MS, Jaakkola JJ. Eur Respir J. Vol. 28. Denmark: 2006. Impact of smoke-free workplace legislation on exposures and health: possibilities for prevention; pp. 397–408. [DOI] [PubMed] [Google Scholar]
- 42.Issa JS, Abe TM, Pereira AC, Megid MC, Shimabukuro CE, Valentin LS, Ferreira MM, Nobre MR, Lancarotte I, Barretto AC. Tob Control. Vol. 20. England: 2011. The effect of Sao Paulo’s smoke-free legislation on carbon monoxide concentration in hospitality venues and their workers; pp. 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004;328:977–980. doi: 10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. Global Youth Tobacco Survey (GYTS) [ http://www.who.int/tobacco/surveillance/gyts/en/]
- 45.Yang Y, Wang JJ, Wang CX, Li Q, Yang GH. Biomed Environ Sci. Vol. 23. United States: The Editorial Board of Biomedical and Environmental Sciences. Published by Elsevier B.V; 2010. Awareness of tobacco-related health hazards among adults in China; pp. 437–444. [DOI] [PubMed] [Google Scholar]
- 46.He Y. Women’s passive smoking and coronary heart disease. Zhonghua Yu Fang Yi Xue Za Zhi. 1989;23:19–22. [PubMed] [Google Scholar]