Abstract
A series of 3′-difluorovinyl taxoids with C10 modifications, as well as those with C2 and C10 modifications, were strategically designed to block the metabolism by cytochrome P-450 3A4 enzyme and synthesized. These novel difluorovinyl taxoids were evaluated for their cytotoxicity against drug-sensitive human breast (MCF7), multidrug-resistant (MDR) human ovarian (NCI/ADR), human colon (HT-29) and human pancreatic (PANC-1) cancer cell lines. 3′-Difluorovinyl taxoids exhibit several to 16 times better activity against MCF7, HT-29 and PANC-1 cell lines and up to three orders of magnitude higher potency against NCI/ADR cell line as compared to paclitaxel. Structure-activity relationship study shows the critical importance of the C2 modifications on the activity against MDR cancer cell line, while the C10 modifications have a rather minor effect on the potency with some exceptions. The effect of the C2 modifications on potency against MCF7 cell line increases in the following order: H < F < Cl <N3. Among the twenty five 3′-difluorovinyl taxoids evaluated, eight taxoids exhibited less than 100 pM IC50 values against MCF7 cell line. Difluorovinyl taxoids induced GTP-independent tubulin polymerization much faster than paclitaxel. Then, the resulting microtubules were stable to Ca2+-induced depolymerization, indicating strong stabilization of microtubules. Molecular modeling study indicated that a difluorovinyl taxoid binds to β-tubulin in a manner that is consistent with the REDOR-Taxol structure. The difluorovinyl group appears to mimic the isobutenyl group to some extent, but with very different electronic property, which may account for the unique activities of difluorovinyl taxoids.
Keywords: Anticancer agent, Taxoid, Fluoro-taxoid, β-Lactam Synthon Method, Difluorovinyl, Baccatin, Structure-activity relationship, Microtubules
1. Introduction
The fact that a large number of fluorinated compounds have been approved by the FDA for medical use clearly demonstrates the importance of fluorine in medicinal chemistry [1–4]. In drug design, fluorine is now recognized as the second “favorite heteroatom”, after nitrogen [5]. The replacement of a C-H or C-O bond with a C-F bond in biologically active compounds often introduces beneficial properties such as higher metabolic stability, increased binding to target molecules, increased lipophilicity and membrane permeability [6, 7].
Paclitaxel (Taxol®) and its semi-synthetic analogue docetaxel (Taxotere®) have been approved for the treatment of advanced ovarian cancer, metastatic breast cancer, melanoma, non-small cell lung cancer and Kaposi’s sarcoma [8, 9]. These drugs have also been approved for the treatment of prostate, neck and cervical cancer [8, 9]. Paclitaxel and docetaxel bind to β-tubulin, one half of the α,β-heterodimer subunit that comprises microtubules, stabilizing the structure and preventing depolymerization. These drugs exert their cytotoxic effects through the disruption of microtubule dynamics, which causes the cell cycle arrest at the G2/M stage, leading to apoptosis through signaling cascade [10, 11].
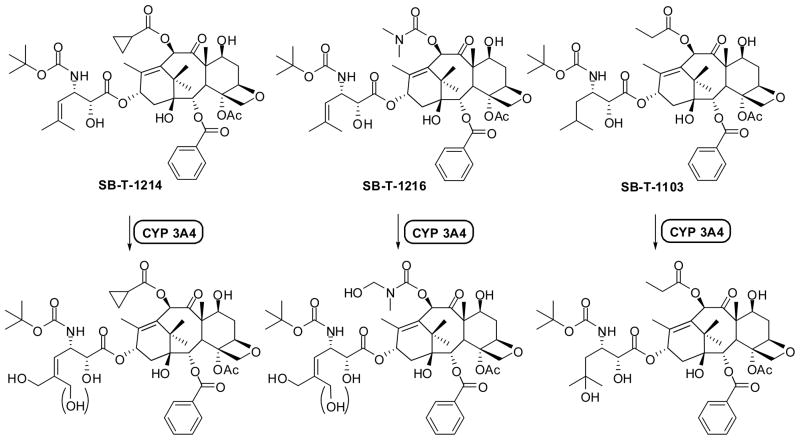
Paclitaxel and docetaxel possess potent antitumor activity, but chemotherapy with these drugs involves numerous undesirable side effects as well as drug resistance. Resistance to paclitaxel is mainly attributed to multi-drug resistance (MDR), which is caused by the over-expression of ABC transporters, such as P-glycoprotein (Pgp), an efflux pump responsible for the removal of cytotoxic agents [12, 13]. In addition to MDR, point mutations in the drug binding pocket of β-tubulin as well as the altered expression of β-tubulin isoforms contribute to drug resistance [14, 15]. Therefore, there is an obvious need to develop new taxane anticancer agents with fewer side effects, superior pharmacological properties, and improved activity against various classes of tumors, especially against drug-resistant human cancer. Our extensive structure-activity relationship (SAR) studies of taxoid anticancer agents have led to the discovery and development of new generation taxoids bearing non-aromatic substituents at the C3′ position (isobutenyl and isobutyl group in particular) and various acyl groups at the C10 position [16, 17] as well as meta-substituted benzoyl groups at the C2 position [17, 18]. These new generation taxoids bearing an isobutenyl or isobutyl group at C3′ and modifications at C10 and/or C2-benzoate exhibit 2–3 orders of magnitude higher potency than paclitaxel and docetaxel against drug-resistant cancer cell lines expressing MDR phenotype [16–18]. We have also developed 3′-trifluoromethyl and 3′-difluoromethyl taxoids with C10 as well as C10/C2 modifications in a similar manner [19–21]. This study has shown that trifluoromethyl and difluoromethyl groups are viable modifiers of the C3′ position and a number of highly potent fluoro-taxoids have been identified. Nevertheless, the isobutenyl group appears to be the best substituent at C3′ as far as the cytotoxicity is concerned. However, our recent study on the metabolic stability of 3′-isobutyl- and 3′-isobutenyl-taxoids revealed that there was a marked difference in metabolism between the new generation taxoids (e.g., SB-T-1214, SB-T-1216 and SB-T-1103) and that of docetaxel and paclitaxel [22]. The CYP 3A4 enzyme in the cytochrome P450 family in humans was found to metabolize these taxoids through hydroxylation primarily at the two allylic methyl groups of the 3′-isobutenyl group and the methyne moiety of the 3′-isobutyl group (Figure 1).
Figure 1.
Primary sites of hydroxylation on the second-generation taxoids by the P450 family of enzymes
This result makes a sharp contrast to the fact that the tert-butyl group of the C3′N-Boc moiety is the single predominant metabolic site for docetaxel and the C3′-phenyl and C6-methylene moieties are the sites for paclitaxel [23]. Accordingly, we have addressed the unique metabolic profiles of the new generation taxoids by designing a series of 3′-difluorovinyl taxoids in order to block the oxidation by CYP 3A4, which should enhance the metabolic stability and activity in vivo. The chemical synthesis, full characterization, biological evaluations, SAR and molecular modeling study of novel 3′-difluorovinyl taxoids are described herein.
2. Results and Discussion
2.1. Synthesis of 3′-difluorovinyl taxoids
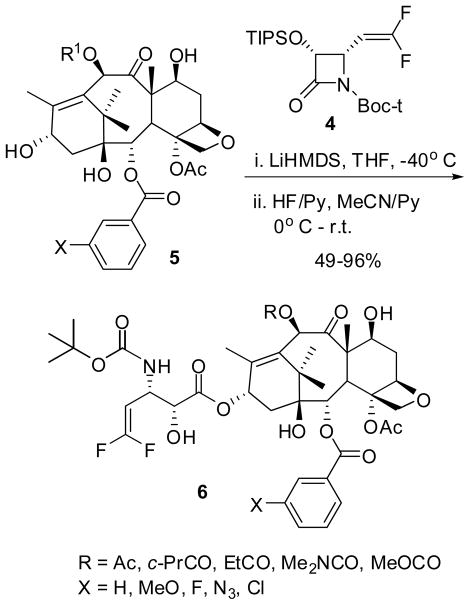
A series of novel 3′-difluorovinyl taxoids 6 were synthesized from (3R,4S)-TIPSO-4-difluorovinyl-β-lactam 4 and modified baccatins 5 using the highly efficient Ojima-Holton coupling protocol based on the “β-Lactam Synthon Method” (Schemes 1–4) [24–27].
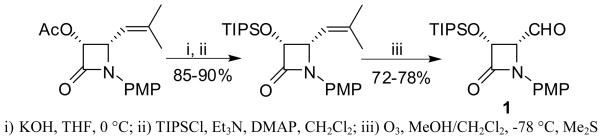
Scheme 1.
Synthesis of (3R,4R)-1-PMP-3-TIPSO-4-formyl-β-lactam (1)
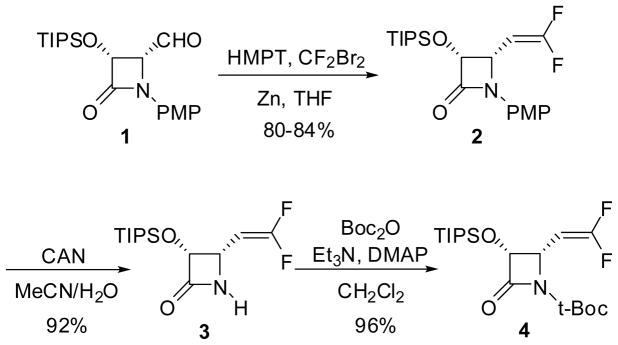
Enantiopure (3R,4S)-4-difluorovinyl-β-lactam 2 was synthesized through a Wittig-type reaction of (3R,4R)-4-formyl-β-lactam 1 (>99% ee) with phosphorus ylide, generated in situ by the reaction of HMPT with CF2Br2, in the presence of zinc (Scheme 2). Difluoromethylenation reaction of ketones and aldehydes using HMPT/CF2Br2 combination has been studied with and without Zn [28–30]. In the reaction of 4-formyl-β-lactam 1, the presence of Zn was found to be critical. Under optimized conditions (1/HMPT/CF2Br2/Zn = 1/10/4/5) (see Experimental Section), difluorovinyl-β-lactam 2 was obtained in 80–84% yield (Scheme 2). (3R,4R)-4-Formyl-β-lactam 1 (>99% ee) was prepared from (3R,4R)-1-PMP-4-acetoxy-4-(2-methylprop-2-enyl)azetidin-2-one obtained via enzymatic optical resolution of the racemic β-lactam, using our published protocol (Scheme 1).[19] Removal of the PMP group in the presence of ceric ammonium nitrate (CAN), followed by the introduction of t-Boc moiety afforded enantiopure (3R,4S)-3-TIPSO-4-difluorovinyl-β-lactam 4 in high overall yield (Scheme 2).
Scheme 2.
Synthesis of (3R,4S)-1-Boc-3-TIPSO-4-difluorovinyl-β-lactam (4)
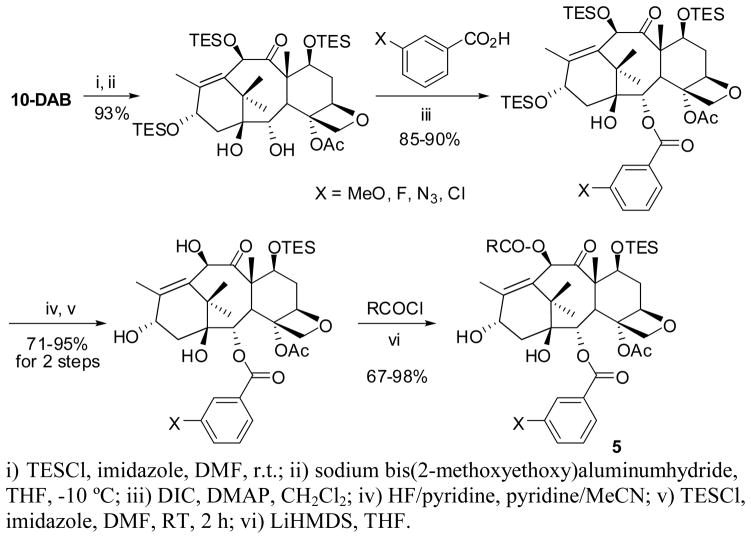
Modifications of 10-deacetylbaccatin III (10-DAB) at C2 and C10 were performed, using our published protocol [17, 21], with four substituents (i.e., MeO, F, N3 and Cl) and five acyl groups at C10 to produce 20 different baccatins 5a-1 ~ 5e-4 (Scheme 3). In addition, five baccatins 5a~d (X = H) with only C10 modifications were prepared, using our published protocol [17].
Scheme 3.
Synthesis of modified baccatin III (5)
The standard Ojima-Holton coupling conditions were used for the ring-opening coupling of 4-difluorovinyl-β-lactam 4 with 25 baccatins 5 in the presence of LiHMDS in THF at −40 °C. Removal of the silyl protecting groups afforded 3′-difluorovinyl taxoids 6 in moderate to excellent overall yield (Scheme 4).
Scheme 4.
Synthesis of 3′-difluorovinyl-taxoids (6)
2.2. Biological evaluation of 3′-difluorovinyl taxoids
2.2.1. Cytotoxicity of 3′-difluorovinyl taxoids
Cytotoxicity of the 3′-difluorovinyl taxoids was evaluated in vitro against drug-sensitive MCF7 human breast cancer cell line and drug-resistant NCI/ADR human ovarian cancer cell line expressing MDR phenotype. Selected difluorovinyl taxoids were also assayed against HT-29 human colon cancer cell line and PANC-1 human pancreatic cancer cell line. As Table 1 shows, all difluorovinyl-taxoids are substantially more potent than paclitaxel against all cancer cell lines examined. The potencies of three representative new generation taxoids, SB-T-1213, SB-T-1214 and SB-T-121303 (3′-isobutenyl in all cases), are also listed for comparison [16, 17]. Marked effect of C2-benzoate modification at the meta position is clearly observed on the potency against drug-sensitive and drug-resistant cell lines. The potency of these difluorovinyl taxoids against NCI/ADR depends on the nature of meta substituents of the C2-benzoate moiety, and the potency increases in the following order: F < Cl ≤ MeO < N3. Several difluorovinlyl taxoids with 2,10-modifications exhibit impressive potency, in that their IC50 values are in <100 pM range (78–92 pM) against MCF7, and in sub-nanomolar range (0.34–0.50 nM) against NCI/ADR, which is three orders of magnitude more potent than paclitaxel (entries 12, 15, 21, 22 and 25). The resistance factor for these difluorovinyl taxoids is 3.7–6.4, while that for paclitaxel is 250. Difluorovinyl taxoid 6b-2 (entry 17) exhibits extremely high potency (IC50 71 pM) against MCF7 cell line, but resistance factor is 24. Difluorovinyl taxoids (6a–d) with unmodified C2-benzoate moiety (entries 6–10) also exhibit substantially enhanced potency against MCF7 and NCI/ADR cell lines as compared to paclitaxel. These difluorovinyl taxoids possess excellent potency against HT-29 and PANC-1 cell lines as well. The potencies of difluorovinyl taxoids with 2,10-modifications have not been evaluated against HT-29 and PANC-1 cell lines yet, but it is reasonable to assume that these novel taxoids would exhibit exceptional potency against these cancer cell lines. It should also be noted that difluorovinyl taxoid 6a (SB-T-12851) showed exceptional potency (IC50 = 5.6 pM) against CFPAC-1 human pancreatic cancer cell line, wherein paclitaxel (IC50 = 15.8 nM) and difluorovinyl taxoid 6d (IC50 = 2.2 nM) indicated the existence of a certain level drug resistance.
Table 1.
In vitro cytotoxicity (IC50 nM)a of 3′-difluorovinyl-taxoids
| Entry | Taxoid | X | R | MCF7b (breast) | NCI/ADRc (ovarian) | R/Sd | HT-29e (colon) | PANC-1f (pancreatic) |
|---|---|---|---|---|---|---|---|---|
| 1 | paclitaxel | H | Ac | 1.2 | 300 | 250 | 3.6 | 25.7 |
| 2 | docetaxel | H | H | 0.83 | 235 | 283 | … | … |
| 3 | SB-T-1213 | H | EtCO | 0.15 | 4.0 | 27 | 0.31 | … |
| 4 | SB-T-121303 | MeO | EtCO | 0.30 | 0.33 | 1.1 | … | 22.6 |
| 5 | SB-T-1214 | H | c-PrCO | 0.17 | 2.1 | 12 | 0.36 | 3.68 |
| 6 | 6a | H | Ac | 0.099 | 0.95 | 9.6 | 0.41 | 1.19 |
| 7 | 6b | H | c-PrCO | 0.12 | 6.03 | 50 | 0.85 | 5.85 |
| 8 | 6c | H | EtCO | 0.12 | 1.2 | 10 | 0.34 | 0.65 |
| 9 | 6d | H | Me2NCO | 0.13 | 4.27 | 33 | 0.46 | 1.58 |
| 10 | 6e | H | MeOCO | 0.14 | 1.29 | 9.2 | … | … |
| 11 | 6a-1 | MeO | Ac | 0.25 | 1.5 | 6.0 | … | … |
| 12 | 6b-1 | MeO | c-PrCO | 0.092 | 0.48 | 5.2 | … | … |
| 13 | 6c-1 | MeO | Et-CO | 0.34 | 0.57 | 1.7 | … | … |
| 14 | 6d-1 | MeO | Me2NCO | 0.11 | 0.96 | 8.7 | … | … |
| 15 | 6e-1 | MeO | MeOCO | 0.078 | 0.50 | 6.4 | … | … |
| 16 | 6a-2 | F | Ac | 0.13 | 1.53 | 12 | … | … |
| 17 | 6b-2 | F | c-PrCO | 0.071 | 1.72 | 24 | … | … |
| 18 | 6c-2 | F | EtCO | 0.23 | 2.54 | 11 | … | … |
| 19 | 6d-2 | F | Me2NCO | 0.17 | 2.25 | 13 | … | … |
| 20 | 6e-2 | F | MeOCO | 0.12 | 1.85 | 15 | … | … |
| 21 | 6a-3 | N3 | Ac | 0.092 | 0.34 | 3.7 | … | … |
| 22 | 6b-3 | N3 | c-PrCO | 0.092 | 0.45 | 4.9 | … | … |
| 23 | 6c-3 | N3 | EtCO | 0.13 | 0.38 | 2.9 | … | … |
| 24 | 6d-3 | N3 | Me2NCO | 0.13 | 0.46 | 3.5 | … | … |
| 25 | 6e-3 | N3 | MeOCO | 0.078 | 0.40 | 5.3 | … | … |
| 26 | 6a-4 | Cl | Ac | 0.14 | 0.70 | 5.0 | … | … |
| 27 | 6b-4 | Cl | c-PrCO | 0.12 | 0.50 | 4.2 | … | … |
| 28 | 6c-4 | Cl | EtCO | 0.13 | 0.45 | 3.5 | … | … |
| 29 | 6d-4 | Cl | Me2NCO | 0.94 | 2.6 | 2.8 | … | … |
| 30 | 6e-4 | Cl | MeOCO | 0.099 | 1.16 | 12 | … | … |
The concentration of compound inhibits 50% of the growth of a cancer cell line after 72 h drug exposure;
human breast carcinona;
multidrug-resistant human ovarian carcinoma;
R/S = drug-resistance factor = IC50 (drug-resistant cell line)/IC50 (drug-sensitive cell line);
human caucasian colon adenocarcinoma;
human pancreatic carcinoma.
2.2.2. Tubulin polymerization and microtubule stabilization by 3′-difluorovinyl taxoids
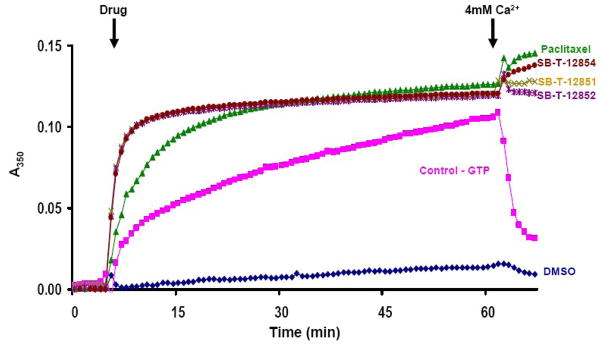
The activities of three difluorovinyl taxoids, SB-T-12851 (6a), SB-T-12853 (6c) and SB-T-12854 (6d), for tubulin polymerization and microtubule stabilization were examined in comparison to paclitaxel. In this spectrophotometric assay, changes in absorbance values provide a direct measure of turbidity, indicating the degree of tubulin polymerization. As Figure 3 show, these three difluorovinyl taxoids induced GTP-independent tubulin polymerization much faster than paclitaxel (Figure 2). Thus, the turbidity of the tubulin solution treated by difluorovinyl taxoids reaches a plateau quickly and does not change with time. The resulting microtubules were stable to Ca2+-induced depolymerization, indicating strong stabilization of microtubules, which is well known for paclitaxel. This observation may suggest that the microtubules formed by these novel difluorovinyl taxoids are different from those formed by paclitaxel.
Figure 2.
Tubulin polymerization with SB-T-12851 (6a), SB-T-12852 (6b), SB-T-12854 (6d), paclitaxel and GTP: microtubule protein 1 mg/mL, 37 °C, GTP 1 mM, Drug 10 μM
2.2.3. Electron microscopy analysis of microtubules treated with selected difluorovinyl taxoids
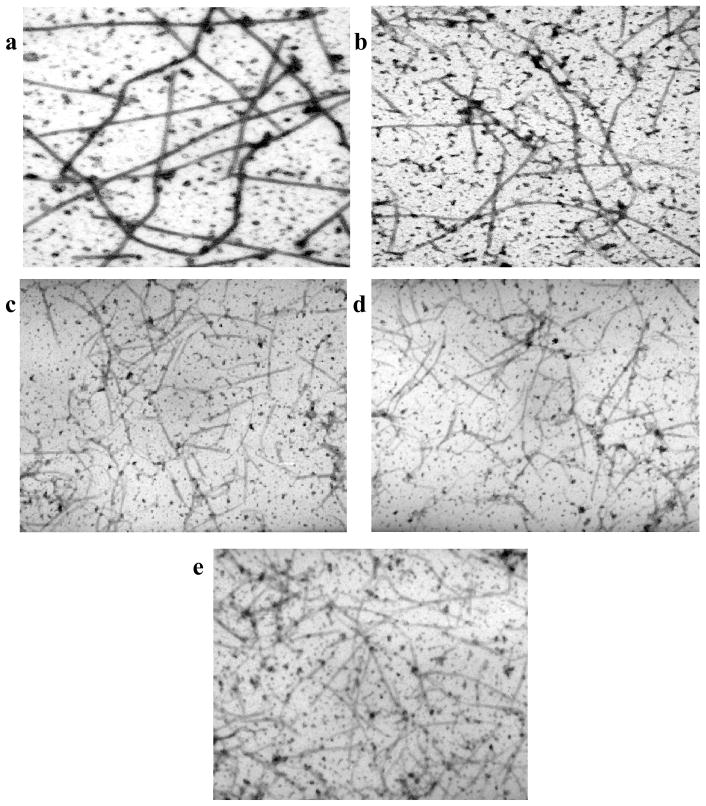
The microtubules formed by treatment of tubulin with the three difluorovinyl taxoids were further analyzed by electron microscopy to study their morphology and structure in comparison to those formed in the presence of GTP or paclitaxel. The electron micrographs of microtubules formed by treatment with SB-T-12851 (6a), SB-T-12852 (6b), SB-T-12854 (6d), paclitaxel and GTP are shown in Figure 3. Microtubules formed in the presence of GTP and paclitaxel are long and thick (Figure 3a and 3b), while those formed by the difluorovinyl taxoids (Figure 3c ~ 3e) appear to be much thinner and shorter in length, which indicates substantial difference in their properties as compared to those formed by paclitaxel. It is strongly suggested that the formation of thinner and shorter microtubules is related to the rapid polymerization of tubulin observed with these difluorovinyl taxoids (see Figure 2). There is some morphological similarity between those microtubules generated by the action of difluorovinyl taxoids and those by second generation taxoids such as SB-T-1213 and SB-T-1214, but the formation of thinner, shorter and straight microtubules appear to be unique to difluorovinyl taxoids.
Figure 3.
Electromicrographs of microtubules: (a) GTP; (b) paclitaxel; (c) 6a; (d) 6b; (e) 6d.
2.3. Molecular modeling study on the bioactive structure of 3′-difluorovinyl taxoids in β-tubulin
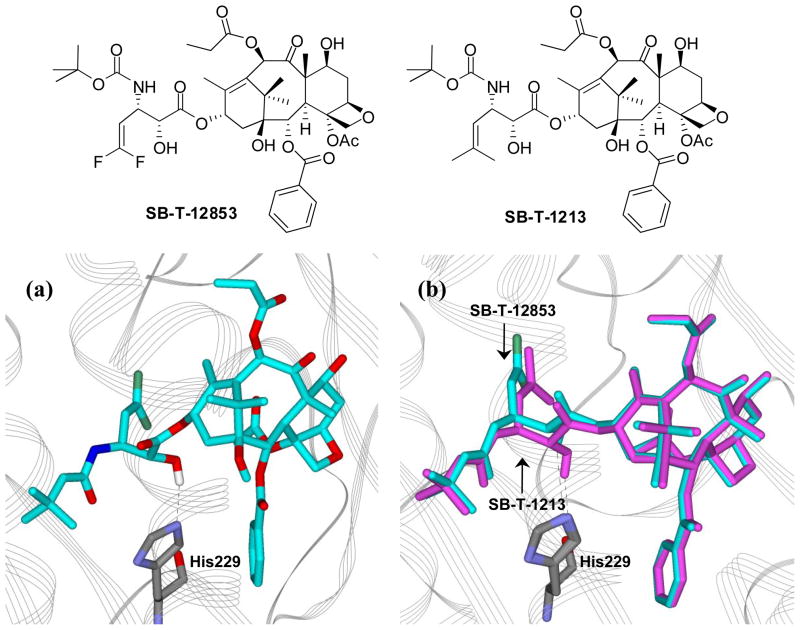
Recently, the “REDOR-Taxol” structure has been shown to be the most plausible bioactive conformation of paclitaxel [31, 32]. To probe the binding conformations of 3′-difluorovinyl taxoids, SB-T-12853 (6c) was selected as representative and docked to the paclitaxel binding pocket of the β-tubulin subunit using the REDOR-Taxol coordinates. With the protein backbone fixed, the energy of the system was minimized (InsightII 2000, CVFF), allowing the side chains to find their lowest energy conformations. A structurally closely related non-fluorinated second-generation taxoid, SB-T-1213, was also docked into β-tubulin following the same protocol for comparison. The resulting computer-generated binding structures of these taxoids and their overlay are shown in Figure 4.
Figure 4.
(a) Proposed binding conformation of SB-T-12853 (6c) in tubulin; (b) overlay of SB-T-12853 (cyan) and SB-T-1213 (magenta) in tubulin
As Figure 4 shows, both SB-T-12853 and SB-T-1213 form a very stable H-bond with His227, consistent with the REDOR-Taxol structure. Their overlay (Figure 4b) shows almost complete overlap in the baccatin moieties of SB-T-12853 and SB-T-1213, with small but appreciable difference in the side chain positions. The result may indicate that difluorovinyl group mimics isobutenyl group, but there is a difference in size and electronic nature between two groups. The difluorovinyl group is between vinyl and isobutenyl groups in size and two fluorine atoms may mimic electronically two hydroxyl groups rather than two methyl groups.
In our recent study on the metabolic stability of difluorovinyl taxoids 6a–d against P-450 family enzymes, almost no appreciable metabolites were detected, which suggests that not only the metabolism at C3′ is effectively blocked, but also oxidative metabolism on other parts of the taxoid molecule, including the C3′N-t-Boc and C6 methylene moieties, is suppressed. The results will be published elsewhere. Thus, our strategic incorporation of a difluorovinyl group in place of an isobutenyl group at C3′ has been proven to be successful to block the observed metabolism of the isobutenyl moiety in second-generation taxoids (see Figure 1).
Accordingly, the difluorovinyl group is a very unique structural component in medicinal chemistry and may serve as a versatile modifier of pharmacological properties in a manner similar to the trifluoromethyl group.
3. Experimental Section
3.1. General Method
1H, 13C and 19F NMR spectra were recorded on Varian 300 MHz or 400 MHz NMR spectrometer in CDCl3. Tetramethylsilane was used as the internal standard for 1H and 13C NMR spectra, while CFCl3 as the standard for 19F NMR spectra. Melting points were measured on a Thomas Hoover Capillary melting point apparatus. Optical rotations were measured on a Perkin-Elmer Model 241 polarimeter. TLC was performed on Merck DC-alufolien with Kieselgel 60F-254 and column chromatography was carried on silica gel 60(Merck; 230–400 mesh ASTM). Chiral HPLC analysis for the determination of enantiomeric excess was carried out with a Waters HPLC assembly. HPLC assembly consisted of a Waters M45 solvent delivery system, A Waters Model 680 gradient controller, and a Water M440 detector (at 254 nm), equipped with a Spectra Physic Model SP4270 integrator and uses a DAICEL-CHIRACEL OD chiral column (25 × 0.46 cm i.d.), employing hexane/2-propanol (99.5/0.5, v/v) as the solvent system with a flow rate of 1.0 ml/min. HPLC analysis for determination of isomeric ratio was carried out with the same Water HPLC assembly using 5μ Spherical Silica column employing hexane/2-propanol/dichloromethane (15/1/1, v/v/v) as the solvent system with a flow rate 1.0 ml/min, or hexane/2-propanol/dichloromethane (10/1/1, v/v/v) as the solvent system with a flow rate 1.4 ml/min. High resolution mass spectra were obtained from the Mass Spectrometry Laboratory, University of Illinois at Urbana-Champaign, Urbana, IL.
3.2. Materials
The chemicals were purchased from Aldrich and Sigma and purified before use by standard methods. Tetrahydrofuran was freshly distilled under nitrogen from sodium metal and benzophenone. Dichloromethane was also distilled immediately prior to use under nitrogen from calcium hydride. 7-Triethylsilylbaccatin III (5a) was prepared by the literature method [33]. 7-Triethylsilyl-10-deacetyl-10-acylbaccatins (5b~e) were prepared by the previously reported method [16]. 2-Debenzoyl-2-(3-methoxybenzoyl)-7-triethylsilylbaccatin III (5a-1), 2-debenzoyl-2-(3-fluorobenzoyl)-7-triethylsilylbaccatin III (5a-2), 2-debenzoyl-2-(3-azidobenzoyl)-7-triethylsilylbaccatin III (5a-3), 2-debenzoyl-2-(3-chlorobenzoyl)-7-triethylsilylbaccatin III (5a-4), 2-debenzoyl-2-(3-methoxybenzoyl)-7-triethylsilyl-10-deacetyl-10-acylbaccatins (5b~e-1), 2-debenzoyl-2-(3-fluorobenzoyl)-7-triethylsilyl-10-deacetyl-10-acylbaccatins (5b~e-2), 2-debenzoyl-2-(3-azidobenzoyl)-7-triethylsilyl-10-deacetyl-10-acylbaccatins (5b~e-3), and 2-debenzoyl-2-(3-chlorobenzoyl)-7-triethylsilyl-10-deacetyl-10-acylbaccatins (5b~e-4) were prepared by the previously reported methods [21].
3.3. Synthesis of (+)-(3R,4S)-1-tert-butoxycarbonyl-3-triisopropylsiloxy-4-(2,2-difluoro-ethenyl)azetidin-2-one (4)
3.3.1. (3R,4S)-1-(4-Methoxyphenyl)-3-triisopropylsiloxy-4-(2,2-difluorovinyl)azetidin-2-one (2)
Hexamethylphosphorous triamide (HMPT) (4.58 mL, 24.6 mmol) was added to a solution of dibromodifluoromethane (1.51 mL, 9.84 mmol) in THF (75 mL) under nitrogen at 0 °C, which immediately yielded white precipitate. To this mixture was added a suspension of 4-formyl-β-lactam 1 (927 mg, 2.46 mmol) and Zn (802 mg, 12.3 mmol) in THF (100 mL) at 0 °C. The ice bath was removed and the the reaction mixture was refluxed for 40 min. Ether was added to the reaction mixture. After cooling to room temperature, the reaction mixture was filtered through Celite and concentrated. The reddish residue was dissolved in ethyl acetate (50 mL), washed with water (20 mL × 3), brine (20 mL × 3), dried over anhydrous MgSO4, and concentrated in vacuo to afford a crude product as an yellow-orange oil. The crude product was purified by flash chromatography on silica gel (hexane/EtOAc = 3/1) to afford 1-PMP-4-difluorovinyl-β-lactam 2 (851 mg, 84% yield) as a white solid: 1H NMR (CDCl3, 300 MHz) δ 1.08–1.15 (m, 21 H), 3.79 (s, 3 H), 4.54 (ddd, J = 1.5 Hz, 6.3 Hz, 16.5 Hz, 1 H), 4.83 (m, 1 H), 5.14 (d, J = 5.1 Hz, 1 H), 6.87 (d, J = 9.0 Hz,2H), 7.32 (d, J = 9.0 Hz, 2H); 13C NMR (CDCl3, 75.5 MHz) 12.1, 17.9, 54.1, 55.8, 75.8, 76.9, 77.4, 114.8, 118.6, 130.9, 156.7, 164.9; 19F NMR (282 MHz, CDCl3) δ −80.80 (d, J = 32.7 Hz, 1F), −86.34 (dd, J = 2.8 Hz, 28.2 Hz, 1F). HRMS (FAB+, m/z): Calcd. for C21H31F2NO3Si H+, 412.2114; Found, 412.2127.
3.3.2. (3R,4S)-3-Triisopropylsilanyloxy-4-(2,2-difluoroethenyl)azetidin-2-one (3)
To a solution of 1-PMP-4-difluorovinyl-β-lactam 2 (688 mg, 1.67 mmol) in acetonitrile (50 mL) and H2O (10 mL) at −10 °C was added dropwise a solution of ceric ammonium nitrate (3.74 g, 6.69 mmol) in water (40 mL) with stirring. The reaction mixture was stirred for 2 h and diluted with water (60 mL) and aqueous saturated Na2SO3 (60 mL). The aqueous layer was extracted with ethyl acetate, and the combined organic layer was washed with water, dried over MgSO4 and concentrated in vacuo. The crude product was purified on a silica gel column (hexanes/EtOAc = 3/1) to give NH free 4-difluorovinyl-β-lactam 3 (469 mg, 92% yield) as a white solid: 1H NMR (CDCl3, 400 MHz) δ 1.03–1.18 (m, 21 H), 4.44–4.54 (m, 2 H), 5.04 (dd, J = 1.6 Hz, 2.4 Hz, 1 H), 6.59 (bs, 1 H); 13C NMR (CDCl3, 100 MHz) 12.1, 17.8, 50.4, 77.1, 79.3, 157.6, 169.4; 19F NMR (282 MHz, CDCl3) δ −82.33 (d, J = 34.7 Hz, 1F), −87.50 (dd, J = 9.3 Hz, 25.7 Hz, 1 F).
3.3.3. (+)-(3R,4S)-1-(tert-Butoxycarbonyl)-3-triisopropylsiloxy-4-(2,2-difluoroethenyl)azetidin-2-one (4)
To a solution of 4-difluorovinyl-β-lactam 3 (469 mg, 1.54 mmol), triethylamine (0.75 mL, 5.38 mmol), and DMAP (43 mg, 0.35 mmol) in CH2Cl2 (9 mL) was added Boc2O (398 mg, 1.77 mmol) at room temperature. The reaction mixture was stirred for 18 h and quenched with water. The reaction mixture was diluted with ethyl acetate and the organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated under reduced pressure. Crude material was purified by flash chromatography on silica to give 1-t-Boc-4-difluorovinyl-β-lactam 4 as colorless oil (599 mg, 96% yield): [α]D20 +24.17 (c 14.4, CHCl3); 1H NMR (CDCl3, 300 MHz): δ 1.04–1.17 (m, 21 H), 1.49 (s, 9 H), 4.49 (ddd, J = 1.6 Hz, 13.8 Hz, 23.7 Hz, 1 H), 4.75 (m, 1 H), 5.04 (d, J = 5.7 Hz, 1 H), 6.59 (bs, 1 H); 13C NMR (CDCl3, 100 MHz): 12.0, 17.8, 28.2, 53.6, 74.5, 77.2, 83.9, 147.9, 158.5, 165.3; 19F NMR (282 MHz, CDCl3): δ −81.20 (d, J = 31.0 Hz, 1 F), −85.83 (dd, J = 5.6 Hz, 29.3 Hz, 1F). HRMS (FAB+, m/z): Calcd. for C19H33F2NO4Si Na+, 428.2039; Found, 428.2050.
3.4. Synthesis of 3′-difluorovinyl taxoids
A typical procedure is described for the synthesis of 10-acetyl-3′-dephenyl-3′-(2,2-difluoroethenyl)docetaxel (6a). All other 3′-difluorovinyl taxoids were synthesized in the same manner.
Difluoromethyl-β-lactam 4 (190 mg, 0.468 mmol) and 5a (234 mg, 0.335 mmol) were dissolved in dry THF (35 mL). The mixture was cooled to −40 °C, and LiHMDS (1M THF solution, 0.67 mL) was added. The reaction mixture was stirred for 2 h, and quenched with aqueous saturated ammonium chloride. The aqueous layer was extracted with ethyl acetate, and the combined organic phases were washed with brine and dried over anhydrous MgSO4. The crude product was purified on silica gel column (hexanes/ethyl acetate = 6/1) to give 7-TES-10-Ac-3′-dephenyl-2′-TIPSO-3′-(2,2-difluoroethenyl)docetaxel (356 mg, 96%).
To a solution of the 7-TES-2′-TIPS-taxoid (356 mg) thus obtained in 14 mL of a 1:1 mixture of pyridine and acetonitrile cooled to 0°C was added 3.5 mL of HF/pyridine (7/3). The reaction mixture was allowed to warm to room temperature and stirred for 24 h. The reaction was then quenched with 10 mL of aqueous saturated sodium bicarbonate and extracted with ethyl acetate. The combined organic layers were washed with copper sulfate and brine, dried over anhydrous MgSO4 and concentrated. The residue was purified by chromatography on silica gel using hexanes/ethyl acetate (1/1) as eluent to afford 6a (SB-T-12851) (263 mg, 96% yield) as a white solid.
3.4.1. 10-Acetyl-3′-dephenyl-3′-(2,2-difluoroethenyl)docetaxel (6a)
Yield 92% for 2 steps; mp 155–160 °C; [α]D20 −74.83 (c 2.86, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.15 (s, 3H), 1.25 (s, 3 H), 1.30 (s, 9H), 1.68 (s, 3 H), 1.75 (bs, 1 H), 1.88 (m, 4 H), 2.24 (s, 3 H), 2.33 (m, 2 H), 2.39 (s, 3H), 2.49 (d, J = 3.6 Hz, 1H), 2.55 (m, 1 H), 3.52 (d, J = 5.6 Hz, 1 H), 3.81 (d, J = 7.2 Hz, 1 H), 4.17 (d, J = 8.4 Hz, 1 H), 4.28 (d, J = 2.8 Hz, 1 H), 4.31 (d, J = 8.4 Hz, 1 H), 4.44 (m, 1 H), 4.58 (ddd, J = 1.2 Hz, 9.6 Hz, 24.8 Hz, 1 H), 4.87 (t, J = 8.8 Hz, 1 H), 4.96 (m, 2 H), 5.66 (d, J = 7.2 Hz, 1 H), 6.24 (t, J = 8.8 Hz, 1 H), 6.30 (s, 1 H), 7.49 (t, J = 7.6 Hz, 2 H), 7.61 (t, J = 7.2 Hz, 1 H), 8.11 (d, J = 7.6 Hz, 2H); 13C NMR (CDCl3, 75.5 MHz) δ 9.8, 14.4, 15.1, 21.1, 22.1, 22.5, 26.9, 28.3, 35.7, 43.5, 45.7, 48.2, 58.8, 72.4, 72.9, 75.8, 76.7, 79.3, 80.7, 81.3, 84.6, 128.9, 129.3, 130.4, 133.4, 133.9, 142.4, 155.1, 156.7, 158.0, 167.3, 170.5, 171.5, 172.7, 203.9; 19F NMR, (CDCl3, 282 MHz) δ −84.29 (dd, J = 25.7 Hz, 36.4 Hz, 1 F), −86.22 (d, J = 34.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H51F2NO15 H+, 836.3300; Found, 836.3278.
3.4.2. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-10-cyclopropanecarbonyldocetaxel (6b)
Yield 88 % for 2 steps; white solid; mp 171–177 °C; [α]D20 −73.71 (c 5.44, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 0.98 (m, 2 H), 1.13 (m, 2 H), 1.15 (s, 3 H), 1.26 (s, 3 H), 1.30 (s, 9 H), 1.66 (s, 3 H), 1.78 (m, 2 H), 1.87 (m, 4 H), 2.31 (m, 2 H), 2.38 (s, 3 H), 2.53 (m, 1 H), 2.59 (d, J = 3.2 Hz, 1 H), 3.57 (bs, 1 H), 3.80 (d, J = 6.8 Hz, 1 H), 4.17 (d, J = 8.4 Hz, 1 H), 4.28 (m, 2 H), 4.40 (m, 1 H), 4.58 (ddd, J = 1.6 Hz, 9.6 Hz, 24.8 Hz, 1 H), 4.87 (t, J = 8.8 Hz, 1 H), 4.97 (m, 2 H), 5.66 (d, J = 7.2 Hz, 1 H), 6.24 (t, J = 8.0 Hz, 1 H), 6.29 (s, 1 H), 7.49 (t, J = 7.6 Hz, 2 H), 7.60 (t, J = 7.6 Hz, 1 H), 8.11 (d, J = 7.2 Hz, 2 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.4, 9.6, 9.8, 13.2, 22.5, 27.0, 28.3, 35.7, 43.5, 45.9, 48.2, 58.8, 72.4, 72.9, 73.3, 75.3, 75.6, 76.6, 79.3, 80.7, 81.3, 84.7, 128.9, 129.3, 130.4, 133.4, 133.9, 142.4, 155.1, 156.7, 167.3, 170.5, 172.6, 175.3, 204.0; 19F NMR, (CDCl3, 282 MHz) δ −84.32 (dd, J = 25.4 Hz, 36.4 Hz, 1 F), −86.30 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C43H53F2NO15 H+, 862.3456; Found, 862.3445.
3.4.3. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-10-propanoyldocetaxel (6c)
Yield 64 % for 2 steps; white solid; mp 175–181 °C; [α]D20 −82.83 (c 5.01, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.14 (s, 3 H), 1.24 (m, 6 H), 1.30 (s, 9 H), 1.67 (s, 3 H), 1.78 (m, 1 H), 1.87 (m, 4 H), 2.31 (m, 2 H), 2.38 (s, 3H), 2.53 (m, 4 H), 3.55 (bs, 1 H), 3.81 (d, J = 6.8 Hz, 1 H), 4.17 (d, J = 8.4 Hz, 1 H), 4.29 (m, 2 H), 4.39 (m, 1 H), 4.56 (ddd, J = 1.6 Hz, 9.6 Hz, 24.8 Hz, 1 H), 4.86 (t, J = 8.8 Hz, 1 H), 4.96 (m, 2 H), 5.66 (d, J = 7.2 Hz, 1 H), 6.25 (t, J = 8.4 Hz, 1 H), 6.30 (s, 1 H), 7.49 (t, J = 7.6 Hz, 2 H), 7.60 (t, J = 7.2 Hz, 1 H), 8.11 (d, J = 7.2 Hz, 2 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.2, 9.8, 15.1, 22.1, 22.5, 26.9, 27.8, 28.4, 35.7, 43.5, 45.9, 48.2, 58.8, 72.2, 72.4, 72.9, 73.3, 75.3, 75.6, 76.6, 77.4, 79.3, 80.7, 81.3, 84.6, 128.9, 129.3, 130.4, 133.5, 133.9, 142.2, 155.1, 156.7, 167.3, 170.5, 172.6, 174.8, 203.9; 19F NMR, (CDCl3, 282 MHz) δ −84.31 (dd, J = 23.7 Hz, 34.7 Hz, 1 F), −86.23 (d, J = 36.4 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H53F2NO15 H+, 850.3456; Found 850.3450.
3.4.4. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-10-dimethylcarbamoyldocetaxel (6d)
Yield 84 % for 2 steps; white solid; mp 166–170 °C; [α]D20 −70.48 (c 6.3, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.15 (s, 3 H), 1.25 (s, 3 H), 1.30 (s, 9 H), 1.67 (s, 3 H), 1.84 (m, 1 H), 1.89 (m, 4 H), 2.31 (m, 2 H), 2.38 (s, 3 H), 2.53 (m, 1 H), 2.96 (s, 3 H), 3.05 (s, 3 H), 3.24 (bs, 1 H), 3.64 (d, J = 5.6 Hz, 1 H), 3.80 (d, J = 6.8 Hz, 1 H), 4.17 (d, J = 8.4 Hz, 1 H), 4.29 (m, 2 H), 4.44 (m, 1 H), 4.57 (dd, J = 10.0 Hz, 25.2 Hz, 1 H), 4.86 (t, J = 8.8 Hz, 1 H), 4.97 (d, J = 9.2 Hz, 1 H), 5.02 (d, J = 9.6 Hz, 1 H), 5.66 (d, J = 7.2 Hz, 1 H), 6.24 (m, 2 H), 7.49 (t, J = 7.6 Hz, 2H), 7.59 (t, J = 7.2 Hz, 1 H), 8.10 (d, J = 7.6 Hz, 2 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.6, 15.1, 22.1, 22.5, 27.1, 28.3, 35.6, 36.3, 36.8, 43.5, 45.8, 48.2, 58.7, 72.6, 72.9, 73.3, 75.4, 76.3, 76.7, 77.4, 79.4, 80.6, 81.3, 84.6, 128.9, 129.4, 130.4, 133.7, 133.9, 142.7, 155.1, 156.3, 156.5, 167.3, 170.4, 171.3, 203.9; 19F NMR, (CDCl3, 282 MHz) δ −84.31 (dd, J = 25.7 Hz, 37.2 Hz, 1 F), −86.23 (d, J = 36.4 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H54F2N2O15 H+, 865.3565; Found 865.3562.
3.4.5. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-10-methoxycarbonyl-docetaxel (6e)
Yield 90 % for 2 steps; white solid; mp 144–148 °C; [α]D20 −77.06 (c 6.8, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.15 (s, 3 H), 1.24 (s, 3 H), 1.29 (s, 9 H), 1.68 (s, 3 H), 1.78 (m, 1 H), 1.88 (m, 1 H), 1.91 (s, 3 H), 2.31 (m, 2 H), 2.39 (s, 3 H), 2.53 (m, 2 H), 3.55 (d, J = 5.6 Hz, 1 H), 3.78 (d, J = 7.2 Hz, 1 H), 3.86 (s, 3 H), 4.17 (d, J = 8.4 Hz, 1 H), 4.29 (m, 2 H), 4.38 (m, 1 H), 4.57 (ddd, J = 1.6 Hz, 9.6 Hz, 24.8 Hz, 1 H), 4.86 (t, J = 8.8 Hz, 1 H), 4.96 (m, 2 H), 5.66 (d, J = 6.8 Hz, 1 H), 6.11 (s, 1 H), 6.23 (t, J = 8.0 Hz, 1 H), 7.49 (t, J = 7.6 Hz, 2 H), 7.60 (t, J = 7.2 Hz, 1 H), 8.10 (d, J = 7.2 Hz, 2 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.7, 15.2, 22.1, 22.5, 26.8, 28.3, 35.8, 43.4, 45.9, 48.2, 55.8, 58.8, 72.1, 72.3, 72.8, 73.3, 75.2, 76.7, 77.4, 78.4, 79.2, 80.7, 81.2, 84.6, 128.9, 129.3, 130.4, 133.0, 133.9, 143.2, 155.1, 155.9, 156.5, 167.3, 170.6, 172.3, 204.1; 19F NMR, (CDCl3, 282 MHz) δ −84.30 (dd, J = 23.7 Hz, 34.7 Hz, 1 F), −86.22 (dd, J = 34.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H51F2NO16 H+, 852.3249; Found 852.3227.
3.4.6. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-methoxybenzoyl)-10-acetyldocetaxel (6a-1)
Yield 76% for 2 steps; white solid; mp °C; [α]D20 −74.42 (c 2.15, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.15 (s, 3 H), 1.26 (m, 3 H), 1.30 (s, 9 H), 1.68 (s, 3 H), 1.76 (s, 1 H), 1.88 (m, 4 H), 2.24 (s, 3 H), 2.33 (m, 2 H), 2.38 (s, 3 H), 2.49 (d, J = 3.5 Hz, 1 H), 2.56 (m, 1 H), 3.47 (bs, 1 H), 3.81 (d, J = 7.5 Hz, 1 H), 3.90 (s, 3 H), 4.15 (d, J = 8.0 Hz, 1 H), 4.27 (m, 1 H), 4.36 (d, J = 8.0 Hz, 1 H), 4.41 (dd, J = 6.0 Hz, 10.5 Hz, 1 H), 4.58 (ddd, J = 1.0 Hz, 9.5 Hz, 24.5 Hz, 1 H), 4.87–4.93 (m, 2 H), 4.97 (dd, J = 2.0 Hz, 9.0 Hz, 1 H), 5.67 (d, J = 7.5 Hz, 1H), 6.24 (t, J = 8.5 Hz, 1 H), 6.30 (s, 1 H), 7.15 (dd, J = 2.0 Hz, 8.0 Hz, 1 H), 7.40 (t, J = 7.5 Hz, 1 H), 7.62 (s, 1 H), 7.72 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 100 MHz) δ 9.5, 14.8, 20.8, 21.8, 22.3, 26.7, 28.1, 35.5, 43.2, 45.6, 55.3, 58.5, 72.1, 72.7, 73.1, 75.1, 75.5, 76.4, 77.2, 79.0, 80.5, 81.2, 84.4, 114.0, 120.7, 122.7, 129.7, 130.3, 133.1, 142.1, 154.8, 159.7, 166.9, 170.2, 171.2, 203.5; 19F NMR, (CDCl3, 282 MHz) δ −84.55 (dd, J = 33.8 Hz, 36.4 Hz, 1 F), −86.24 (d, J = 36.4 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H53F2NO16 H+, 866.3405; Found, 866.3439.
3.4.7. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-methoxybenzoyl)-10-cyclopropanecarbonyldocetaxel (6b-1)
Yield 96% for 2 steps; white solid; [α]D20 −77.03 (c 5.79, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 0.99 (m, 2 H), 1.10 (m, 2 H), 1.15 (s, 3 H), 1.25 (s, 3 H), 1.28 (s, 9 H), 1.66 (s, 3 H), 1.74–1.81 (m, 2 H), 1.83–1.89 (m, 1 H), 1.87 (s, 3 H), 2.31 (m, 2 H), 2.37 (s, 3 H), 2.53 (m, 1 H), 2.59 (d, J = 3.5 Hz, 1 H), 3.55 (bs, 1 H), 3.79 (d, J = 7.5 Hz, 1 H), 3.88 (s, 3 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.26 (d, J = 2.5 Hz, 1 H), 4.34 (d, J = 8.5 Hz, 1 H), 4.40 (m, 1 H), 4.57 (dd, J = 10.0 Hz, 24.5 Hz, 1 H), 4.86 (t, J = 8.5 Hz, 1 H), 4.96 (m, 2 H), 5.65 (d, J = 7.0 Hz, 1 H), 6.23 (t, J = 9.0 Hz, 1 H), 6.28 (s, 1 H), 7.13 (dd, J = 2.0 Hz, 8.0 Hz, 1 H), 7.38 (t, J = 7.5 Hz, 1 H), 7.63 (s, 1 H), 7.79 (d, J = 7.5 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.2, 9.4, 9.5, 13.0, 14.8, 22.0, 22.3, 22.4 26.7, 28.1, 35.5, 43.2, 45.6, 55.3, 58.5, 72.1, 72.6, 73.1, 75.1, 75.3, 76.4, 77.2, 79.0, 80.4, 81.0, 84.4, 114.0, 120.6, 122.7, 129.7, 130.3, 133.2, 142.1, 154.8, 156.4, 159.7, 166.9, 170.2, 172.4, 175.1, 203.8; 19F NMR, (CDCl3, 282 MHz) δ −84.62 (dd, J = 25.7 Hz, 36.7 Hz, 1 F), −86.29 (d, J = 36.4 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C44H55F2NO16 H+, 892.3562; Found, 892.3599.
3.4.8. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-methoxybenzoyl)-10-propanoyldocetaxel (6c-1)
Yield 58% for 2 steps; white solid; [α]D20 −79.78 (c 3.66, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.15 (s, 3 H), 1.22–1.25 (m, 6 H), 1.30 (s, 9 H), 1.67 (s, 3 H), 1.71 (s, 1 H), 1.79 (s, 1 H), 1.86–1.91 (m, 4 H), 2.32 (m, 2 H), 2.38 (s, 3 H), 2.38–2.59 (m, 3 H), 3.52 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 3.89 (s, 3 H), 4.18 (d, J = 8.5 Hz, 1 H), 4.27 (bs, 1 H), 4.35 (d, J = 8.5 Hz, 1 H), 4.40 (m, 2 H), 4.58 (ddd, J = 1.5 Hz, 10.0 Hz, 25.0 Hz, 1 H), 4.87 (t, J = 9.0 Hz, 1 H), 4.96 (m, 2 H), 5.66 (d, J = 6.5 Hz, 1 H), 6.24 (t, J = 9.0 Hz, 1 H), 6.31 (s, 1 H), 7.15 (dd, J = 2.5 Hz, 8.5 Hz, 1 H), 7.39 (t, J = 7.5 Hz, 1 H), 7.65 (s, 1 H), 7.71 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.0, 9.5, 14.8, 21.9, 22.3, 26.7, 27.5, 28.1, 35.4, 43.2, 45.6, 55.3, 58.3, 72.1, 72.6, 73.1, 75.1, 75.3, 76.4, 77.2, 79.0, 81.1, 84.4, 114.0, 122.7, 129.7, 130.3, 133.2, 141.9, 154.8, 159.7, 166.9, 170.2, 174.6, 203.7; 19F NMR, (CDCl3, 282 MHz) δ −84.58 (dd, J = 25.7 Hz, 36.7 Hz, 1 F), −86.26 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C43H55F2NO16 H+, 880.3562; Found 880.3578.
3.4.9. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-methoxybenzoyl)-10-dimethylcarbamoyldocetaxel (6d-1)
Yield 74% for 2 steps; white solid; 1H NMR (CDCl3, 500 MHz) δ 1.15 (s, 3H), 1.25 (s, 3 H), 1.29 (s, 9 H), 1.67 (s, 3 H), 1.76 (bs, 1 H), 1.88 (m, 1 H), 1.89 (s, 3 H), 2.31 (m, 2 H), 2.38 (s, 3 H), 2.53 (m, 1 H), 2.96 (s, 3 H), 3.04 (s, 3H), 3.52 (bs, 1 H), 3.81 (d, J = 7.0 Hz, 1 H), 3.90 (s, 3 H), 4.17 (d, J = 8.0 Hz, 1 H), 4.27 (s, 1 H), 4.35 (d, J = 8.0 Hz, 1 H), 4.45 (dd, J = 6.0 Hz, 10.5 Hz, 1 H), 4.58 (ddd, J = 1.5 Hz, 10.0 Hz, 25.0 Hz, 1 H), 4.87 (t, J = 8.5 Hz, 1 H), 4.97 (m, 2 H), 5.65 (d, J = 7.0 Hz, 1 H), 6.25 (m, 2 H), 7.14 (dd, J = 1.5 Hz, 7.5 Hz, 1 H), 7.39 (t, J = 8.0 Hz, 1 H), 7.65 (s, 1 H), 7.71 (d, J = 7.5 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.3, 14.9, 22.3, 26.9, 28.1, 35.4, 36.0, 36.6, 43.2, 45.5, 55.3, 58.5, 72.4, 72.7, 73.1, 75.2, 76.1, 76.4, 79.2, 80.4, 81.2, 84.6, 114.0. 120.7, 122.7, 129.7, 130.3, 133.5, 133.7, 142.4, 154.8, 156.1, 159.7, 166.9, 170.1, 205.6; 19F NMR, (CDCl3, 282 MHz) δ −84.60 (dd, J = 23.9 Hz, 34.9 Hz, 1 F), −86.30 (d, J = 36.4 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C43H56F2N2O16 H+, 895.3671; Found 895.3676.
3.4.10. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-methoxybenzoyl)-10-methoxycarbonyldocetaxel (6e-1)
Yield 89% for 2 steps; white solid; [α]D20 −68.98 (c 4.61, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.15 (s, 3 H), 1.24 (m, 3 H), 1.29 (s, 9 H), 1.69 (s, 3 H), 1.78 (bs, 1 H), 1.88 (m, 1 H), 1.91 (s, 3 H), 2.32 (m, 2 H), 2.38 (s, 3 H), 2.49 (d, J = 4.5 Hz, 1 H), 2.56 (m, 1 H), 3.53 (bs, 1 H), 3.78 (d, J = 6.5 Hz, 1 H), 3.87 (s, 3 H), 3.89 (s, 3 H), 4.18 (d, J = 8.5 Hz, 1 H), 4.27 (d, J = 3.0 Hz, 1 H), 4.36 (d, J = 8.5 Hz, 1 H), 4.41 (m, 1 H), 4.59 (dd, J = 9.5 Hz, 24.5 Hz, 1 H), 4.86 (t, J = 9.0 Hz, 1 H), 4.95 (m, 2 H), 5.66 (d, J = 7.5 Hz, 1 H), 6.12 (s, 1 H), 6.24 (t, J = 8.5 Hz, 1 H), 7.15 (d, J = 2.0 Hz, 7.5 Hz, 1 H), 7.39 (t, J = 8.0 Hz, 1 H), 7.64 (s, 1 H), 7.71 (d, J = 7.0 Hz, 1 H); 13C NMR (CDCl3, 100 MHz) δ 9.5, 14.9, 21.8, 22.3, 26.6, 28.1, 35.3, 43.1, 45.6, 55.6, 55.6, 58.5, 72.0, 72.6, 73.1, 75.0, 77.2, 78.2, 79.0, 80.5, 81.1, 84.4, 114.0, 120.7, 129.7, 130.3, 132.8, 142.9, 154.8, 155.7, 159.7, 166.9, 170.3, 203.9; 19F NMR, (CDCl3, 282 MHz) δ −84.60 (dd, J = 25.7 Hz, 36.7 Hz, 1 F), −86.27 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H53F2NO17 H+, 882.3354; Found 882.3353.
3.4.11. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-fluorobenzoyl)-10-acetyldocetaxel (6a-2)
Yield 72% for 2 steps; white solid; mp 165–168 °C; [α]D20 −73.29 (c 7.0, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.15 (s, 3H), 1.26 (s, 3 H), 1.31 (s, 9 H), 1.68 (m, 4 H), 1.89 (m, 4 H), 2.24 (s, 3 H), 2.33 (m, 2 H), 2.39 (s, 3 H), 2.47 (bs, 1 H), 2.56 (m, 1 H), 3.46 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.28 (s, 1 H), 4.31 (d, J = 8.5 Hz, 1 H), 4.42 (dd, J = 6.5 Hz, 11.0 Hz, 1 H), 4.58 (ddd, J = 1.0 Hz, 9.0 Hz, 26.0 Hz, 1 H), 4.90 (m, 2H), 4.97 (dd, J = 2.0 Hz, 9.0 Hz, 1 H), 5.64 (d, J = 7.0 Hz, 1 H), 6.23 (t, J = 8.0 Hz, 1 H), 6.30 (s, 1 H), 7.31 (dt, J = 2.0 Hz, 8.0 Hz, 1 H), 7.49 (ddd, J = 6.0 Hz, 8.5 Hz, 13.5 Hz, 1 H), 7.80 (d, J = 9.0 Hz, 1 H), 7.91 (d, J = 7.5 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.5, 14.8, 20.8, 21.8, 22.2, 26.7, 28.1, 35.5, 43.2, 45.6, 58.5, 72.1, 72.5, 73.1, 75.5, 76.3, 79.1, 80.4, 80.9, 84.4, 117.0, 120.8, 125.9, 130.4, 131.2, 132.9, 142.2, 154.9, 156.7, 160.9, 164.2, 165.9, 170.2, 171.2, 203.5; 19F NMR, (CDCl3, 282 MHz) δ −84.23 (dd, J = 25.7 Hz, 34.7 Hz, 1 F), −86.21 (d, J = 36.7 Hz, 1 F), −111.7 (dd, J = 9.3, 14.6 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H50F3NO15 H+, 854.3205; Found, 854.3207.
3.4.12. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-fluorobenzoyl)-10-cyclopropane-carbonyldocetaxel (6b-2)
Yield 78% for 2 steps; white solid; mp 157–161 °C; [α]D20 −77.04(c 7.1, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 0.99 (m, 2 H), 1.12 (m, 2 H), 1.15 (s, 3 H), 1.26 (s, 3 H), 1.30 (s, 9 H), 1.66 (s, 3 H), 1.77(m, 2 H), 1.86 (m, 1 H), 1.87 (s, 3 H), 2.33 (m, 2 H), 2.39 (s, 3 H), 2.53 (m, 1 H), 2.61 (bs, 1 H), 3.55 (bs, 1 H), 3.80 (d, J = 7.0 Hz, 1 H), 4.17 (d, J = 8.0 Hz, 1 H), 4.28 (s, 1 H), 4.29 (d, J = 8.0 Hz, 1 H), 4.40 (dd, J = 7.0 Hz, 10.5 Hz, 1 H), 4.58 (dd, J = 8.5 Hz, 23.5 Hz, 1 H), 4.86 (m, 1 H), 4.97 (m, 2 H), 5.63 (d, J = 7.0 Hz, 1 H), 6.24 (t, J = 9.5 Hz, 1 H), 6.29 (s, 1 H), 7.49 (dt, J = 2.5 Hz, 8.5 Hz, 1 H), 7.60 (ddd, J = 6.0 Hz, 8.0 Hz, 13.5 Hz, 1 H), 7.78 (d, J = 9.5 Hz, 1 H), 7.90 (d, J = 7.5 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.2, 9.5, 13.0, 14.7, 21.9, 22.2, 26.7, 28.1, 35.5, 43.2, 45.6, 48.0, 58.5, 72.1, 72.5, 73.1, 75.3, 75.5, 76.3, 79.1, 80.4, 80.9, 84.4, 117.0, 120.8, 125.9, 130.4, 131.3, 133.0, 142.2, 154.8, 160.9, 164.2, 165.8, 170.2, 175.1, 203.7; 19F NMR, (CDCl3, 282 MHz) δ −84.22 (dd, J = 23.7 Hz, 36.7 Hz, 1 F), −86.20 (d, J = 34.7 Hz, 1 F), −111.72 (dd, J = 9.3 Hz, 14.6 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C43H52F3NO15 H+, 880.3362; Found, 880.3346.
3.4.13. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-fluorobenzoyl)-10-propanoyldocetaxel (6c-2)
Yield 71% for 2 steps; white solid; mp 161–165 °C; [α]D20 −71.57(c 8.3, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.14 (s, 3 H), 1.24 (m, 6 H,), 1.31 (s, 9 H), 1.67 (s, 3 H), 1.72 (bs, 1 H), 1.88 (m, 4 H), 2.32 (m, 2 H), 2.39 (s, 3 H), 2.53 (m, 3 H), 3.51 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.28 (d, J = 1.5 Hz, 1 H), 4.30 (d, J = 8.5 Hz, 1 H), 4.42 (dd, J = 6.5 Hz, 10.5 Hz, 1 H), 4.58 (ddd, J = 1.5 Hz, 9.0 Hz, 24.5 Hz, 1 H), 4.87 (m, 1 H), 4.96 (m, 2 H), 5.64 (d, J = 6.5 Hz, 1 H), 6.23 (t, J = 8.0 Hz, 1 H), 6.31 (s, 1 H), 7.31 (dt, J = 2.0 Hz, 7.0 Hz, 1 H), 7.48 (ddd, J = 5.5 Hz, 7.5 Hz, 13.0 Hz, 1 H), 7.80 (d, J = 8.5 Hz, 1 H), 7.91 (d, J = 7.5 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 8.9, 9.5, 14.8, 21.9, 22.2, 26.7, 27.5, 28.1, 35.5, 43.2, 45.6, 58.5, 72.1, 72.5, 73.1, 75.3, 75.4, 76.3, 79.1, 80.9, 84.4, 116.9, 120.8, 125.9, 130.4, 131.3, 133.0, 142.1, 154.6, 160.9, 164.2, 165.8, 170.2, 174.6, 203.6; 19F NMR, (CDCl3, 282 MHz) δ −84.24 (dd, J = 23.9 Hz, 34.9 Hz, 1 F), −86.22 (d, J = 36.4 Hz, 1 F); −111.72 (dd, J = 9.3 Hz, 14.6 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H52F3NO15 H+, 868.3362; Found 868.3352.
3.4.14. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-fluorobenzoyl)-10-dimethyl-carbamoyldocetaxel (6d-2)
Yield 71% for 2 steps; white solid; [α]D20 −85.33 (c 1.5, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.16 (s, 3 H), 1.26 (s, 3 H), 1.31 (s, 9 H), 1.60 (bs, 1 H), 1.67 (s, 3 H), 1.84 (m, 1 H), 1.89 (m, 1 H), 1.91 (s, 3 H), 2.33 (m, 2 H), 2.40 (s, 3 H), 2.55 (m, 1 H), 2.97 (s, 3 H), 3.05 (s, 3 H), 3.40 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 4.17 (d, J = 7.5 Hz, 1 H), 4.29 (s, 1 H), 4.31 (d, J = 7.5 Hz, 1 H), 4.45 (dd, J = 6.0 H, 10.5 Hz, 1 H), 4.58 (ddd, J = 1.5 Hz, 9.0 Hz, 24.5 Hz, 1 H), 4.87 (m, 2 H), 4.99 (d, J = 8.0 Hz, 1 H), 5.64 (d, J = 7.0 Hz, 1 H), 6.26 (m, 2 H), 7.32 (dt, J = 2.0 Hz, 8.0 Hz, 1 H), 7.49 (ddd, J = 6.0 Hz, 8.5 Hz, 13.5 Hz, 1 H), 7.81 (d, J = 9.5 Hz, 1 H), 7.92 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.3, 14.7, 22.3, 26.9, 28.1, 35.4, 36.0, 36.6, 43.2, 45.5, 58.5, 72.4, 72.7, 73.1, 75.6, 76.1, 76.4, 77.2, 79.3, 80.4, 81.1, 84.6, 110.7, 120.8, 125.9, 130.4, 131.3, 133.9, 142.7, 155.1, 157.4, 161.2, 164.1, 166.1, 170.2, 172.3, 205.5; 19F NMR, (CDCl3, 282 MHz) δ −84.13 (dd, J = 25.4 Hz, 36.4 Hz, 1 F), −86.13 (d, J = 36.6 Hz, 1 F), −111.73 (dd, J = 9.3 Hz, 14.6 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H53F3N2O15 H+, 883.3471; Found 883.3433.
3.4.15. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-fluorobenzoyl)-10-methoxycarbonyldocetaxel (6e-2)
Yield 72 % for 2 steps; white solid; mp 157–160 °C; [α]D20 −70.31 (c 6.5, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.15 (s, 3 H), 1.25 (s, 3 H), 1.29 (s, 9 H), 1.65 (bs, 1 H), 1.69 (s, 3 H), 1.88 (m, 1 H), 1.92 (s, 3 H), 2.31 (m, 2 H), 2.39 (s, 3 H), 2.46 (bs, 1 H), 2.57 (m, 1 H), 3.48 (bs, 1 H), 3.79 (d, J = 7.5 Hz, 1 H), 3.87 (s, 3 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.28 (s, 1 H), 4.31 (d, J = 8.5 Hz, 1 H), 4.39 (dd, J = 7.0 Hz, 10.5 Hz, 1 H), 4.58 (dd, J = 9.0 Hz, 24.5 Hz, 1 H), 4.87–4.94 (m, 2H), 4.97 (d, J = 8.0 Hz, 1 H), 5.65 (d, J = 7.0 Hz, 1 H), 6.12 (s, 1 H), 6.23 (t, J = 9.0 Hz, 1 H), 7.31 (dt, J = 2.5 Hz, 8.5 Hz, 1 H), 7.49 (ddd, J = 5.0 Hz, 7.5 Hz, 13.5 Hz, 1 H), 7.79 (d, J = 8.5 Hz, 1 H), 7.91 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.4, 14.9, 21.8, 22.2, 26.6, 28.1, 35.3, 35.6, 43.1, 45.6, 47.9, 55.6, 58.5, 72.1, 72.5, 73.1, 75.4, 76.3, 78.2, 79.2, 80.5, 81.0, 84.6, 117.1, 120.8, 126.0, 130.4, 131.3, 132.7, 143.1, 154.9, 155.7, 160.9, 164.2, 165.9, 170.3, 203.8; 19F NMR, (CDCl3, 282 MHz) δ −84.15 (dd, J = 25.4 Hz, 36.4 Hz, 1 F), −86.17 (d, J = 36.7 Hz, 1 F), −111.71 (dd, J = 9.3 Hz, 14.6 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H50F3NO16 H+, 870.3154; Found 870.3146.
3.4.16. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-azidobenzoyl)-10-acetyl-docetaxel (6a-3)
Yield 49% for 2 steps; white solid; [α]D20 −70.59 (c 3.23, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.15 (s, 3 H), 1.26 (s, 3 H), 1.30 (s, 9 H), 1.67 (s, 3 H), 1.69 (bs, 1 H), 1.88 (m, 4 H), 2.24 (s, 3 H), 2.32 (m, 2 H), 2.39 (s, 3 H), 2.49 (bs, 1 H), 2.56 (m, 1 H), 3.48 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.26 (s, 1 H), 4.33 (d, J = 8.5 Hz, 1 H), 4.42 (dd, J = 6.5 Hz, 10.5 Hz, 1 H), 4.57 (ddd, J = 1.5 Hz, 9.5 Hz, 25.0 Hz, 1 H), 4.85 (t, J = 8.5 Hz, 1 H), 4.93 (d, J = 9.5 Hz, 1 H), 4.98 (d, J = 7.5, 1 H), 5.66 (d, J = 7.5 Hz, 1 H), 6.22 (t, J = 9.0 Hz, 1 H), 6.29 (s, 1 H), 7.23 (dd, J = 1.5 Hz, 8.0 Hz, 1 H), 7.48 (t, J = 8.0 Hz, 1 H), 7.81 (s, 1 H), 7.89 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 100 MHz) δ 9.5, 14.9, 20.8, 21.9, 22.4, 26.7, 28.1, 35.5, 43.2, 45.6, 58.5, 72.1, 72.6, 73.1, 75.4, 75.5, 76.4, 79.1, 80.4, 81.0, 84.4, 120.1, 124.4, 126.7, 130.2, 133.0, 140.9, 142.2, 154.8, 166.1, 170.3, 171.2, 203.6; 19F NMR, (CDCl3, 282 MHz) δ −83.99 (dd, J = 25.7 Hz, 36.7 Hz, 1 F), −86.12 (d, J = 35.0 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H50F2N4O15 H+, 877.3314; Found, 877.3351.
3.4.17. 3′-Dephenyl-3′-(2,2-difluoroethenyl)--2-debenzoyl-2-(3-azidobenzoyl)-10-cyclopropanecarbonyldocetaxel (6b-3)
Yield 78% for 2 steps; white solid; [α]D20 −67.39 (c 5.09, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.02 (m, 2 H), 1.15 (m, 2 H), 1.17 (s, 3 H), 1.28 (m, 3 H), 1.32 (s, 9 H), 1.68 (s, 3 H), 1.76 (bs, 1 H), 1.79 (m, 1 H), 1.86–1.91 (m, 1 H), 1.90 (s, 3 H), 2.34 (m, 2 H), 2.41 (s, 3 H), 2.56 (m, 1 H), 2.62 (bs, 1H), 3.54 (d, J = 5.5 Hz, 1 H), 3.83 (d, J = 7.5 Hz, 1 H), 4.18 (d, J = 8.5 Hz, 1 H), 4.28 (d, J = 3.0 Hz, 1 H), 4.35 (d, J = 8.5 Hz, 1 H), 4.43 (dd, J = 6.5 Hz, 10.0 Hz, 1 H), 4.58 (ddd, J = 1.5 Hz, 9.0 Hz, 24.5 Hz, 1 H), 4.87 (t, J = 8.5 Hz, 1 H), 4.98 (m, 2 H), 5.67 (d, J = 7.0 Hz, 1 H), 6.24 (t, J = 8.0 Hz, 1 H), 6.31 (s, 1 H), 7.25 (dd, J = 1.5 Hz, 8.0 Hz, 1 H), 7.49 (t, J = 8.0 Hz, 1 H), 7.82 (s, 1 H), 7.90 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 100 MHz) δ 9.2, 9.4, 9.5, 13.0, 14.9, 22.0, 22.3, 26.7, 28.1, 35.5, 43.2, 45.6, 58.5, 72.1, 72.6, 73.1, 75.3, 75.5, 76.4, 79.1, 80.4, 81.0, 84.5, 120.1, 124.4, 126.7, 130.2, 130.8, 133.1, 140.9, 142.3, 154.8, 166.1, 170.3, 175.1, 203.7; 19F NMR, (CDCl3, 282 MHz) δ −84.02 (dd, J = 25.7 Hz, 34.9 Hz, 1 F), −86.14 (d, J = 34.9 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C43H52F2N4O15 H+, 903.3470; Found, 903.3469.
3.4.18.3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-azidobenzoyl)-10-propanoyldocetaxel (6c-3)
Yield 93%; white solid; [α]D20 −67.77 (c 3.32, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.14 (s, 3 H), 1.22–1.25 (m, 6 H), 1.30 (s, 9 H), 1.67 (s, 3 H), 1.71 (s, 1 H), 1.85–1.91 (m, 4 H), 2.32 (m, 2 H), 2.39 (s, 3 H), 2.47–2.59 (m, 3 H), 3.48 (d, J = 4.5 Hz, 1 H), 3.82 (d, J = 7.5 Hz, 1 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.26 (bs, 1H), 4.33 (d, J = 8.5 Hz, 1 H), 4.43 (dd, J = 7.0 Hz, 11.0 Hz, 1 H), 4.56 (ddd, J = 1.5 Hz, 9.0 Hz, 24.5 Hz, 1 H), 4.86 (t, J = 8.0 Hz, 1 H), 4.92 (d, J = 9.5 Hz, 1 H), 4.98 (d, J = 8.0 Hz, 1 H), 5.66 (d, J = 7.5 Hz, 1 H), 6.22 (t, J = 8.0 Hz, 1 H), 6.31 (s, 1 H), 7.23 (dd, J = 1.5 Hz, 7.5 Hz, 1 H), 7.48 (t, J = 7.5 Hz, 1 H), 7.81 (s, 1 H), 7.89 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.0, 9.5, 14.9, 21.7, 22.4, 26.7, 27.5, 28.1, 35.5, 43.2, 45.6, 58.5, 72.1, 72.6, 73.1, 75.1, 75.3, 75.4, 76.4, 79.1, 81.0, 84.5, 120.1, 124.4, 126.7, 130.2, 130.8, 133.2, 140.9, 154.8, 159.7, 166.1, 170.3, 174.6, 203.7; 19F NMR, (CDCl3, 282 MHz) δ −83.99 (dd, J = 25.7 Hz, 34.7 Hz, 1 F), −86.12 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H52F2N4O15 H+, 891.3470; Found 891.3473.
3.4.19. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-azidobenzoyl)-10-dimethylcarbamoyldocetaxel (6d-3)
Yield 79% for 2 steps; white solid; [α]D20 −75.39 (c 4.51, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.15 (s, 3 H), 1.25 (s, 3 H), 1.30 (s, 9 H), 1.66 (s, 3 H), 1.75 (bs, 1 H), 1.87 (m, 1 H), 1.90 (s, 3 H), 2.31 (m, 2 H), 2.39 (s, 3 H), 2.54 (ddd, J = 7.0 Hz, 10.0 Hz, 15.5 Hz, 1 H), 2.96 (s, 3 H), 3.04 (s, 3 H), 3.23 (bs, 1 H), 3.56 (bs, 1 H), 3.81 (d, J = 7.0 Hz, 1 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.27 (s, 1 H), 4.32 (d, J = 8.5 Hz, 1 H), 4.45 (dd, J = 6.5 Hz, 10.5 Hz, 1 H), 4.57 (ddd, J = 1.0 Hz, 9.0 Hz, 24.0 Hz, 1 H), 4.85 (t, J = 8.0 Hz, 1 H), 4.97 (m, 2 H), 5.65 (d, J = 7.0 Hz, 1 H), 6.23 (t, J = 9.5 Hz, 1 H), 6.25 (s, 1 H), 7.23 (dd, J = 1.5 Hz, 8.5 Hz, 1 H), 7.47 (t, J = 8.0 Hz, 1 H), 7.80 (s, 1 H), 7.89 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.3, 14.9, 22.3, 26.9, 28.1, 35.4, 36.0, 36.6, 43.2, 58.5, 72.4, 72.6, 73.1, 75.6, 76.1, 76.3, 79.2, 80.1, 84.7, 120.7, 120.2, 124.3, 126.7, 130.2, 130.9, 133.4, 140.9, 142.6, 154.9, 156.1, 166.1, 170.3, 205.5; 19F NMR, (CDCl3, 282 MHz) δ −84.06 (dd, J = 25.7 Hz, 34.7 Hz, 1 F), −86.17 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H53F2N5O15 H+, 906.3579; Found 906.3588.
3.4.20. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-azidobenzoyl)-10-methoxycarbonyldocetaxel (6e-3)
Yield 77% for 2 steps; white solid; [α]D20 −66.67 (c 3.9, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.15 (s, 3 H), 1.24 (s, 3 H), 1.29 (s, 9 H), 1.69 (s, 3 H), 1.71 (bs, 1 H), 1.89 (m, 1 H), 1.92 (s, 3 H), 2.32 (m, 2 H), 2.39 (s, 3 H), 2.49 (bs, 1 H), 2.56 (m, 1 H), 3.51 (bs, 1 H), 3.79 (d, J = 7.0 Hz, 1 H), 3.87 (s, 3 H), 4.17 (d, J = 8.5 Hz, 1 H), 4.26 (s, 1 H), 4.33 (d, J = 8.5 Hz, 1 H), 4.40 (dd, J = 7.0 Hz, 11.0 Hz, 1 H), 4.57 (ddd, J = 1.0 Hz, 9.0 Hz, 24.5 Hz, 1 H), 4.85 (t, J = 8.5 Hz, 1 H), 4.96 (m, 2 H), 5.66 (d, J = 7.5 Hz, 1 H), 6.12 (s, 1 H), 6.22 (t, J = 9.0 Hz, 1 H), 7.23 (dd, J = 1.5 Hz, 6.5 Hz, 1 H), 7.48 (t, J = 7.5 Hz, 1 H), 7.81 (s, 1 H), 7.89 (d, J = 8.0 Hz, 1 H); 13C NMR (CDCl3, 100 MHz) δ 9.5, 14.9, 21.8, 22.4, 26.6, 28.1, 35.4, 43.1, 45.6, 55.6, 55.6, 58.5, 72.0, 72.5, 73.1, 75.4, 76.3, 78.2, 79.1, 80.4, 81.0, 84.3, 120.1, 124.4, 126.7, 130.2, 130.8, 132.7, 140.9, 143.0, 154.7, 155.7, 166.1, 170.4, 203.8; 19F NMR, (CDCl3, 282 MHz) δ −84.01 (dd, J = 25.7 Hz, 36.7 Hz, 1 F,), −86.13 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H50F2N4O16 H+, 893.3263; Found 893.3269.
3.4.21. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-chlorobenzoyl)-10-acetyl-docetaxel (6a-4)
Yield 57% for 2 steps; white solid; mp 170–175 °C; [α]D20 −72.93 (c 4.1, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 1.14 (s, 3 H), 1.25 (s, 3 H), 1.31 (s, 9 H), 1.67 (s, 3 H), 1.73 (s, 1 H), 1.89 (m, 4 H), 2.24 (s, 3 H), 2.33 (m, 2 H), 2.39 (s, 3 H), 2.51 (bs, 1 H), 2.55 (m, 1 H), 3.51 (bs, 1 H), 3.81 (d, J = 7.5 Hz, 1 H), 4.15 (d, J = 8.5 Hz, 1 H), 4.29 (m, 2 H), 4.41 (dd, J = 6.5 Hz, 11.0 Hz, 1 H), 4.58 (ddd, J = 1.0 Hz, 10.0 Hz, 24.5 Hz, 1 H), 4.86 (m, 1 H), 4.93 (d, J = 9.0 Hz, 1 H), 4.98 (d, J = 7.5, 1 H), 5.62 (d, J = 7.5 Hz, 1 H), 6.21 (t, J = 9.5 Hz, 1 H), 6.30 (s, 1 H), 7.45 (t, J = 7.5 Hz, 1 H), 7.58 (dd, J = 1.5 Hz, 8.5 Hz, 1 H), 8.00 (d, J = 7.5 Hz, 1 H), 8.12 (s, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.5, 14.8, 20.8, 21.8, 22.1, 26.7, 28.1, 35.4, 43.1, 45.6, 58.5, 72.1, 72.5, 73.1, 75.5, 76.3, 79.2, 80.4, 80.9, 84.3, 128.2, 130.0, 130.3, 130.9, 132.9, 134.8, 142.2, 154.8, 165.7, 170.2, 171.2, 203.5; 19F NMR, (CDCl3, 282 MHz) δ −84.15 (dd, J = 25.7 Hz, 36.7Hz, 1 F), −86.12 (dd, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H50ClF2NO15 H+, 870.2910; Found, 870.2891.
3.4.22. 3′-Dephenyl-3′-(2,2-difluorovinyl)-2-debenzoyl-2-(3-chlorobenzoyl)-10-cyclopropanecarbonyldocetaxel (6b-4)
Yield 73% for 2 steps; white solid; mp 161–166 °C; [α]D20 −78.97(c 5.8, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.02 (m, 2 H), 1.15 (m, 2 H), 1.16 (s 3 H), 1.28 (m, 3 H), 1.32 (s, 9 H), 1.68 (s, 3 H), 1.70 (bs, 1 H), 1.80 (m, 1 H,), 1.86–1.89 (m, 4 H), 2.33 (m, 2 H), 2.41 (s, 3 H), 2.56–2.59 (m, 2H), 3.51 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 4.16 (d, J = 8.5 Hz, 1 H), 4.29 (s, 1 H), 4.31 (d, J = 8.5 Hz, 1 H), 4.41 (dd, J = 7.0 Hz, 10.5 Hz, 1 H), 4.59 (dd, J = 9.0 Hz, 25.0 Hz, 1 H), 4.89 (m, 1 H), 4.98 (m, 2 H), 5.63 (d, J = 7.0 Hz, 1 H), 6.24 (t, J = 9.0 Hz, 1 H), 6.31 (s, 1 H), 7.46 (t, J = 8.5 Hz, 1 H), 7.60 (dd, J = 1.0 Hz, 7.0 Hz, 1 H), 8.01 (d, J = 7.5 Hz, 1 H), 8.13 (s, 1 H); 13C NMR (CDCl3, 100 MHz) δ 9.2, 9.4, 13.0, 14.9, 21.9, 22.2, 26.7, 28.1, 35.4, 43.2, 45.6, 48.0, 58.5, 72.1, 72.6, 73.1, 75.3, 75.5, 76.3, 77.2, 79.2, 80.4, 81.0, 84.4, 128.3, 130.0, 130.3, 130.9, 133.0, 133.7, 134.8, 142.3, 154.8, 165.7, 170.2, 175.1, 203.7; 19F NMR, (CDCl3, 282 MHz) δ −84.15 (dd, J = 25.7 Hz, 34.7 Hz, 1 F), −86.16 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C43H52ClF2NO15 H+, 896.3066; Found, 896.3036.
3.4.23. 3′-Dephenyl-3′-(2,2-difluorovinyl)-2-debenzoyl-2-(3-chlorobenzoyl)-10-propanoyldocetaxel (6c-4)
Yield 72% for 2 steps; white solid; mp 154–158 °C; [α]D20 −76.73(c 4.9, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 1.14 (s, 3 H), 1.22–1.25 (m, 6 H), 1.31 (s, 9 H), 1.67 (s, 3 H), 1.71 (s, 1 H), 1.86–1.91 (m, 4 H), 2.32 (m, 2 H), 2.39 (s, 3 H), 2.40–2.59 (m, 3 H), 3.49 (bs, 1 H), 3.82 (d, J = 7.0 Hz, 1 H), 4.15 (d, J = 8.5 Hz, 1 H), 4.29 (m, 2 H), 4.40 (dd, J = 6.0 Hz, 10.5 Hz, 1 H), 4.58 (ddd, J = 1.5 Hz, 9.0 Hz, 24.5 Hz, 1 H), 4.87 (m, 1 H), 4.92 (d, J = 9.0 Hz, 1 H), 4.98 (dd, J = 1.5 Hz, 9.5 Hz, 1 H), 5.62 (d, J = 7.5 Hz, 1 H), 6.22 (t, J = 9.5 Hz, 1 H), 6.31 (s, 1 H), 7.45 (t, J = 8.0 Hz, 1 H), 7.56 (dd, J = 1.0 Hz, 8.0 Hz, 1 H), 8.00 (d, J = 7.5 Hz, 1 H), 8.12 (s, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 8.9, 9.5, 14.9, 21.8, 22.2, 26.7, 27.5, 28.1, 35.5, 43.2, 45.7, 58.5, 72.2, 72.6, 73.1, 75.3, 75.5, 76.3, 79.2, 80.4, 81.0, 84.4, 128.3, 130.1, 130.3, 130.9, 133.1, 133.7, 134.8, 142.1, 154.8, 165.7, 170.2, 174.6, 203.6; 19F NMR, (CDCl3, 282 MHz) δ −84.14 (dd, J = 23.7 Hz, 34.7 Hz, 1 F), −86.13 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H52ClF2NO15 H+, 884.3066; Found 884.3057.
3.4.24. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-chlorobenzoyl)-10-dimethylcarbamoyldocetaxel (6d-4)
Yield 91% for 2 steps; white solid; mp 170–173 °C; [α]D20 −88.09 (c 2.1, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.17 (s, 3 H), 1.27 (s, 3 H), 1.33 (s, 9 H), 1.62 (bs, 1 H), 1.69 (s, 3 H), 1.84–1.90 (m, 2 H), 1.93 (s, 3H), 2.34 (m, 2H,), 2.42 (s, 3 H), 2.56 (m, 1 H), 2.98 (s, 3 H), 3.06 (s, 3 H), 3.25 (d, J = 2.5 Hz, 1 H), 3.52 (d, J = 5.5 Hz, 1 H), 3.83 (d, J = 7.0 Hz, 1 H), 4.18 (d, J = 8.5 Hz, 1 H), 4.29 (s, 1 H), 4.32 (d, J = 8.5 Hz, 1 H), 4.46 (dd, J = 6.5 Hz, 11.0 Hz, 1 H), 4.58 (ddd, J = 1.5 Hz, 10.0 Hz, 24.5 Hz, 1 H), 4.93 (m, 2 H), 5.01 (d, J = 10.0 Hz, 1 H), 5.63 (d, J = 7.5 Hz, 1 H), 6.25 (t, J = 9.0 Hz, 1 H), 6.27 (s, 1 H), 7.47 (t, J = 7.5 Hz 1 H), 7.62 (dd, J = 1.0 Hz, 9.5 Hz, 1 H), 8.03 (d, J = 8.0 Hz, 1 H), 8.15 (s, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.3, 14.9, 22.2, 26.9, 28.1, 35.4, 36.0, 36.6, 43.2, 45.5, 58.5, 72.4, 72.6, 73.1, 75.6, 76.1, 76.3, 79.3, 80.4, 81.1, 84.6, 128.3, 130.1, 130.4, 130.9, 133.3, 133.7, 134.8, 142.6, 154.8, 156.1, 165.7, 171.1, 205.5; 19F NMR, (CDCl3, 282 MHz) δ −84.16 (dd, J = 23.9 Hz, 34.9 Hz, 1 F), −86.17 (d, J = 36.7 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C42H53ClF2N2O15 H+, 899.3175; Found 899.3151.
3.4.25. 3′-Dephenyl-3′-(2,2-difluoroethenyl)-2-debenzoyl-2-(3-chlorobenzoyl)-10-methoxycarbonyldocetaxel (6e-4)
Yield 70% for 2 steps; white solid; mp 161–165 °C; [α]D20 −72.09 (c 4.3, CHCl3); 1H NMR (CDCl3, 500 MHz) δ 1.16 (s, 3H), 1.26 (m, 3 H), 1.32 (s, 9 H), 1.63 (bs, 1 H), 1.71 (s, 3 H), 1.91 (m, 1 H), 1.94 (s, 3 H), 2.33 (m, 2 H), 2.42 (s, 3 H), 2.46 (bs, 1 H), 2.59 (m, 1 H), 3.46 (d, J = 6.0 Hz, 1 H), 3.81 (d, J = 7.0 Hz, 1 H), 3.87 (s, 3 H), 4.17 (d, J = 8.5 Hz, 1 H), 4.29 (d, J = 5.5 Hz, 1 H), 4.32 (d, J = 8.5 Hz, 1 H), 4.39 (m, 1 H), 4.59 (dd, J = 8.0 Hz, 24.5 Hz, 1 H), 4.89 (m, 2 H), 5.00 (d, J = 8.5 Hz, 1 H), 5.64 (d, J = 7.5 Hz, 1 H), 6.13 (s, 1 H), 6.23 (t, J = 8.5 Hz, 1 H), 7.49 (t, J = 8.0 Hz, 1 H), 7.60 (d, J = 7.5 Hz, 1 H), 8.01 (d, J = 8.0 Hz, 1 H), 8.14 (s, 1 H); 13C NMR (CDCl3, 75.5 MHz) δ 9.4, 14.9, 21.7, 22.2, 26.6, 28.1, 35.3, 35.6, 43.1, 45.6, 55.6, 58.5, 72.1, 72.5, 73.2, 75.4, 76.3, 78.2, 79.2, 80.5, 80.9, 84.4, 128.3, 130.1, 130.3, 130.9, 132.6, 133.7, 134.9, 143.1, 154.9, 155.7, 165.7, 170.2, 203.8; 19F NMR, (CDCl3, 282 MHz) δ −84.16 (dd, J = 23.7 Hz, 34.7 Hz, 1 F), −86.17 (dd, J = 34.9 Hz, 1 F); HRMS (FAB+, m/z): Calcd. for C41H50ClF2NO16 H+, 886.2859; Found 886.2845.
Tubulin polymerization assay
Assembly and disassembly of calf brain microtubule protein (MTP) was monitored spectrophotometrically (Beckman Coulter DU 640, Fullerton, CA) by recording changes in turbidity at 350 nm at 37 °C [34, 35]. MTP was diluted to 1mg/mL in MES buffer containing 3 M glycerol. Tubulin stored in liquid nitrogen was centrifuged just before use and protein concentration adjusted to 1 mg/mL. The concentration of tubulin in MTP is 85% and that is taken into consideration when the ratios of tubulin to drug are presented in Figures 1. Microtubule assembly was carried out with 10 μM SB-T-12851, SB-T-12852 and SB-T-12854. Paclitaxel (10 μM) was also used for comparison purpose. Calcium chloride (6 mM) was added to the assembly reaction after 50 min to follow the calcium-induced microtubule depolymerization.
Electron microscopy
Aliquots (50 μL) were taken from in vitro polymerization assays at the end of the reaction and placed onto 300-mesh carbon-coated, formavar-treated copper grids. Samples were then stained with 20 μL of 2% uranyl acetate and viewed with a JEOL model 100CX electron microscope.
Computational methods
The structures of SB-T-12853 and SB-T-1213 in the 1JFF coordinate [36] were produced by directly changing the groups of REDOR-Taxol in the 1JFF complexes using the Builder module in the InsightII 2000 program (CVFF). Then, these structures were energy-minimized in 5000 steps or until the maximum derivative was found to be < 0.001 kcal/A by means of the conjugate gradients method using the CVFF force field and a distance-dependent dielectric. The backbone of the protein was fixed throughout the energy minimization. After the energy minimization, the snapshots were overlaid by superimposing the backbones of the proteins.
Highlights.
A series of 3′-difluorovinyl taxoids were strategically designed to block the metabolism by P-450 3A4 enzyme and synthesized.
Difluorovinyl taxoids exhibit up to 3 orders of magnitude higher potency against MDR cell line as compared to paclitaxel.
Difluorovinyl taxoids induced GTP-independent tubulin polymerization much faster than paclitaxel.
Molecular modeling study indicates that a difluorovinyl taxoid binds to β-tubulin consistent with the REDOR-Taxol structure.
Difluorovinyl group’s unique stereoelectronic property may account for the high potency of difluorovinyl taxoids.
Acknowledgments
This work has been supported by grants from the National Institutes of Health (GM42798 and CA103314 to I.O.; CA083185 and CA077263 to S.B.H.; CA 73872 to R.J.B.). Generous support from Indena SpA, is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. Wiley-Blackwell; Chichester: 2009. [Google Scholar]
- 2.Müller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 3.Begue J-P, Bonnet-Delpon D. J Fluor Chem. 2006;127:992–1012. [Google Scholar]
- 4.Isanbor C, O’Hagan D. J Fluor Chem. 2006;127:303–319. [Google Scholar]
- 5.Cottet F, Marull M, Lefebvre O, Schlosser M. Eur J Org Chem. 2003:1559–1568. [Google Scholar]
- 6.Kirk KL. J Fluorine Chem. 2006;127:1013–1029. [Google Scholar]
- 7.Yamazaki T, Taguchi T, Ojima I. Unique Properties of Fluorine and Their Relevance to Medicinal Chemistry and Chemical Biology. In: Ojima I, editor. Fluorine in Medicinal Chemistry and Chemical Biology. Wiley-Blackwell; Chichester: 2009. pp. 3–46. [Google Scholar]
- 8.Rowinsky EK. Ann Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 9.FDA. 2004 http://www.fda.gov/cder/approval/t.htm.
- 10.Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 11.Jordan MA, Toso RJ, Wilson L. Proc Natl Acad Sci. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling V. Ann N Y Acad Sci. 1987;507:7–8. doi: 10.1111/j.1749-6632.1987.tb45786.x. [DOI] [PubMed] [Google Scholar]
- 13.Chevillard S, Pouillart P, Beldjord C, Asselain B, Beuzeboc P, Magdelenat H, Vielh P. Cancer. 1996;77:292–300. doi: 10.1002/(SICI)1097-0142(19960115)77:2<292::AID-CNCR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Giannakakou P, Sackett DL, Kang Y-K, Zhan Z, Buters JTM, Fojo T, Poruchynsky MS. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 15.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 16.Ojima I, Slater JC, Michaud E, Kuduk SD, Bounaud P-Y, Vrignaud P, Bissery M-C, Veith J, Pera P, Bernacki RJ. J Med Chem. 1996;39:3889–3896. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 17.Ojima I, Chen J, Sun L, Borella CP, Wang T, Miller ML, Lin S, Geng X, Kuznetsova LV, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-St Clair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. J Med Chem. 2008;51:3202–3221. [Google Scholar]
- 18.Ojima I, Wang T, Miller ML, Lin S, Borella CP, Geng X, Pera P, Bernacki RJ. Bioorg Med Chem Lett. 1999;9:3423–3428. doi: 10.1016/s0960-894x(99)00629-0. [DOI] [PubMed] [Google Scholar]
- 19.Ojima I, Lin S, Slater JC, Wang T, Pera P, Bernacki RJ, Ferlini C, Scambia G. Bioorg Med Chem. 2000;8:1619–1628. doi: 10.1016/s0968-0896(00)00093-6. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsova L, Ungureanu IM, Pepe A, Zanardi I, Wu X, Ojima I. J Fluor Chem. 2004;125:487–500. [Google Scholar]
- 21.Kuznetsova LV, Pepe A, Ungureanu IM, Pera P, Bernacki RJ, Ojima I. J Fluor Chem. 2008;129:817–828. doi: 10.1016/j.jfluchem.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gut I, Ojima I, Vaclavikova R, Simek P, Horsky S, Linhart I, Soucek P, Kondrova E, Kuznetsova LV, Chen J. Xenobiotica. 2006;36:772–792. doi: 10.1080/00498250600829220. [DOI] [PubMed] [Google Scholar]
- 23.Vuilhorgne M, Gaillard C, Sanderlink GJ, Royer I, Monsarrat B, Dubois J, Wright M. In: ACS Symp Ser. Georg GI, Chen TT, Ojima I, Vyas DM, editors. Vol. 583. American Chemical Society; Washington, D. C: 1995. pp. 98–110. [Google Scholar]
- 24.Ojima I, Sun CM, Zucco M, Park YH, Duclos O, Kuduk SD. Tetrahedron. 1992;48:6985–7012. [Google Scholar]
- 25.Ojima I, Sun CM, Zucco M, Park YH, Duclos O, Kuduk SD. Tetrahedron Lett. 1993;34:4149–4152. [Google Scholar]
- 26.Ojima I. Acc Chem Res. 1995;28:383–389. and references cited therein. [Google Scholar]
- 27.Holton RA, Biediger RJ, Boatman PD. Semisynthesis of Taxol and Taxotere. In: Suffness M, editor. Taxol®: Science and Applications. CRC Press; New York: 1995. pp. 97–121. [Google Scholar]
- 28.Lim MH, Kim HO, Moon HR, Chun MW, Jeong LS. Org Lett. 2002;4:529–531. doi: 10.1021/ol017112v. [DOI] [PubMed] [Google Scholar]
- 29.Bhadury PS, Palit M, Sharma M, Raza SK, Jaiswal DK. J Fluor Chem. 2002;116:75–80. [Google Scholar]
- 30.Yamazaki T, Hiraoka S, Sakamoto J, Kitazume T. Org Lett. 2001;3:743–746. doi: 10.1021/ol007060u. [DOI] [PubMed] [Google Scholar]
- 31.Geney R, Sun L, Pera P, Bernacki Ralph J, Xia S, Horwitz Susan B, Simmerling Carlos L, Ojima I. Chem Biol. 2005;12:339–348. doi: 10.1016/j.chembiol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Simmerling C, Ojima I. ChemMedChem. 2009;4:719–731. doi: 10.1002/cmdc.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denis J-N, Greene AE, Guénard D, Guéritte-Voegelein F, Mangatal L, Potier PA. J Am Chem Soc. 1988;110:5917–5919. [Google Scholar]
- 34.Weisenberg RC. Science. 1972;177:1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- 35.Shelanski ML, Gaskin F, Cantor CR. Proc Nat Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe J, Li H, Downing KH, Nogales E. J Mol Bol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]