Abstract

A zinc carbenoid-initiated chain extension reaction provides access to an organometallic intermediate, which can be used to capture activated imines. Deprotection of the nitrogen and reduction provides access to racemic derivatives of β-proline. The relative stereochemistry of the β-proline can be controlled through use of different activating groups on the imine nitrogen.
Keywords: Chain Extension, Zinc Carbenoid, β-Proline, Mannich reaction
Introduction
The development of stereoselective synthetic approaches to β-amino acids and their derivatives has been the focus of significant research effort,1 of which one of the most common strategies has involved the addition of enolates to imine functionalities. Chiral enolates and chiral imines have been studied for the purpose of diastereocontrol,2 and enantioselective methods have also been investigated.3 Application of this synthetic approach to β-proline and its derivatives, however, is complicated by the cyclic nature of the skeleton. Development of a facile and stereoselective approach that uses Mannich reactions in the formation of a variety of β-proline derivatives would provide an alternative synthetic approach to these unusual β-amino acids.
Our research group has developed a zinc carbenoid-mediated chain extension reaction4 that culminates in the formation of an intermediate organometallic 2 that is capable of trapping various electrophiles, including aldehydes,5 electrophilic carbenoids,6, and halogens.7 We anticipated that activated imines would be suitable electrophilic partners for this type of tandem reaction process, and that the presence of a γ-keto functionality in the product would open the possibility for the formation of β-proline derivatives through reductive cyclization.
Results and Discussion
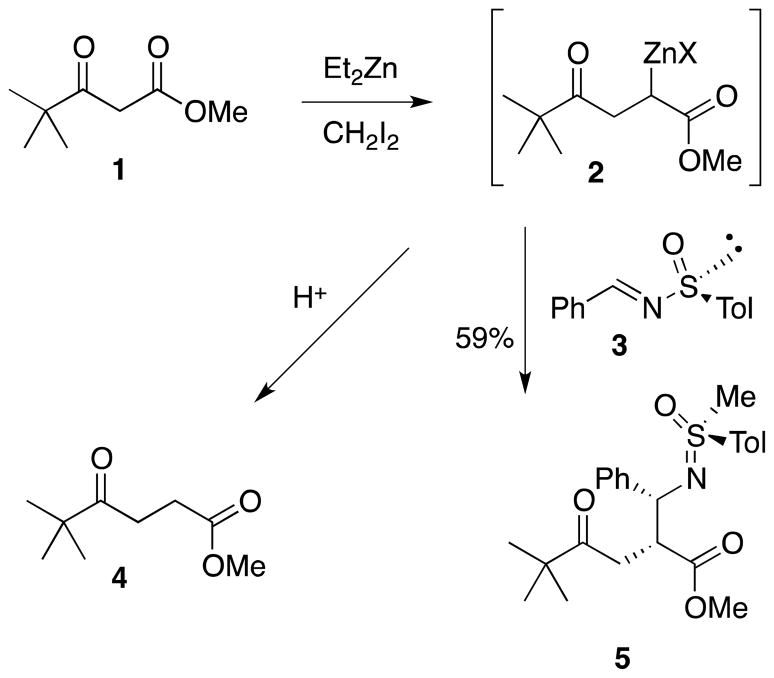
The availability of enantiopure sulfinimines8 encouraged the exploration of their utility in a tandem chain extension-imine capture reaction. Treatment of methyl pivaloylacetate (1) with three equivalents of the Furukawa reagent (ethyl(iodomethyl)zinc)9 was followed by the addition of sulfinimine 3. All efforts to trap the enolate with the sulfinimine resulted initially in the isolation of chain extended material (4) with no evidence of imine incorporation. A lengthening of the reaction time and modification of the stoichiometry to use a three-fold excess of the enolate eventually led to the formation of a product in which the imine had been trapped with the intermediate zinc enolate (Scheme 1). Surprisingly, the incorporation of an additional methyl group was revealed through NMR analysis. The N-methylation of amide functionality with the Furukawa reagent had been reported by Charette,10 and we had observed O-methylation in an earlier study of tandem chain extension-aldol reactions.5 In an effort to prevent methylation of the product, a reduction in the equivalencies of carbenoid was proposed; however, this resulted in a decreased yield of the methylated product and no formation of the non-methylated material. Based upon the assumed presence of the methylated nitrogen, cleavage of the sulfoxamines was expected to provide access to a methylamine derivative and eventually to a heterocycle. Deprotection of the sulfoxamines is reported to proceed under mildly acidic conditions,2c,11 but the starting material was returned unchanged after treatment under a wide variety of reaction conditions, including excess trifluoroacetic acid in refluxing methanol for 24 hours. Exposure of the β-amino ester to conc HCl in methanol eventually resulted in consumption of starting material, but no β-amino acid derivative was evident. The unanticipated difficulty in deprotection of the presumed sulfoxamine derivative led us to reconsider the structural assignment.
Scheme 1.
Tandem Chain Extension-Mannich reaction with Sulfinimine 3
Crystallization of the product (5) from methanol eventually provided an X-ray grade crystal that revealed S-methylation of a syn-Mannich product had taken place. Control studies revealed that the starting sulfinimine (3) was not affected by the Furukawa carbenoid, therefore S-methylation took place after nucleophilic addition to the imine. Literature reports suggest that nitrogen anions of sulfoximines are prone to N-alkylation,12 so S-methylation is atypical and may be a result of carbenoid use and/or the presence of the zinc-counter ion. In an effort to generate the targeted β-proline derivatives, imines that lack the capacity for S-nucleophilic behavior were investigated.
Diphenylphosphinoyl imine (6) was prepared via a literature method13 and added to the chain extension reaction mixture using similar reaction conditions as developed for the tandem chain extension aldol reaction. Low yields of a β-amino acid (7) were isolated, with the bulk of the material isolated from the reaction mixture being the chain extended material (4).14 Addition of an aldehyde to the imine-containing solution prior to the aqueous quench resulted in formation of an aldol product,5 which confirmed that reactive enolate was still present and suggested that the diphenylphosphinoyl imine reacts slowly with the zinc organometallic. Longer reaction times (>48 h) eventually resulted in the formation of the β-amino acid target (7) in modest yields as a mixture of two stereoisomers (Scheme 2). Chain extension aldol reactions performed on β-keto ester starting materials have typically favored formation of syn-aldol products, and the vicinal 1H NMR coupling constants between the α- and β-protons have proven to be good indicators of the diastereocontrol in the tandem chain extension aldol reactions. This NMR-based predictive tool was not viewed as reliable for these β-amino acids, so an alternate method for assessing stereoselectivity was required. Deprotection of the imine functionality provided access to a mixture of two diastereomeric cyclic imines, one derived from the syn-Mannich product and the other from the anti-Mannich product, and the imines were immediately reduced with NaCNBH315 to yield β-proline derivatives 8. These diastereomeric β-proline derivatives were formed in a ratio of 1:4:7, as determined by 1H NMR integration, when using the unpurified Mannich products as starting materials. The minor diastereomer, which is not illustrated in Scheme 2, is generated from the anti-Mannich product, while the two major diastereomers arise from the syn-Mannich product (7). The cyclic imine derived from the syn-Mannich product would be expected to undergo reduction with unremarkable facial selection due the trans relationship of the phenyl and carboxylate ester functionalities. Modest selectivity observed in the cyclization-reduction sequence resulting in a 4:7 ratio is consistent with this analysis. Even though isolation of the major diastereomeric product of this reaction sequence was possible via chromatography, assignment of the relative stereochemistry of the t-butyl substituent was not possible.
Scheme 2.
Tandem Chain Extension-Mannich Reaction with Diphenylphosphinoyl Imine 6
The long reaction times required for nucleophilic addition to the diphenylphosphinoyl imine and the modest stereoselectivity of the Mannich reaction prompted the exploration of another activated imine. Utilization of a Boc-activated imine (9) was attractive due to the reports16 of high reactivity of the imine and the formation of a Boc-protected β-amino acid as the product. The reaction between the zinc organometallic, resulting from chain extension of 1, and the Boc-activated imine was much more rapid than the phosphinoyl imine reaction. Capture of compound 9a provided access to a mixture of the desired β-amino acid derivative (10a) and the deprotected cyclic imine (11a). The premature removal of the Boc-group was not considered a major concern since the next step in the preparation of the β-proline derivative involved Boc-deprotection. The crude mixture of products was subjected to trifluoroacetic acid, which converted the remaining β-amino acid to the cyclic imine (11a). Reduction with NaCNBH3 in acidic methanol gave rise to β-proline derivative (12a) (Scheme 3).
Scheme 3.
Formation of racemic β-proline derivatives 12 through use of N-Boc imines 9
The ratio of diastereomeric β-proline derivatives, as determined by NMR analysis of the crude reaction mixture, was 9:1:2. The major isomer (12a) was identical to the minor diastereoisomer generated in the reaction of the diphenylphosphinoyl imine, while the two minor diastereomers created in the reaction sequence utilizing Boc-imine (9a) corresponded to the major products (8) of the phosphinoyl imine reaction. Since the reaction conditions for the reduction were identical to those used with the phosphinoyl imine substrate, diastereocontrol in the enolate addition to the Boc-imine was responsible for the significant differences in diastereoselectivity. A syn–Mannich product, as proposed for the phosphinoyl imine reaction, would produce a cyclic imine with trans substituents (13). Poor facial selectivity in a NaCNBH3 reduction would be expected. In contrast, reduction of the cyclic imine produced from the anti-Mannich product would be expected to be more selective due to the presence of both substituents on the same face of the heterocycle (11a) (Figure 1). This hypothesis proved to be correct when X-ray analysis of the major product (10a) indicated that the stereochemical relationship established in the Mannich reaction was, in fact, anti.
Figure 1.
Divergent Diastereocontrol based upon Imines
The tandem reaction was also studied with other aromatic imines (9b and 9c).3b β-Proline derivatives derived from p-methoxybenzaldehyde (12b) and p-tolualdehyde (12c) were produced with similar yields and selectivities. An X-ray grade crystal of 10c, derived from the p-toluene-derived Boc-imine, confirmed the anti relationship for the Mannich reaction.
The divergent stereoselectivity when using the phosphinoyl imine and the Boc-imines is a key feature of the method. Tandem chain extension aldol reactions are syn-selective as a result of a closed transition state involving a Z-enolate that is selectively generated through ketone interaction with the organometallic intermediate.5 Identical chain extension reaction conditions are utilized for enolate formation in the tandem-aldol and the tandem-Mannich reactions; therefore, the enolate intermediate generated in the presence of the imine is expected to be very similar.
Changes in facial selectivity in the reaction with the two imines through a closed transition state could account for the divergence in diastereocontrol. The anti selectivity observed in the reaction of a Z-enolate with an E-imine has been proposed to proceed through a chair-like closed transition state, which accounts for the anti selectivity observed in the Boc-imine reactions.17 In order for a closed chair-like transition state to be operative in the phosphinoyl imine reaction and result in the formation of the syn product, the imine must isomerize to a Z-imine. Computational investigation of the E-Z isomerization at the 321-G* level suggests that the energy difference between the E and Z isomers is quite small, on the order of 2 kcal/mol. Furthermore, the barrier (approx 11 kcal/mol) for imine inversion is easily accessible at the reaction temperature. The opportunity, therefore, exists for the Z-phosphinoyl imine to react with the Z enolate to provide the syn product. Alternatively, the steric size of the diphenylphosphinoyl groups18 as presented in an E-imine may encounter excessive 1,3-diaxial steric interaction when reacting with a Z-enolate. Alleviation of this steric interaction by reaction through a twist boat conformation would also account for formation of the syn product. The operation of an open-transition state mechanism, similar to that proposed for aldol reaction when performed in the presence of excess Lewis base, could also be considered.19
β-Keto imides have been subjected to tandem chain extension aldol reactions with high anti-aldol selectivity being favored, presumably through the operation of an open transition state.5,19 The starting β-keto imide (16)20 can be synthesized through acetylation of Meldrum’s acid (14),21 followed by treatment with 2-oxazolidinone (15).22
The bis-carbenoid provided an enhanced yield in the tandem chain extension reaction of β-keto imide 16. Analysis by NMR indicated that the open chain form of the β-amino acid derivative was not present, whereas a 13C NMR resonance at 90 ppm was consistent with the presence of a hemi-aminal (17) (Scheme 4). X-ray crystallographic analysis revealed that the anti-β-amino acid was generated in the tandem reaction and that the compound crystallized in the N-Boc hemiaminal form. The reduction of N-Boc hemiaminals through treatment with triethylsilane and BF3•OEt2 provided access to N-Boc protected β-proline derivative (18) with outstanding diastereocontrol (Scheme 4).23
Scheme 4.
Synthesis of β-proline derivative 18 from β-keto imide 16
The strategies described above for generating β-proline derivatives, both Boc-protected and unprotected, are direct, versatile, and practical; however, the method at this time is limited to aryl imines. Although the diastereocontrol is not exceptional, the N-Boc-imines and N-phosphinoyl imines provide access to racemic β-proline derivatives with complementary diastereocontrol. Furthermore, the sense of the diastereoselection is opposite to that observed with the catalytic asymmetric Mannich-type reactions using the same imines.24 Studies targeted at the application of this method and absolute stereocontrol are underway.
Experimental Section
General Experimental Methods
All reactions were run in oven-dried glassware under nitrogen atmosphere and stirred with teflon-coated magnetic stir-bars. The terms ‘concentrated in vacuo or under reduced pressure’ refer to the use of a rotary-evaporator. All commercially available starting materials were used as received, unless otherwise described. Diethylzinc was used as a 1.0 M solution in hexanes. (Neat diethylzinc can also be used successfully for these reactions, although extreme caution should be used when using the neat reagent since diethylzinc is pyrophoric.) Methylene iodide (CH2I2) was purchased from commercial suppliers and non-oxidized copper wire was added as a stabilizer. Tetrahydrofuran and dichloromethane were dried by passing through an alumina column under pressure. Ethyl acetate and hexanes were distilled prior to use. Column chromatography was performed on EM Science flash silica gel (35–75μm), and the mobile phases were used as noted. 1H and 13C NMR spectra were referenced to internal tetramethylsilane (TMS). Melting points are uncorrected. High Resolution Mass Spectroscopy was performed at the University of Notre Dame Mass Spectrometry Facility. Crystallographica data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC 885735 – 885738. Computational work was performed using the Spartan© suite of programs.
Detailed Experimental Procedures
Product (5) of Tandem Chain Extension-Mannich Reaction with Sulfoximine
Into a 100-mL round-bottomed flask, equipped with a magnetic stir bar and and a nitrogen inlet, was charged with 25 mL of dichloromethane and 12 mL of a diethylzinc solution (1 M in hexanes, 12 mmol). The mixture was cooled to 0 °C in an ice bath and methylene iodide (0.95 mL, 12 mmol)) was added dropwise by syringe. After stirring for 10 min, 0.48 mL of methyl pivaloylacetate 1 (3 mmol) was added dropwise by syringe to the reaction mixture. After stirring for another 20 min, (S)-(+)-benzylidene-p-toluenesulfinate 3 (0.365 g, 1 mmol) dissolved in a minimal amount of dichloromethane was added. The reaction mixture was stirred for three h, while monitoring for the disappearance of (S)-(+)-benzylidene-p-toluenesulfinate by TLC. The reaction mixture was quenched with 20 mL of saturated ammonium chloride. Ethyl acetate (15 mL) was added amd the organic layer removed. A second portion of ethyl acetate (15 mL) was used to extract the aqueous layer. The combined organic layers were dried with anhydrous MgSO4, filtered, and concentrated under reduced pressure. The residue was chromatographed on silica (hexane: ethyl acetate; 4: 1) to provide 247 mg (59%) of 5 as a yellowish oil. A crystal suitable for X–ray crystallographic analysis was prepared by crystallization from methanol. 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 4.8 Hz, 2H), 7.12–7.51 (m, 7H), 4.61 (d, J = 5.6 Hz, 1H), 3.45 (s, 3H), 3.06–3.28 (m, 2H), 2.93 (s, 3H), 2.70 dd, J = 17.6, 2.4 Hz, 1H), 2.45 (s, 3H), 1.09 (s, 9H); 13C NMR (400 MHz, CDCl3) δ 215.3, 174.3, 143.9, 137.2, 130.0, 128.4, 127.3, 60.6, 59.7, 51.7, 50.2, 44.9, 44.2, 35.3, 26.7, 21.7, 21.3, 14.4;
(±)-(S)-Methyl 2-((S)-((diphenylphosphoryl)amino)(phenyl)methyl)-5,5-dimethyl-4-oxohex-anoate (7)
An oven-dried, one-necked, 100-mL round-bottomed flask was equipped with a magnetic stir bar and a rubber septum and was then charged with dichloromethane (20 mL) via syringe, and flushed with nitrogen through a needle in a septum. The flask was placed in an ice water bath, cooled to 0 °C, and neat diethylzinc (5.00 mmol, 0.50 mL, 5.00 equiv) was added via syringe in one portion followed by slow addition, over 30 sec, of diiodomethane (5.00 mmol, 0.38 mL, 5.00 equiv) via syringe. The resulting solution was stirred for approximately 10 min. Methyl pivaloylacetate (1) (1.00 mmol, 0.14 mL, 1.00 equiv) was added via syringe and the solution was stirred for 30 min. N-Phosphinoylimine 6 (0.50 mmol, 0.15 g, 0.50 equiv) was mixed with dichloromethane (5 mL) and dried with 4Å molecular sieves. The solution was added to the round-bottomed flask via syringe and the solution was stirred for 48 h. The solution was quenched with saturated ammonium chloride (2 mL), extracted with dichloromethane (2 × 25 mL), dried over anhydrous sodium sulfate, filtered, and concentrated on a rotary evaporator (35 °C, 25 mmHg). The crude reaction mixture was purified via column chromatography (15:2, hexanes: ethyl acetate, Rf=0.22) to afford 0.13 g (0.27 mmol, 27% yield) of the product (7) as a white solid (mp = 168–172 °C). 1H NMR (400 MHz, CDCl3) δ: 7.80-7.73 (m, 2H), 7.68-7.61 (m, 2H), 7.50-7.36 (m, 5H), 7.31–7.36 (m, 4H), 7.14-7.08 (m, 2H), 4.37 (dt, J=12.3, 6.2 Hz, 1H), 4.09 (br t, J= 10.6 Hz, 1H), 3.46-3.35 (m, 4H), 3.05 (dd, J=6.5, 3.3 Hz, 2H), 1.11 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 214.7, 173.4, 141.4 (d, JC-P=3.7 Hz), 132.9 (d, JC-P=9.8 Hz), 132.1 (d, JC-P=12.0 Hz), 131.8 (d, JC-P=9.6 Hz), 128.7 (d, JC-P=15.2 Hz), 128.5 (d, JC-P=12.8 Hz), 127.8, 126.9, 57.2, 51.9, 48.5 (d, JC-P=3.7 Hz), 44.2, 36.9, 26.7. IR (neat) ν 3250, 3059, 2950, 2904, 1771, 1741, 1725, 1698, 1474, 1434, 1366, 1304, 1184, 1106 cm −1. HRMS (ESI) m/z: calcd. for C28H33NO4P [M+H] 478.2142; found [M+H] 478.2134.
(±)-(2S,3S)-Methyl 5-tert-butyl-2-phenylpyrrolidine-3-carboxylate (8)
An oven-dried, one-necked, 100-mL round-bottomed flask was equipped with a magnetic stir bar and a rubber septum. The flask was charged with methanol (50 mL) via syringe, and flushed with nitrogen through a needle in a rubber septum. Thionyl chloride (0.5–1.0 mL) was added via syringe and the solution became strongly acidic (pH ~1–2). α-Substituted γ-keto ester 7 (0.27 mmol, 0.13 g, 1.00 equiv) was added as a solution in methanol (1–2 mL) and allowed to stir for 12 h. Sodium cyanoborohydride (0.05 g, 0.81 mmol, 3.0 equiv) was added in one portion and the solution was stirred for 16 h. The reaction mixture was diluted with water (15 mL) and basified (pH ~12–13) with sodium hydroxide (20%). The basic mixture was extracted with dichloromethane (3 × 25 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated on a rotary evaporator (35 °C, 25 mmHg). The crude reaction mixture was purified via column chromatography (5:1, hexanes: ethyl acetate, Rf=0.17) to afford 0.04 g (0.15 mmol, 56% yield) of the product (8) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ: 7.47-7.42 (m, 2H), 7.34-7.28 (m, 2H), 7.24 (m, 1H), 4.38 (d, J=8.5 Hz, 1H), 3.63 (s, 3H), 3.13 (t, J=7.9 Hz, 1H), 2.73 (dd, J=18.0, 7.9 Hz, 1H), 2.10-1.93 (m, 2H), 0.94 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 175.4, 143.3, 128.5, 127.6, 127.1, 66.8, 65.8, 52.1, 51.9, 33.7, 31.4, 26.4. IR (neat) ν 2952, 2867, 1733, 1636, 1493, 1453 1435, 1392, 1361, 1243, 1165, 898 cm −1. HRMS (ESI) m/z: calcd. for C16H24NO2 [M+H] 262.1802; found [M+H] 262.1806.
(±)-(S)-Methyl 2-((R)-(tert-butoxycarbonylamino)(phenyl)methyl)-5,5-dimethyl-4-oxohex-anoate (10a)
An oven-dried, one-necked, 100-mL round-bottomed flask was equipped with a magnetic stir bar and a rubber septum and was then charged with dichloromethane (20 mL) via syringe, and flushed with nitrogen through a needle in a septum. The flask was placed in an ice water bath, cooled to 0 °C, and neat diethylzinc (3.00 mmol, 0.30 mL, 3.00 equiv) was added via syringe in one portion followed by slow addition, over 30 sec, of diiodomethane (3.00 mmol, 0.24 mL, 3.00 equiv) via syringe. The resulting solution was stirred for approximately 10 min. Methyl pivaloylacetate (1) (1.00 mmol, 0.24 mL, 1.00 equiv) was added via syringe and the solution was stirred for 30 min. Freshly prepared N-Boc imine 9a (1.00 mmol, 0.21 mL, 1.00 equiv) was added as a solution in 3.00 mL of dichloromethane in one portion via syringe and the solution was stirred for 15 h, during which the reaction slowly warmed to room temperature. The solution was quenched with saturated ammonium chloride (2 mL) and extracted with dichloromethane (3 × 25 mL). The organic layers were combined, dried over anhydrous sodium sulfate, filtered, and concentrated on a rotary evaporator (35 °C, 25 mmHg). The crude reaction mixture was purified via column chromatography on alumina (5:1, hexanes: ethyl acetate, Rf=0.27) to afford 0.20 g (0.52 mmol, 52% yield) of the product 10a as a colorless oil. The crude reaction mixture was a mixture of the desired product (10a) and the deprotected cyclic imine (11a). The mixture could be used for further chemistry without purification as well. 1H-NMR (400 MHz, CDCl3) δ: 7.36-7.30 (m, 2H), 7.27-7.23 (m, 3H), 5.76 (d, J=8.4 Hz, 1H), 4.86 (d, J=7.1 Hz, 1H), 3.54 (s, 3H), 3.33 (s, 1H), 2.93 (dd, J=18.2, 8.9 Hz, 1H), 2.80 (m, 1H), 1.42 (s, 9H), 1.11 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 213.4, 174.5, 155.4, 140.7, 128.7, 127.7, 126.3, 79.8, 55.1, 52.1, 46.2, 44.2, 37.4, 28.5, 26.5. IR (neat) ν 3380, 3062, 3030, 2974, 2907, 2875, 1707, 1498, 1455, 1436, 1391, 1366, 1283, 1168, 737 cm−1.
(±)-(S)-Methyl 2-((R)-((tert-butoxycarbonyl)amino)(4-methoxyphenyl)methyl)-5,5-dimethyl-4-oxohexanoate (10b)
The identical procedure as used for the formation of 10a was used; Methyl pivaloylacetate (1) (1.00 mmol, 0.24 mL, 1.00 equiv) and 9b (1 mmol, 235 mg, 1 equiv) were used. 57% yield, colorless oil. 1H-NMR (400 MHz, CDCl3) δ: 7.16 (d, J=8.6 Hz, 2H), 6.88-6.80 (m, 2H), 5.67 (m, 1H), 4.80 (m, 1H), 3.78 (s, 3H), 3.60-3.52 (m, 3H), 2.92 (dd, J=18.2, 8.2 Hz, 1H), 2.17 (d, J=2.8 Hz, 1H), 1.39 (s, 9H), 1.11 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 213.5, 174.6, 159.1, 155.4, 127.5, 114.1, 55.5, 54.6, 54.0, 46.3, 44.2, 37.4, 29.5, 28.5, 26.5. IR (neat) ν 3386, 2970, 1706, 1610, 1512, 1366, 1292, 1247, 1168, 1030, 888, 835 cm−1. HRMS (ESI) m/z: calcd. for C22H34NO6 [M+H] 408.2381; found [M+H] 408.2383.
(±)-(S)-Methyl 2-((R)-((tert-butoxycarbonyl)amino)(p-tolyl)methyl)-5,5-dimethyl-4-oxohex-anoate (10c)
The identical procedure as used for the formation of 10a was used; Methyl pivaloylacetate (1) (1.00 mmol, 0.24 mL, 1.00 equiv) and 9c (1.00 mmol, 0.22 mL, 1.00 equiv) were used. 55% yield, colorless oil. 1H-NMR (400 MHz, CDCl3) δ: 7.13-7.11 (m, 4H), 5.69 (m, 1H), 4.81 (m, 1H), 3.55 (d, J=2.2 Hz, 1H), 3.30 (s, 3H), 2.92 (dd, J=18.2, 8.1 Hz, 1H), 2.75 (d, J=14.1 Hz, 1H), 2.32, (d, J=2.7 Hz, 3H), 2.16 (s, 3H), 1.41 (s, 9H), 1.10 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 213.5, 174.5, 163.1, 155.4, 137.3, 129.4, 126.5, 126.2, 79.7, 54.9, 54.0, 52.0, 46.2, 44.2, 37.4, 36.3, 31.1, 29.9, 28.5, 26.6, 26.5, 21.2. IR (neat) ν 3330, 3131, 3094, 3050, 2968, 2874, 2733, 1902, 1698, 1651, 1556, 1455, 1366, 1285, 1044, 880 cm−1. HRMS (ESI) m/z: calcd. for C22H34NO5 [M+H] 392.2431; found [M+H] 392.2424.
(±)-(2R, 3S)-Methyl 5-tert-butyl-2-phenyl-3,4-dihydro-2H-pyrrole-3-carboxylate (11a)
A 20-mL scintillation vial was charged with α-substituted γ-keto ester 10a (0.38 g, 1.00 mmol, 1.00 equiv), dichloromethane (10 mL) and a magnetic stir bar. The vial was place in an ice-water bath and trifluoroacetic acid (1.5 mL) was added dropwise over a 5 min period. The ice-water bath was removed and the reaction was stirred for 8 h. The mixture was diluted with dichloromethane (10 mL), extracted with water (2 × 15 mL), washed with saturated sodium bicarbonate (3 × 15 mL), dried over sodium sulfate, filtered and concentrated on a rotary evaporator (35 °C, 25 mmHg). The crude reaction mixture was purified via flash column chromatography (5:1 hexanes: ethyl acetate, Rf=0.15) to afford 0.23 g of the product (0.89 mmol, 89%). 1H-NMR (400 MHz, CDCl3) δ: 7.26-7.17 (m, 3H), 7.09-7.04 (m, 2H), 5.51 (d, J=9.3 Hz, 1H), 3.53 (td, J=9.3, 6.5 Hz, 1H), 3.20 (ddd, J=17.3, 6.5, 1.9 Hz, 1H), 3.12 (s, 3H), 2.76 (m, 1H), 1.28 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 185.8, 172.9, 138.9, 128.1, 127.7. 127.5, 77.8, 51.4, 48.6, 36.8, 36.4 28.5. IR (neat) ν 3062, 3029, 2962, 2926, 2868, 1737, 1638, 1493, 1454, 1435, 1363, 1268, 1202, 1174, 1104, 1076, 1033, 1006, 913, 734 cm−1. HRMS (ESI) m/z: calcd. for C16H22NO2 [M+H] 260.1645; found [M+H] 260.1649.
(±)-(2R,3S)-Methyl 5-(tert-butyl)-2-(4-methoxyphenyl)-3,4-dihydro-2H-pyrrole-3-carboxylate (11b)
The identical procedure as used for the formation of 11a was performed on a 1 mmol scale using 10b (0.41 g) as the starting material to yield 0.27 g (92%) of 11b. 1H-NMR (400 MHz, CDCl3) δ: 7.02-6.97 (m, 2H), 6.83-6.76 (m, 2H), 5.47 (d, J=9.2 Hz, 1H), 3.76 (s, 3H), 3.51 (td, J=9.4, 6.8 Hz, 1H), 3.23-3.17 (m, 4H), 2.76 (ddd, J=17.3, 9.4, 0.7 Hz, 1H), 1.29 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 185.3, 172.9, 159.1, 130.9, 128.6, 113.5, 77.3, 55.4, 51.5, 48.5, 36.6, 36.3, 28.5. IR (neat) ν 2962, 2869, 2838, 1735, 1637, 1612, 1585, 1512, 1461, 1438, 1363, 1248, 1176, 1036, 833, 732 cm−1. HRMS (ESI) m/z: calcd. for C17H24NO3 [M+H] 290.1751; found [M+H] 290.1755.
(±)-(2R,3S)-Methyl 5-(tert-butyl)-2-(p-tolyl)-3,4-dihydro-2H-pyrrole-3-carboxylate (11c)
The identical procedure as used for the formation of 11a was performed on a 1 mmol scale using 10c (0.39 g) as the starting material to yield 0.26 g (95%) of 11c. 1H-NMR (400 MHz, CDCl3) δ: 7.06 (d, J=7.8 Hz, 2H), 6.95 (d, J=8.0 Hz, 2H), 5.48 (d, J=9.2 Hz, 1H), 3.53 (ddt, J=8.4, 7.0, 1.4 Hz, 1H), 3.18 (s, 4H), 2.76 (ddd, J=17.3, 9.4, 0.8 Hz, 1H), 2.29 (s, 3H), 1.29 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 185.4, 172.9, 137.1, 135.7, 128.8, 127.4, 77.6, 51.4, 48.5, 36.7, 36.3, 28.5, 21.3. IR (neat) ν 3350, 3131, 3094, 3050, 2968, 2874, 2733, 1902, 1698, 1651, 1556, 1455, 1366, 1285, 1079, 1044, 1019 cm−1. HRMS (ESI) m/z: calcd. for C17H24NO2 [M+H] 274.1802; found [M+H] 274.1796.
(±)-(2R, 3S, 5S)-Methyl 5-tert-butyl-2-phenylpyrrolidine-3-carboxylate (12a)
An oven-dried, one-necked, 100-mL round-bottomed flask was equipped with a magnetic stir bar and rubber septum. The flask was charged with methanol (50 mL) via syringe, and flushed with nitrogen through a needle in a rubber septum. Thionyl chloride (0.5–1 mL) was added via syringe and the solution became strongly acidic (pH ~1–2). Cyclic imine 8 (0.08 g, 0.27 mmol, 1.00 equiv) was added as a solution in methanol (1–2 mL) and allowed to stir for 5 min. Sodium cyanoborohydride (0.05 g, 0.81 mmol, 3.00 equiv) was added in one portion and the solution was stirred for 16 h. The reaction mixture was diluted with water (15 mL) and basified (pH ~12–13) with sodium hydroxide (20%). The basic mixture was extracted with dichloromethane (3 × 25 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated on a rotary evaporator (35 °C, 25 mmHg) to afford 0.05 g (0.21 mmol, 79% yield) of the product as a colorless oil. The crude reaction mixture required no further purification. 1H-NMR (400 MHz, CDCl3) δ: 7.33-7.25 (m, 4H), 7.23-7.17 (m, 1H) 4.49 (d, J=9.0 Hz, 1H), 3.30 (dt, J=8.7, 7.5 Hz, 1H), 3.12 (s, 3H), 2.99 (dd, J=10.2, 6.7 Hz, 1H), 2.29 (s, 1H), 2.00 (ddq, J=12.8, 9.4, 7.1 Hz, 1H), 1.04 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 174.4, 140.8, 128.1, 127.4, 127.3, 68.7, 65.1, 51.2, 49.9, 33.1, 30.5, 26.9. IR (neat) ν 3350, 3062, 3030, 2955, 2927, 2867, 1960, 1899, 1737, 1623, 1577, 1493, 1450, 1436, 1364, 1299, 1206, 1174, 1106, 696 cm−1. HRMS (ESI) m/z: calcd. for C16H24NO2 [M+H] 262.1802; found [M+H] 262.1805.
(±)-(2R,3S,5S)-Methyl 5-(tert-butyl)-2-(4-methoxyphenyl)pyrrolidine-3-carboxylate (12b)
The identical procedure as used for the formation of 12a was performed on a 0.27 mmol scale using 11b (0.08 g) as the starting material to yield 0.06 g (82%) of 12b; colorless oil. 1H-NMR (400 MHz, CDCl3) δ: 7.25-7.22 (m, 2H), 6.83-6.79 (m, 2H), 4.45 (d, J=9.1 Hz, 1H), 3.78 (s, 3H), 3.25 (m, 1H), 3.17 (s, 3H), 2.96 (dd, J=10.2, 6.5 Hz, 1H), 2.04 (ddd, J=12.7, 10.4, 7.7 Hz, 1H), 1.99-1.87 (m, 2H), 1.03 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 174.5, 158.9, 133.2, 128.5, 113.4, 68.7, 64.5, 55.4, 51.2, 49.9, 33.1, 30.4, 26.9. IR (neat) ν 2954, 2929, 2869, 1734, 1637, 1610, 1584, 1511, 1462, 1394, 1301, 1244, 1173, 1033, 933, 828 cm−1. HRMS (ESI) m/z: calcd. for C17H26NO3 [M+H] 292.1907; found [M+H] 292.1904.
(±)-(2R,3S,5S)-Methyl 5-(tert-butyl)-2-(p-tolyl)pyrrolidine-3-carboxylate (12c)
The identical procedure as used for the formation of 12a was performed on a 0.27 mmol scale using 11c (0.08 g) as the starting material to yield 0.60 g (80%) of 12c. 80% yield, colorless oil. 1H-NMR (400 MHz, CDCl3) δ: 7.19 (d, J=8.0 Hz, 2H), 7.07 (d, J=7.9 Hz, 2H), 4.45 (d, J=9.0 Hz, 1H), 3.27 (dt, J=8.7, 7.6 Hz, 1H), 3.15 (s, 3H), 2.97 (dd, J=10.2, 6.6 Hz, 1H), 2.30 (s, 3H), 2.10-1.98 (m, 2H), 1.93 (ddd, J=12.8, 8.5, 6.6 Hz, 1H), 1.03 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 174.5, 137.9, 136.9, 128.7, 127.3, 68.7, 64.9, 51.2, 49.9, 33.1, 30.6, 27.0, 21.3. IR (neat) ν 3346, 2952, 2926, 2859, 1738, 1513, 1435, 1366, 1201, 1165, 1108, 1042, 934, 820, 718 cm. HRMS (ESI) m/z: calcd. for C17H26NO2 [M+H] 276.1958; found [M+H] 276.1948.
1-(2-Oxooxazolidin-3-yl)butane-1,3-dione (16)
A 250-mL round-bottomed flask was equipped with a magnetic stir bar and a reflux condenser. The flask was charged with acetylated Meldrum’s acid21 (11.76 g, 62.2 mmol, 1.25 equiv), oxazolidine (4.33 g, 49.8 mmol, 1.00 equiv), and toluene (100 mL) and was flushed with nitrogen through a needle in a septum. The solution was allowed to reflux for 8 h and the solution was concentrated under reduced pressure (30 °C, 25 mmHg). The crude reaction mixture was recrystallized from ethanol (2 × 95%) to afford 6.90 g (40.3 mmol, 81% yield) of the product as a dark brown solid (M.P. = 54.0–57.0 °C, Lit. mp.20 = 61–63 °C). 1H NMR (400 MHz, CDCl3) δ: 4.45 (t, J=8.2 Hz, 2H), 4.09-4.05 (m, 4H), 2.29 (s, 3H). 13C NMR (100 MHz, CDCl3) δ: 201.1, 166.7, 153.9, 62.5, 51.2, 42.3, 30.3. IR (neat) ν 2985, 2904, 2853, 1768, 1724, 1701, 1526, 1477, 1420, 1396, 1362, 1334, 1256, 1188, 1047, 1014, 962, 880 cm−1. HRMS (ESI) m/z: calcd. for C7H9NNaO4 [M+Na] 194.0424; found [M+Na] 194.0416.
(±)-(2R,4S,5S)-tert-Butyl 2-hydroxy-2-methyl-4-(2-oxooxazolidine-3-carbonyl)-5-phenyl-pyrrolidine-1-carboxylate (17)
An oven-dried, one-necked, 100-mL round-bottomed flask was equipped with a magnetic stir bar and a rubber septum and was then charged with dichloromethane (20 mL) via syringe, and flushed with nitrogen through a needle in a septum. The flask was placed in an ice water bath, cooled to 0 °C, and neat diethylzinc (3.00 mmol, 0.30 mL, 3.00 equiv) was added via syringe in one portion followed by slow addition, over 30 sec, of diiodomethane (6.00 mmol, 0.48 mL, 6.00 equiv) via syringe. The resulting solution was stirred for approximately 10 min. Freshly prepared β-keto imide 16 (1.00 mmol, 0.17 g, 1.00 equiv) was added as a solution in 3 mL of dichloromethane via syringe and the solution was stirred for 30 min. Freshly prepared N-Boc imine 9a (1.00 mmol, 0.21 mL, 1.00 equiv) was added as a solution in 3 mL of dichloromethane in one portion via syringe and the solution was stirred for 15 h, during which the reaction slowly warmed to room temperature. The solution was quenched with saturated ammonium chloride (2 mL), extracted with dichloromethane (3 × 25 mL), dried over anhydrous sodium sulfate, filtered, and concentrated on a rotary evaporator (35 °C, 25 mmHg). The crude reaction mixture was recrystallized from hexanes to afford 0.23 g (0.59 mmol, 59% yield) of the product as a crystalline solid, which converted to a mixture of hemiaminal isomers in solution. Mixture of isomers: 1H-NMR (400 MHz, CDCl3) δ: 7.39-7.19 (m, 5H), 5.76 (d, J=9.5 Hz, 1H), 5.33 (d, J=8.7 Hz, 1H), 5.19 (s, 1H), 4.87-4.77 (m, 1H), 4.71 (m, 1H), 4.47-4.20 (m, 4H), 4.05-3.93 (m, 2H), 3.89-3.73 (m, 1H), 3.38-3.25 (m, 1H), 3.11 (dd, J=18.1, 11.1 Hz, 2H), 3.01-2.89 (m, 1H), 2.15-1.98 (m, 2H), 1.76 (s, 3H), 1.08 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 169.6, 154.1, 153.5, 140.6, 128.3, 127.8, 126.8, 89.9, 80.7, 62.8, 62.5, 60.6, 45.2, 43.1, 42.7, 39.1, 28.5, 28.1, 27.2, 21.2, 14.4. IR (neat) ν 2977, 1779, 1693, 1672, 1478, 1455, 1363, 1310, 1272, 1221, 1166, 1105, 1037, 909, 843 cm−1. Representative 1H resonances for the major hemiacetal isomeric form 1H-NMR (400 MHz, CDCl3) δ: 7.39-7.19 (m, 5H), 5.33 (d, J=8.7 Hz, 1H), 5.19 (s, 1H), 4.45-4.20 (m, 4H), 3.87-3.71 (m, 1H), 3.37-3.28 (m, 1H), 3.01-2.87 (m, 1H), 2.15-1.99 (m, 2H), 1.76 (s, 3H), 1.08 (s, 9H). HRMS (ESI) m/z: calcd. for C20H27N2O6 [M+H] 391.1864; found [M+H] 391.1853.
(±)-(2R,3S)-tert-Butyl 5-methyl-3-(2-oxo-oxazolidine-3-carbonyl)-2-phenylpyrrolidine-1-carboxylate (18)
An oven-dried, one-necked, 50-mL round-bottomed flask was equipped with a magnetic stir bar and a rubber septum and was then charged with dichloromethane (15 mL) via syringe, N-Boc hemiaminal 17 (0.72 mmol, 0.28 g, 1.00 equiv) and flushed with nitrogen through a needle in a septum. The flask was placed in dry ice-acetone bath and was cooled to −78 °C. Boron trifluoride etherate (0.78 mmol, 0.10 mL, 1.10 equiv) was added via syringe in one portion and allowed to stir for 10 min. Triethylsilane (0.72 mmol, 0.12 mL, 1.00 equiv) was added via syringe in one portion and allowed to stir for 2 h. The solution was quenched with saturated sodium bicarbonate (5 mL) and was extracted with dichloromethane (3 × 20 mL), dried over anhydrous sodium sulfate, filtered and concentrated on a rotary evaporator (35 °C, 25 mmHg). The crude reaction mixture was purified via flash column chromatography (4:1 hexanes: ethyl acetate, Rf=0.19) to afford 0.20 g (0.53 mmol, 74% yield) of 18 as a sticky white solid. Mixture of Diastereomers (dr = 5:1); 1H-NMR (400 MHz, CDCl3) δ: 7.31-7.18 (m, 3H), 7.14 (d, J=7.1 Hz, 2H), 5.41 (d, J=9.3 Hz, 1H) 5.32 (d, J=9.0 Hz, 1H), 4.40 (ddd, J=11.9, 9.3, 7.1 Hz, 1H), 4.29 (m, 1H), 4.20 (dt, J=16.2, 8.2 Hz, 1H), 3.93 (dt, J=17.2, 6.1, Hz, 1H), 3.78 (ddd, J=16.5, 9.5, 7.1 Hz, 1H), 3.26 (ddd, J=10.8, 9.3, 6.6 Hz, 1H), 2.48-2.35 (m, 1H), 2.16 (dt, J=13.4, 6.8 Hz, 1H), 1.61 (d, J=6.0 Hz, 3H), 1.38-1.18 (br s, 9H), 1.06 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 170.2, 154.9, 153.4, 141.1, 128.2, 127.7, 126.9, 79.8, 64.2, 62.4, 53.8, 47.3, 42.7, 34.2, 28.4, 21.3 IR (neat) ν 2972, 2929, 1774, 1686, 1601, 1478, 1455, 1363, 1341, 1299, 1268, 1220, 1125, 1036, 1004, 977, 882 cm−1. Representative 1H Resonances for the Major Diastereomer 1H-NMR (400 MHz, CDCl3) δ: 7.30-7.18 (m, 3H), 7.16-7.13 (m, 2H), 5.41 (d, J=9.3 Hz, 1H), 4.40 (ddd, J=11.9, 9.3, 7.1 Hz, 1H), 4.29 (tt, J=11.0, 5.5 Hz, 1H), 4.20 (dt, J=16.2, 8.2 Hz, 1H), 3.94 (tt, J=12.2, 6.2 Hz, 1H), 3.78 (ddd, J=16.5, 9.5, 7.1 Hz, 1H), 3.26 (ddd, J=10.8, 9.3, 6.6 Hz, 1H), 2.48-2.34 (m, 1H), 2.16 (dt, J=13.4, 6.8 Hz, 1H), 1.61 (d, J=6.0 Hz, 3H), 1.27 (s, 9H). HRMS (ESI) m/z: calcd. for C20H27N2O5 [M+H] 375.1914; found [M+H] 375.1909.
Supplementary Material
Acknowledgments
The support of the NIH (R15 GM-060967) is appreciated. RBJ acknowledges the NSF MRI program (grant No. CHE-0619278) for funds to purchase the X-ray diffractometer. Professor Franklin Davis is acknowledged for a generous gift of compound 3.
Footnotes
Supporting Information Available 1H and 13C NMR spectra for all compounds
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Sewald N. Angew Chem Int Ed. 2003;42:5794–5795. doi: 10.1002/anie.200301692. [DOI] [PubMed] [Google Scholar]; (b) Weiner B, Szymanski W, Janssen DB, Minnaard AJ, Feringa BL. Chem Soc Rev. 2010;39:1656–1691. doi: 10.1039/b919599h. [DOI] [PubMed] [Google Scholar]; (c) Sleebs BE, Van Nguyen TT, Hughes AB. Org Prep Proced Int. 2009;41:429–478. [Google Scholar]
- 2.(a) Davis FA, Song M. Org Lett. 2007;9:2413–2416. doi: 10.1021/ol0708166. [DOI] [PubMed] [Google Scholar]; (b) Moumne R, Larregola M, Boutadla Y, Lavielle S, Karoyan P. Tetrahedron Lett. 2008;49:4704–4707. [Google Scholar]; (c) Tang TP, Ellman JA. J Org Chem. 2002;67:7819–7832. doi: 10.1021/jo025957u. [DOI] [PubMed] [Google Scholar]
- 3.(a) Deiana L, Zhao GL, Dziedzic P, Rios R, Vesely J, Ekstroem J, Cordova A. Tetrahedron Lett. 2010;51:234–237. [Google Scholar]; (b) Wenzel AG, Jacobsen EN. J Am Chem Soc. 2002;124:12964–12965. doi: 10.1021/ja028353g. [DOI] [PubMed] [Google Scholar]
- 4.(a) Brogan JB, Zercher CK. J Org Chem. 1997;62:6444–6446. [Google Scholar]; (b) Eger W, Zercher CK, Williams C. J Org Chem. 2010;75:7322–7331. doi: 10.1021/jo101590t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai SJ, Zercher CK, Jasinski JP, Reid SN, Staples RJ. Org Lett. 2001;3:4169–4171. doi: 10.1021/ol016788n. [DOI] [PubMed] [Google Scholar]
- 6.Hilgenkamp R, Zercher CK. Org Lett. 2001;3:3037–3040. doi: 10.1021/ol016485t. [DOI] [PubMed] [Google Scholar]
- 7.Ronsheim MD, Zercher CK. J Org Chem. 2003;68:4535–4538. doi: 10.1021/jo026299g. [DOI] [PubMed] [Google Scholar]
- 8.Davis FA, Zhang Y, Andemichael Y, Fang T, Fanelli DL, Zhang H. J Org Chem. 1999;64:1403–1406. [Google Scholar]
- 9.(a) Furukawa J, Kawabata N, Nishimura J. Tetrahedron Lett. 1966:3353–3354. [Google Scholar]; (b) Furukawa J, Kawabata N, Nishimura J. Tetrahedron. 1968;24:53–58. [Google Scholar]
- 10.Charette AB, Juteau H, Lebel H, Molinaro C. J Am Chem Soc. 1998;120:11943–11952. [Google Scholar]
- 11.Davis FA, Prasad KR, Carroll PJ. J Org Chem. 2002;67:7802–7806. doi: 10.1021/jo020302e. [DOI] [PubMed] [Google Scholar]
- 12.(a) Aggarwal VK, Castro AMM, Mereu A, Adams H. Tetrahedron Lett. 2002;42:1577–1581. [Google Scholar]; (b) Colonna S, Germinario G, Manfredi A, Stirling CJM. J Chem Soc, Perkin Trans 1. 1988:1695–1698. [Google Scholar]
- 13.Desrosiers JN, Cote A, Boezio AA, Charette AB. Org Synth. 2006;83:5–17. [Google Scholar]
- 14.Ronsheim MD, Hilgenkamp R, Zercher CK. Org Synth. 2002;79:146–153. [Google Scholar]
- 15.Hutchins RO, Su WY, Sivakumar R, Cistone F, Stercho YP. J Org Chem. 1983;48:3412–3422. [Google Scholar]
- 16.Yang JW, Pan CP, List B. Org Synth. 2009;86:11–17. [Google Scholar]
- 17.Adrian JC, Jr, Barkin JL, Fox RJ, Chick JE, Hunter AD, Nicklow RA. J Org Chem. 2000;65:6264–6267. doi: 10.1021/jo005555r. [DOI] [PubMed] [Google Scholar]
- 18.Jaurista E, Lopez-Nunez NA, Glass RS, Petsom A, Hutchins RO, Stercho JP. J Org Chem. 1986;51:1357–1360. [Google Scholar]
- 19.Danda H, Hansen MM, Heathcock CH. J Org Chem. 1990;55:173–181. [Google Scholar]
- 20.Palombi L, Feroci M, Orsini M, Inesi A. Tetrahedron Asymm. 2002;13:2311–2316. [Google Scholar]
- 21.Oikawa Y, Yoshioka T, Sugano K, Yonemitsu O. Org Synth. 1985;63:198–200. [Google Scholar]
- 22.Marchi C, Trepat E, Moreno-Manas M, Vallribera A, Molins E. Tetrahedron. 2002;58:5699–5708. [Google Scholar]
- 23.Pedregal C, Ezquerra J, Carreno MC, Garcia R, Ruano JLG. Tetrahedron Lett. 1994;35:2053–2056. [Google Scholar]
- 24.Trost BM, Jaratjaroonphong J, Reutrakul V. J Am Chem Soc. 2006;128:2778–2779. doi: 10.1021/ja057498v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.