Abstract
Plant stomata function in disease resistance by restricting bacteria entry inside leaves. During plant-bacteria interactions, stomatal closure is initiated by the recognition of Microbe-Associated Molecular Patterns (MAMPs). Recently, we have shown that the Lectin Receptor Kinase V.5 (LecRK-V.5) negatively regulates bacterium- and MAMP-induced stomatal closure upstream of Reactive Oxygen Species (ROS) production mediated by abscisic acid signaling. Closed stomata in lecrk-V.5 mutants are correlated with constitutive high level of ROS in guard cells. Consequently, lecrk-V.5 mutants are more resistant to hemi-biotrophic pathogen Pseudomonas syringae pv tomato DC3000 (Pst DC3000). In this report, we further investigate the role of LecRK-V.5 in resistance against necrotrophic bacteria Pectobacterium carotovorum ssp. carotovorum (Pcc). Upon surface-inoculation lecrk-V.5 mutants exhibited enhanced resistance against Pcc whereas a wild-type level of resistance was observed using infiltration-inoculation, an inoculation method that bypasses the epidermal barrier. Enhanced resistance of dip-inoculated lecrk-V.5 mutants against necrotrophic bacteria, that induce different defense responses than hemi-biotrophic bacteria, further suggests a possible role for LecRK-V.5 in stomatal immunity.
Keywords: Arabidopsis thaliana, abscisic acid, bacteria, innate immunity, lectin receptor kinase, reactive oxygen species, stomatal immunity
Stomatal closure as part of the Pattern-Triggered Immunity (PTI) response is one of the first lines of plant defense against bacterial invasion.1 Pseudomonas syringae pv tomato DC3000 (Pst DC3000), Xanthomonas campestris and Escherichia coli bacteria indeed induce stomatal closure in Arabidopsis within 1 to 2 h post inoculation.2,3 Pst DC3000 is able to reopen stomata 3 to 4 h after infection through the action of the virulence factor coronatine (COR).3 Recently, a genetic screen identified several susceptible to coronatine-deficient (scord) mutants defective in stomatal closure upon COR-deficient Pst DC3000 inoculation and two of them showed wild-type (WT) apoplastic defense.4 The promotion of stomatal closure by bacteria is mediated by the perception of Microbe-Associated Molecular Patterns (MAMPs) such as the flagellin peptide 22 (flg22).3 Flagellin Insensitive 2 (FLS2) is required for flg22-induced stomatal closure and plays an essential role in stomatal closure mediated by Pst DC3000.3,5
We have recently identified the Lectin Receptor Kinase V.5 (LecRK-V.5) as an important negative regulator of stomatal immunity.6 LecRK-V.5 expression is rapidly induced in guard cells after flg22 and Pst DC3000 treatments. Overexpression of LecRK-V.5 leads to defects in MAMP-induced stomatal closure and Reactive Oxygen Species (ROS) production in guard cells.6 By contrast, lecrk-V.5 mutants exhibit constitutive stomatal closure that is correlated with a high level of ROS in stomata. Upon PTI activation, lecrk-V.5 mutants are insensitive to COR-mediated stomatal reopening and lecrk-V.5 stomata are still closed at 3 h post inoculation with Pst DC3000.6 As a biological consequence, lecrk-V.5 mutants are more resistant than WT to Pst DC3000 dip-inoculation. However, using infiltration inoculation, no difference in symptoms and in bacterial growth were observed between mutants and WT plants indicating that disease resistance is linked to stomatal defense.6 By contrast, LecRK-V.5 overexpression (OE) lines are more susceptible than WT plants to Pst DC3000 dip-inoculation probably because of faster stomatal reopening.6
LecRK-V.5 Negatively Regulates Disease Resistance to the Necrotrophic Bacteria Pectobacterium carotovorum ssp. carotovorum
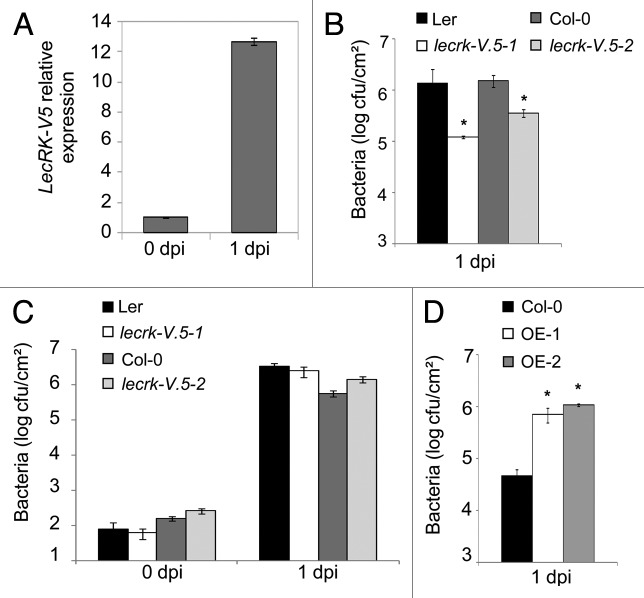
To evaluate the possible contribution of LecRK-V.5 to disease resistance against necrotrophic bacteria, that activate a different Arabidopsis defense response than hemi-biotrophic Pst DC3000,7 we first analyzed LecRK-V.5 expression after Pectobacterium carotovorum ssp. carotovorum (formerly Erwinia carotovora ssp. carotovora) strain WPP14 (Pcc) infection.8 Analysis of LecRK-V.5 expression by qRT-PCR6 revealed that LecRK-V.5 was induced more than 10-fold at 1 d after Pcc dip-inoculation (Fig. 1A). We also dip-inoculated lecrk-V.5 mutants with Pcc and quantified in planta bacterial growth. At 1 d post Pcc dip-inoculation, growth of bacteria in lecrk-V.5–1 and lecrk-V.5–2 mutants was about 5 to 10-fold lower than in WT control plants (Fig. 1B), indicating that both lecrk-V.5 mutants were resistant to Pcc. When bacteria were directly infiltrated into the apoplast, lecrk-V.5 mutants did not show any significant differences in bacterial titers when compared with WT control plants (Fig. 1C). These results suggest that enhanced resistance of lecrk-V.5 mutants to Pcc is not linked to an increase in apoplastic defense but is likely due to constitutive stomatal closure, confirming our observations with Pst DC3000.6 By contrast, lines overexpressing LecRK-V.5 exhibited enhanced susceptibility to Pcc dip-inoculation with bacterial titers about 10-fold higher than WT plants (Fig. 1D). Lines overexpressing LecRK-V.5 are therefore more susceptible to both Pcc and Pst DC3000.6 Collectively, these results indicate that LecRK-V.5 negatively regulates Arabidopsis resistance to both hemi-biotrophic and necrotrophic bacteria, possibly through negatively regulating Arabidopsis stomatal immunity.
Figure 1. A negative role for LecRK-V.5 in Arabidopsis resistance to Pcc. (A) Analysis of LecRK-V.5 expression by qRT-PCR in Arabidopsis thaliana (Ler ecotype) at 0 and 1 d after dip-inoculation with 1 × 105 cfu.ml−1Pectobacterium carotovorum (Pcc) strain WPP14. Expression level was normalized to EF-1 and compared with time 0 with a defined expression value of 1. Data represent average values ± SE (n = 3). (B) Bacterial growth in lecrk-V.5–1 and lecrk-V.5–2 mutants and corresponding Ler and Col-0 WT plants at 1 d after dip-inoculation with 1 × 105 cfu.ml−1Pcc. (C) Bacterial growth in lecrk-V.5 mutants and WT (Ler and Col-0) infiltrated-inoculated with 1 × 104 cfu.ml−1Pcc. (D) Bacterial growth assessed at 1 d after dip-inoculation with Pcc in WT (Col-0) and LecRK-V.5 overexpression lines (OE-1 and OE-2). Bacterial quantification was determined as described earlier.6,11 Data represent average values ± SD. Statistical differences between WT controls and mutant or plants overexpressing LecRK-V.5 are detected with a t-test (p < 0.01, n = 6). All experiments were repeated at least twice with similar results. dpi, day post inoculation; cfu, colony forming units.
Conclusions
An effective stomatal closure upon MAMPs perception is critical for plant resistance to bacteria.1 We showed that LecRK-V.5 is a negative regulator of MAMP-triggered stomatal closure.6 In this report we demonstrate that LecRK-V.5 represses resistance to necrotrophic Pcc bacteria possibly through action on stomatal immunity. Similar divergent phenotypes after surface- or infiltration-inoculation were previously observed using the hemi-biotrophic bacteria Pst DC3000.6 Moreover, a recent study using mutant and overexpression lines of LecRK-VI.2 suggested the importance of stomatal immunity in resistance against both Pcc and Pst DC3000 infection.9 Necrotrophic and hemi-biotrophic pathogens have different lifestyles that induce a different set of defense responses in Arabidopsis.7 However, as the first barrier against microbes, stomatal immunity is important for plant defense against pathogenic and non-pathogenic bacteria that penetrate into leaves via natural opening.10 At later stage of infection, other PTI responses occur preferentially in mesophyll cells.4,5 This study provides additional evidences that upon MAMP perception stomatal immunity is effective against a wide range of pathogenic bacteria with different lifestyles.
Acknowledgments
This work was supported by the National Science Council of Taiwan grants 96–2628-B-002–112-MY3 and 99–2628-B-002–053-MY3 (to L.Z.) and the Frontier and Innovative Research grant of the National Taiwan University code number 99R70436 (to L.Z.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21013
References
- 1.Zeng W, Melotto M, He SY. Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr Opin Biotechnol. 2010;21:599–603. doi: 10.1016/j.copbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudesblat GE, Torres PS, Vojnov AA. Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. 2009;149:1017–27. doi: 10.1104/pp.108.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–80. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Zeng W, Brutus A, Kremer JM, Withers JC, Gao X, Jones AD, et al. A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 2011;7:e1002291. doi: 10.1371/journal.ppat.1002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng W, He SY. A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 2010;153:1188–98. doi: 10.1104/pp.110.157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desclos-Theveniau M, Arnaud D, Huang TY, Lin GJC, Chen WY, Lin YC, et al. The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 2012;8:e1002513. doi: 10.1371/journal.ppat.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–27. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 8.Yap M-N, Barak JD, Charkowski AO. Genomic diversity of Erwinia carotovora subsp. carotovora and its correlation with virulence. Appl Environ Microbiol. 2004;70:3013–23. doi: 10.1128/AEM.70.5.3013-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh P, Kuo YC, Mishra S, Tsai CH, Chien CC, Chen CW, et al. The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell. 2012;24:1256–70. doi: 10.1105/tpc.112.095778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melotto M, Underwood W, He SY. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol. 2008;46:101–22. doi: 10.1146/annurev.phyto.121107.104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal S, Eriksson ARB, Montesano M, Denecke J, Palva ET. Cell wall-degrading enzymes from Erwinia carotovora cooperate in the salicylic acid-independent induction of a plant defense response. Mol Plant Microbe Interact. 1998;11:23–32. doi: 10.1094/MPMI.1998.11.1.23. [DOI] [Google Scholar]