Abstract
Plant defense mechanisms respond to diverse environmental factors and play key roles in signaling pathways. The phospholipidic signaling pathway forms part of the plant response to several phytoregulators, such as salicylic acid (SA) and methyl jasmonate (MJ), which have been widely used to stimulate secondary metabolite production in cell cultures.1 Furthermore, it has been reported that the levels of such phytoregulators as SA and MJ can increase in response to stressful conditions.2,3 The phospholipidic signal transduction system involves the generation of second messengers by the hydrolysis of phospholipids. In this study, we examined how phospholipidic signaling can be modulated depending on the growth stage of the culture, and we focused on two key lipases having relevant roles in the signaling cascades in plants. An evaluation was made of the effects of SA and MJ on the phospholipase activities in Capsicum chinense Jacq. suspension cells at different phases of the culture cycle. The treatment with SA differentially modified the phospholipase C (PLC) (EC: 3.1.4.3) and phospholipase D (PLD) (EC: 3.1.4.4) activities in a dose-dependent manner that also depended on the day of the culture cycle. In contrast, the treatment with MJ resulted in a biphasic behavior of the PLC and PLD activities. We conclude that the enzymatic activities in the phospholipidic signaling pathways are modified differentially depending on the day of the culture’s growth cycle; accordingly, the response capacity to such environmental factors as phytoregulators is variable at different stages of growth and the physiology of the cells.
Keywords: Capsicum chinense, methyl jasmonate, phospholipase C, phospholipase D, salicylic acid
Results and Discussion
In the mechanisms of cellular transduction, phospholipid signal transduction participates as the main pathway leading to the cascade of phospholipidic signals. The phospholipid system consists of the generation of messengers by several reactions, such as phospholipid hydrolysis, phosphorylation and dephosphorylation. After a signal is perceived, several enzymes involved in the signaling cascades are activated, including PLC, which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), generating two products: inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG). Ins(1,4,5)P3 is an important second messenger that causes the liberation of Ca2+ ions into the cytosol4,5 as a consequence of the opening of calcium channels. Another enzyme, PLD, generates the second messenger phosphatidic acid (PA) via the hydrolysis of membrane phospholipids, such as phosphatidylcholine (PC); PA can also be generated by the action of diacylglycerol kinase (DGK)6 on DAG. PA has a key function as a second messenger in plants and modulates the activities of kinases, phosphatases, phospholipases and other proteins involved in membrane trafficking, calcium signaling and biotic and abiotic stress responses.7,8
In plants, the regulation of phospholipase activities is closely related to and plays an important role in the generation of second messengers in response to a broad range of biotic of abiotic stresses. The regulation of several processes during growth and the PLC activity are modified by the presence of aluminum in Catharanthus roseus9 and in response to abscisic acid (ABA)10 and auxin.11 PLC is associated with increased phytoalexin production in cultured carrot cells,12 pisatin accumulation in pea,13 the improvement drought tolerance in maize14 and osmotic stress tolerance in tobacco.15 PLD has been linked to a wide range of cellular processes of biotechnological importance and that mediate signaling and stress responses.16,17 The PLD activity also is involved in elicitor-induced phytoalexin accumulation in rice cells.18 It has also been reported that phospholipase activities are involved in responses to other phytoregulators, such as cytokinins,19 SA,20 ABA,21,22 MJ and ethylene.23 SA and MJ mediate cell responses to stress, and these compounds have been used as elicitors to study secondary metabolite production in suspension cell cultures of different plants, including Capsicum.1,24,25
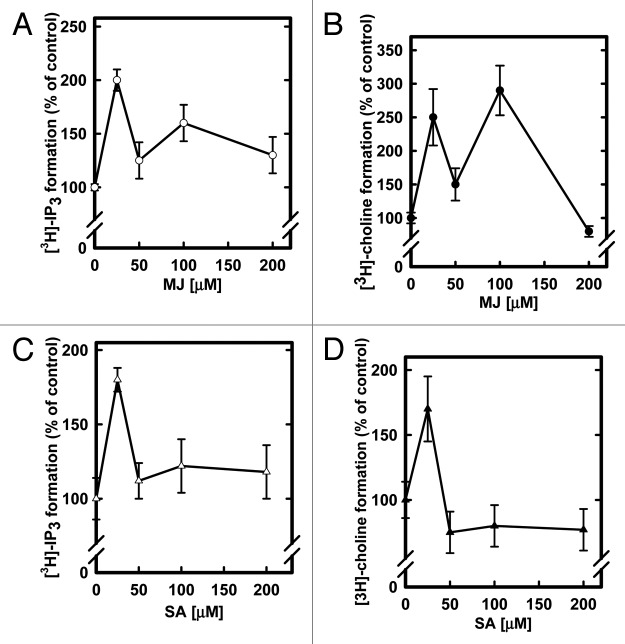
The main goal of this work was to investigate the effects of SA and MJ on the activities of two phospholipases (PLC and PLD) in Capsicum chinense cells on different days of the culture cycle to complement data reported in.1 We used an enzymatic in vitro assay with a radiolabeled substrate in both assays,3[H]-PtdIns(4,5)P2 and3[H]-PC, measuring the formation of Ins(1,4,5)P3 for the PLC activity and choline for the PLD activity, respectively. The experimental procedure was performed as previously reported.1 The results obtained (Fig. 1) show that the PLC activity in the cells on day 7 of the culture was stimulated at 25 μM and 100 μM MJ concentrations (Fig. 1A). In contrast, the PLD activity on day 7 presented an activation with a biphasic behavior, showing two points of high levels of activity, stimulated to 250% and 290% above the control, when the cells were incubated with 25 and 100 μM of MJ, respectively (Fig. 1B). PLC activity in cultured cells for 14 d was stimulated mainly with 25 μM SA. When using higher SA concentrations, PLC activity values remained above the control ones (Fig. 1C), whereas the formation of 3H-choline in the cells at 14 d in the presence of 25 μM of SA was stimulated by 70%, and then there was a decrease in the stimulation of PLD activity.
Figure 1. Effects of increasing concentrations of MJ on day 7 and SA on day 14 of the cell culture for 30 min on the PLC (A, C) and PLD (B, D) activities, respectively. The conditions of the treatments and the enzyme activities measurements are as reported.1 The results shown are the means of five separated experiments ± SE and are expressed as a percentage of the phospholipase specific activity in the absence of MJ and SA.
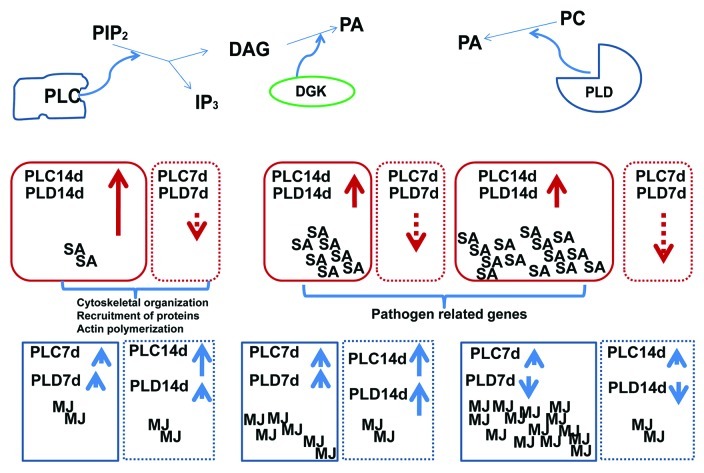
In summary, our data show that the phytoregulator compounds involved in stress responses, including SA and MJ, can modulate the PLC and PLD activities through different phospholipid signal transduction pathways (Fig. 2, top panel) and at different time points (bottom panel) during growth, thus affecting various biochemical processes in the cells (Fig. 2, bottom panel). We also noted a differential response depending on the day of the culture cycle, as observed by a decrease in PLC activity on day 7 in the presence of SA [1] compared with the increased activity in cells on day 14 of the culture cycle (Fig. 1, panel C), resulting in the modulation of several physiological events. The PLD activities detected on days 7 [1] and 14 (Fig. 1, panel D) of the culture cycle also presented differential responses to increasing SA concentrations. Such differential effects on enzyme activities by key growth and defense regulators are congruent with the complex interconnections between several metabolic pathways that exert control at the genetic to structural organization levels in plants (Fig. 2). The effect produced by MJ in the suspension cells produced a similar pattern for the PLC activity on day 7 (Fig. 1, panel A) or 14 [1] of the culture cycle after increasing the phytoregulator concentrations. This effect was similar for the PLD activity on both of these days of the culture cycle, but the stimulation of the enzymatic activity in the first stage of the culture cycle (Fig. 1, panel B) was 2-fold compared with the activity of the cells at 14 d of growth.[1] The PLC and PLD enzymes are involved in the process of cytoskeletal organization, post-translational modification or defensive metabolite production, and our results demonstrate their differential responses to phytoregulators SA and MJ, which are key in several mechanisms of defense (Fig. 2, bottom panel). During the growth cycle, constant metabolic changes are occurring in the cells, and the function of phospholipases could be an outstanding mode to control those processes. We conclude that SA and MJ modulate phospholipidic signaling at different stages of the culture cycle and that these molecules may have diferentially regulatory effects in the general response to phytoregulators in Capsicum chinense J. cells (Fig. 2).
Figure 2. The effects of phytoregulators (MJ, SA) on phospholipase signaling. The effects of these molecules on the PLC or PLD activities are dependent on the day of the culture and can modulate several downstream processes in C. chinense cells. These effects can include responses at the biochemical and molecular levels (reference 1 and the present study).
Li MY, Hong YY, Wang XM. Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim Biophys Acta. 2009;1791:927–35. doi: 10.1016/j.bbalip.2009.02.017.
Acknowledgments
This work was supported by a Consejo Nacional de Ciencia y Tecnología grant (98352) and a fellowship granted to A. A-M (204969).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21220
References
- 1.Altúzar-Molina AR, Muñoz-Sánchez JA, Vázquez-Flota F, Monforte-González M, Racagni-Di Palma G, Hernández-Sotomayor SMT. Phospholipidic signaling and vanillin production in response to salicylic acid and methyl jasmonate in Capsicum chinense J. cells. Plant Physiol Biochem. 2011;49:151–8. doi: 10.1016/j.plaphy.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Clarké JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in arabidopsis. Plant Cell. 2000;12:2175–90. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glazebrook J, Chen W, Estes B, Chang HS, Nawrath C, Métraux JP, et al. Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J. 2003;34:217–28. doi: 10.1046/j.1365-313X.2003.01717.x. [DOI] [PubMed] [Google Scholar]
- 4.Meijer HJG, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 5.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–65. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 6.Laxalt AM, Munnik T. Phospholipid signalling in plant defence. Curr Opin Plant Biol. 2002;5:332–8. doi: 10.1016/S1369-5266(02)00268-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang X. Lipid signaling. Curr Opin Plant Biol. 2004;7:329–36. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Shah J. Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu Rev Phytopathol. 2005;43:229–60. doi: 10.1146/annurev.phyto.43.040204.135951. [DOI] [PubMed] [Google Scholar]
- 9.Piña-Chable ML, Hernandez-Sotomayor SMT. Phospholipase C activity from Catharanthus roseus transformed roots: aluminum effect. Prostag Oth Lipid M. 2001;65:45–56. doi: 10.1016/S0090-6980(01)00113-7. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez JP, Chua NH. Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell. 2001;13:1143–54. doi: 10.1105/tpc.13.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue H, Chen X, Li G. Involvement of phospholipid signaling in plant growth and hormone effects. Curr Opin Plant Biol. 2007;10:483–9. doi: 10.1016/j.pbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Kurosaki F, Tsurusawa Y, Nishi A. Breakdown of phosphatidylinositol during the elicitation of phytoalexin production in cultured carrot cells. Plant Physiol. 1987;85:601–4. doi: 10.1104/pp.85.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyoda K, Shiraishi T, Yamada T, Ichinose Y, Oku H. Rapid changes in polyphosphoinositide metabolism in pea in response to fungal signals. Plant Cell Physiol. 1993;34:729–35. [Google Scholar]
- 14.Wang CR, Yang AF, Yue GD, Gao Q, Yin HY, Zhang JR. Enhanced expression of phospholipase C 1 (ZmPLC1) improves drought tolerance in transgenic maize. Planta. 2008;227:1127–40. doi: 10.1007/s00425-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Sano H. A plasma-membrane linker for the phosphoinositide-specific phospholipase C in tobacco plants. Plant Signal Behav. 2009;4:26–9. doi: 10.4161/psb.4.1.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 17.Li G, Xue HW. Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 2007;19:281–95. doi: 10.1105/tpc.106.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi T, Minami E, Ueki J, Shibuya N. Elicitor-induced activation of phospholipases plays an important role for the induction of defense responses in suspension-cultured rice cells. Plant Cell Physiol. 2005;46:579–87. doi: 10.1093/pcp/pci065. [DOI] [PubMed] [Google Scholar]
- 19.Repp A, Mikami K, Mittmann F, Hartmann E. Phosphoinositide-specific phospholipase C is involved in cytokinin and gravity responses in the moss Physcomitrella patens. Plant J. 2004;40:250–9. doi: 10.1111/j.1365-313X.2004.02205.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu HT, Huang WD, Pan QH, Weng FH, Zhan JC, Liu Y, et al. Contributions of PIP(2)-specific-phospholipase C and free salicylic acid to heat acclimation-induced thermotolerance in pea leaves. J Plant Physiol. 2006;163:405–16. doi: 10.1016/j.jplph.2005.04.027. a. [DOI] [PubMed] [Google Scholar]
- 21.Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot. 2006;57:3337–47. doi: 10.1093/jxb/erl098. b. [DOI] [PubMed] [Google Scholar]
- 22.Krinke O, Ruelland E, Valentová O, Vergnolle C, Renou JP, Taconnat L, et al. Phosphatidylinositol 4-kinase activation is an early response to salicylic acid in Arabidopsis suspension cells. Plant Physiol. 2007;144:1347–59. doi: 10.1104/pp.107.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung T, Lee JH, Cho MH, Kim WT. Induction of 1-aminocyclopropane-1-carboxylate oxidase mRNA by ethylene in mung bean roots: possible involvement of Ca2+ and phosphoinositides in ethylene signaling. Plant Cell Environ. 2000;23:205–13. doi: 10.1046/j.1365-3040.2000.00534.x. [DOI] [Google Scholar]
- 24.Zhao J, Guo YQ, Kosaihira A, Sakai K. Rapid accumulation and metabolism of polyphosphoinositol and its possible role in phytoalexin biosynthesis in yeast elicitor-treated Cupressus lusitanica cell cultures. Planta. 2004;219:121–31. doi: 10.1007/s00425-003-1198-x. [DOI] [PubMed] [Google Scholar]
- 25.Sudha G, Ravishankar GA. Influence of methyl jasmonate and salicylic acid in the enhancement of capsaicin production in cell suspension cultures of Capsicum frutescens. Mill. Curr Sci. 2003;85:1212–7. doi: 10.1078/0176-1617-00928. [DOI] [PubMed] [Google Scholar]