Abstract
Plants have evolved complex signaling networks to respond to their fluctuating environment and adapt their growth and development. Calcium-dependent signaling pathways play key role in the onset of these adaptive responses. In plant cells, the intracellular calcium transients are triggered by numerous stimuli and it is supposed that the large repertory of calcium sensors present in higher plants could contribute to integrate these signals in physiological responses. Here, we present data on CML9, a calmodulin-like protein that appears to be involved in plant responses to both biotic and abiotic stress. Using a reverse genetic approach based on gain and loss of function mutants, we present here data indicating that this CML might also be involved in root growth control in response to the flagellin, a pathogen-associated molecular pattern (PAMP) also involved in plant immunity.
Keywords: Arabidopsis thaliana, Calmodulin-like protein, flagellin, gibberellins, root growth
Many stimuli such as hormones and stress factors elicit changes in intracellular calcium content that serve to convey information and activate appropriate responses.1 These Ca2+ signals are perceived by different Ca2+ sensors, and calmodulin (CaM) is one of the best characterized Ca2+ sensors in eukaryotes. Calmodulin-like (CML) proteins extend the Ca2+-toolkit in plants; CMLs share sequence similarity with the ubiquitous and highly conserved CaM, however, except for some of them, their roles at physiological and molecular levels remain largely unknown.2,3 In our group, we reported data on Arabidopsis thaliana CML9 that exhibits 46% amino acid sequence identity with CaM.4,5 We showed that CML9 transcripts are found in all organs and that CML9 gene is rapidly induced by both abiotic and biotic stress. In a recent publication in Plant Journal, we demonstrated that CML9 expression is also rapidly induced by the phytopathogenic bacteria Pseudomonas syringae pv tomato DC3000 (Pst DC3000)4 and that this upregulation belongs to salicylic acid (SA) production and to the flagellin perception receptor FLS2. Moreover, exogenous applications of SA or flg22, the biological active peptide of flagellin, are also able to induce rapid and transient CML9 gene expression and using a reverse genetic approach, we established that CML9 participates in plant innate immunity through a flagellin-dependent signaling pathway.4
In addition to mediate plant innate immunity, flagellin and flg22 are also known to inhibit root elongation and seedling growth.6 Thus, we explored this facet of flagellin effect and we bring here new informations on plant growth behavior of CML9 overexpressing and knockout lines upon flg22 treatments and discuss the possible involvement of CML9 in plant growth control through hormonal compounds.
The cml9 genotypes exhibit altered responses to flagellin
As previously showed, cml9 mutants or CML9 overexpressors exhibit respectively an enhanced susceptibility and a better resistance against phytopathogenic bacteria.4 Using the non-host strain of Pseudomonas syringae pv phaseolicola mutated in the fliC subunit of the flagellum, we established that plant defense behavior of the cml9 genotypes (KO and OE-CC) mainly depends on the ability of plants to respond to the flagellin perception.4 This suggests that CML9 is involved in the enhancement of PAMP responses leading to set up faster and/or more robust defense responses. Different physiological effects have been associated to the flagellin-derived peptide flg22 such as the increase of antibacterial resistance7 but also the inhibition of seedling growth6 upon perception of flg22 by the FLS2 receptor.
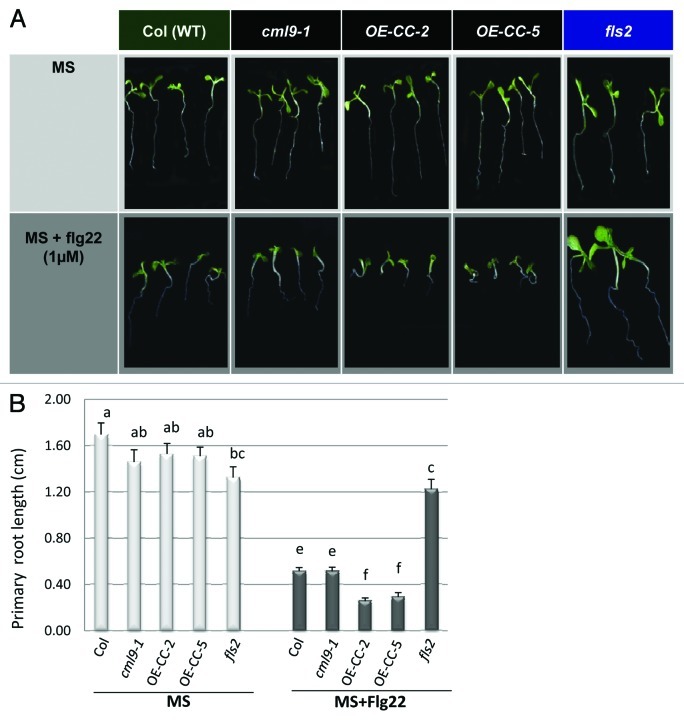
To investigate this possibility, we used a bioassay based on flg22-mediated inhibition of seedling growth to evaluate the involvement of CML9 in this process.6Three-day old wild-type Col, knockout cml9–1, overexpressing CML9 lines (OE-CC-2 and 5) and fls2 mutant seedlings were transferred from agar plates to liquid growth media supplemented or not with 1 μM flg22. Untreated cml9 genotypes (KO and OE-CCs) seedlings were indistinguishable from wild type Col (Fig. 1A, upper panel). A quantitative analyses based on the measurement of the primary root growth confirm these observations because no significative difference was observed between WT and cml9 genotypes when the plants are grown in MS medium (Fig. 1B, MS). Following flg22 treatment, an inhibition of seedlings root growth is observed for all the genotypes except, as expected, for the fls2 mutant which is unable to perceive the flg22 peptide (Fig. 1A, lower panel). Primary root length measurements show a growth inhibition of about 70% for the WT and cml9–1 KO after flg22 application (Fig. 1B, MS+flg22). The cml9 mutant line display similar enhanced PAMP-induced growth inhibition as in the WT (Fig. 1A and B). In contrast, root growth of OE-CCs seedlings was severely stunted after 10 d of growth in the presence of flg22 (Fig. 1A, + flg22) as compared with plants grown in the absence of the PAMP (Fig. 1A, MS). Because CML9 belongs to a multigenic family and although CML9 is one of the CMLs mostly-induced by flg22 application, we cannot exclude some functional redundancies between CML9 and other CMLs that might explain the absence of visible phenotype in the KO mutant. The transgenic CML9 overexpressing lines (OE-CC-2 and 5) had shorter roots than Col (Fig. 1A) and they exhibited a significant enhanced sensitivity (2-fold more important) than Col ecotype or knockout mutant to flg22-mediated inhibition of root growth at 1µM flg22 (Fig. 1B).
Figure 1. Primary root growth analyses of CML9 genotype in reponse to flagellin treatment (A) Wild-type (Col), cml9 genotypes (KO cml9–1 and OE-CCs lines) and fls2 mutant seedlings grown for 7 d in MS medium (control, upper panel) in presence or not of flg22 1µM (lower panel). (B) Quantitative analyses of the primary root growth under control condition (MS) or after flagellin treatment (MS + flg22). The experiments were performed using three independent biological replicates and each histogram represents the mean root length (± SEM) analyzed using 20 to 24 independent roots per genotype. Statistical differences between the genotypes treated or not by flg22 are detected by ANOVA analysis followed by Tukey’s HSD test, at p < 0.05.
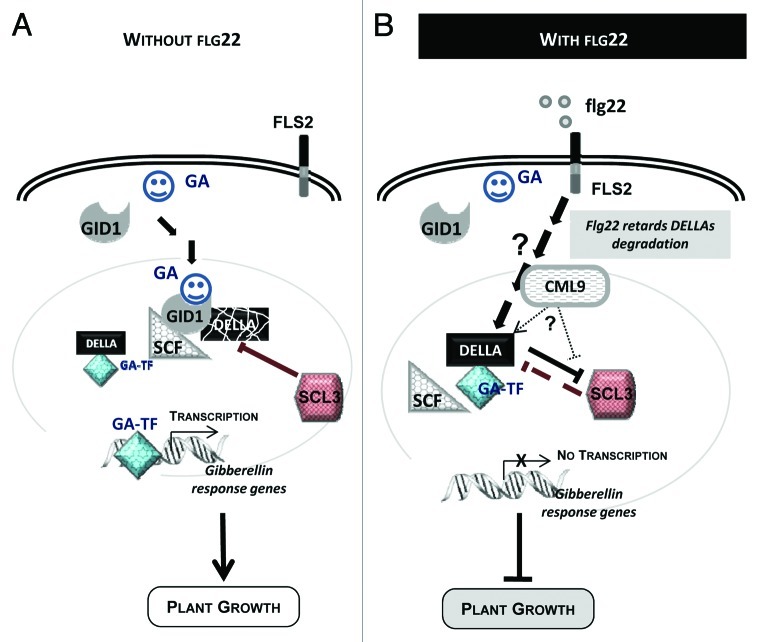
Collectively, these data question about the contribution of CML9 in flg22-induced root growth inhibition process. Interestingly, Navarro et al.8 established that altered growth inhibition upon fl22 treatments were only detected in mutants affected in gibberellins (GAs) biosynthesis or signaling. These data clearly illustrate the major contribution of both GAs and the key regulatory elements of the GA cascade in growth of plants exposed to flagellin.8 It is well known that GAs play central roles in the control of plant growth and development by modulating cell division and cell elongation.9 These past few years, molecular components of GA signaling have been identified and well characterized (Fig. 2A). The soluble GA receptors GID1 (Gibberellin insensitive Dwarf 1) interact with DELLA proteins (DELLAs), that are considered as major negative regulators of GA signaling.10 The DELLAs are conserved repressors of GA signaling that act immediately downstream the GA receptor to modulate all aspects of GA-induced growth and development. DELLAs accumulate when bioactive GA levels are low, whereas degradation of DELLA is accelerated when GAs are elevated9 (Fig. 2A). In response to the flg22 treatment, the accumulation and/or stabilization of the DELLA proteins can be observed and this could explain the flagellin-induced root growth inhibition.8
Figure 2. Hypothetical model involving CML9 in flg22-induced plant growth inhibition through GA-dependent signaling pathway. (A) Model of plant growth control through GA-dependent signaling. Upon GA binding to its soluble receptor (GID1), signaling pathway is activated; DELLA proteins are degraded via the ubiquitin-proteasome pathway. The major players of this GA-signaling cascade are the GA receptors (GID1), the DELLA repressor proteins and the F-box protein part of the SCF E3 Ubiquitin ligase complex. DELLAs are nuclear transcriptional regulators, which interact with other transcription factors (i.e. GA-TF, GA-dependent transcription factor) to modulate expression of GA-responsive genes. (B) Working model of flg22-induced plant growth inhibition that involve GA signaling cascade components and CML9.
What could be the relationship between CML9 and the flagellin-inhibition of root growth?
To go further in the contribution of AtCML9 to plant immunity and plant growth inhibition by flagellin, the identification of the cellular processes controlled by this CML is needed. It is well known that the typical calmodulin act as Ca2+ relay by interacting and modulating the activity of target proteins.1 Experimental evidences demonstrate the ability of CML9 to bind Ca2+ ions11 and CML9 was shown to fulfil under certain conditions the role of CaM in yeast.12 More recently, Perochon et al.13,14 and Popescu et al.15 identified CML9-interacting proteins suggesting that CML9 participates in Ca2+-regulated processes in plant. It was reported by various approaches (two-hybrid screens, in vitro or in planta interactions) that several transcription factors could be the downstream targets of CML9.14,15 Among these nuclear interacting partners, TGA3, TGA2 and WRKY53 were shown to be involved in plant defense16,17 and interestingly, others such as the transcription regulators SCL (Scarecrow-like) could be involved in plant growth control.18 It was shown that CML9 could in vitro interact with SCL3 (identified by an in vitro cDNA library screening),13 SCL4 and SCL21,15 three transcription regulators belonging to the GRAS family. CML9 and SCL3 exhibit a similar gene expression pattern in primary root5,19 and these SCLs exhibit a nuclear localization18,20 which is consistent with the nucleo-cytoplasmic localization of CML9 protein in plant cells.14 Functional analyses using scl3 null mutants indicate that SCL3 acts as a positive regulator of GA signaling that integrate GA signaling in the root to ultimately coordinate cell division and cell expansion.21 Thus, to explain the contribution of CML9 to plant growth control under flg22 treatment, we can hypothesize that CML9 interacts with SCL3 to negatively regulate its activity. According to this model, the accumulation of DELLA might occur leading to root growth inhibition (Fig. 2B).
In the future, the challenge will aim at a better understanding of the biological meaning of the interactions between CML9 and transcription factors associated to plant defenses and to plant growth control. This work will precise the multiple roles played by CML9 and by calcium on the activity of CML9 targets. These researches might lead to new findings that would be integrated into the complex picture of calcium signaling system involved in plant stress responses and plant growth and this will contribute to a better knowledge of CML function in plant physiology.
Material and Methods
Plant material
We used in this study, a T-DNA insertional mutant, cml9–1 (background Col-8)5 and two overexpressing lines (Over expressor of CML9 in Col-8 named OE-CC-2 and OE-CC-5) harbouring constitutive and stronger expression of the CML9 gene compared with the WT.4 Under standard culture conditions, neither the mutation, as reported by Magnan et al. (2008), nor the overexpression of AtCML9 are responsible for significant effect on growth and morphology of transgenic plants as compared with WTs.4 The fls2 Arabidopsis mutant in Columbia ecotype altered in flagellin perception was purchased from the NASC and used as a control in the in vitro plant growth assay.
Flagellin-induced plant growth inhibition
Surface sterilized seeds of different Arabidopsis genotypes were sown in liquid MS medium (0.5x pH 5.7, 1% sucrose) in microplates (24-well) and cultivated for two days into a growth chamber under 16h light. Once germinated, the seedlings were transferred in MS liquid medium supplemented or not with 1µM flg22. The effects of the flg22 or mock treatment on seedling growth were analyzed after 10 d by a quantitative analysis of primary root growth using ImageJ software. All the experiments were performed three times with three independent biological replicates and statistical analyses were treated by using the software Statgraphics Centurion XV (SigmaPlus).
Acknowledgments
University Paul Sabatier (Toulouse III), the CNRS supported this work. This work has been done in the lab UMR5546, part of the “Laboratoire d'Excellence” (LABEX) entitled TULIP (ANR -10-LABX-41).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21308
References
- 1.Reddy AS, Ali GS, Celesnik H, Day IS. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell. 2011;23:2010–32. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack E, Tsai YC, Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–9. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Perochon A, Aldon D, Galaud JP, Ranty B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie. 2011;93:2048–53. doi: 10.1016/j.biochi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Leba L-J, Cheval C, Ortiz-Martín I, Ranty B, Beuzón CR, Galaud J-P, et al. CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway. Plant J. 2012 doi: 10.1111/j.1365-313X.2012.05045.x. [DOI] [PubMed] [Google Scholar]
- 5.Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008;56:575–89. doi: 10.1111/j.1365-313X.2008.03622.x. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Gómez L, Felix G, Boller T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999;18:277–84. doi: 10.1046/j.1365-313X.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 7.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–7. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 8.Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol. 2008;18:650–5. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 9.Sun TP. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. Arabidopsis Book. 2008;6:e0103. doi: 10.1199/tab.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol. 2007;58:183–98. doi: 10.1146/annurev.arplant.58.032806.103830. [DOI] [PubMed] [Google Scholar]
- 11.Köhler C, Neuhaus G. Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Lett. 2000;471:133–6. doi: 10.1016/S0014-5793(00)01383-1. [DOI] [PubMed] [Google Scholar]
- 12.Zielinski RE. Characterization of three new members of the Arabidopsis thaliana calmodulin gene family: conserved and highly diverged members of the gene family functionally complement a yeast calmodulin null. Planta. 2002;214:446–55. doi: 10.1007/s004250100636. [DOI] [PubMed] [Google Scholar]
- 13.Perochon A. Signalisation calcium chez les plantes: Identification et caractérisation de partenaires de CML9, une protéine réceptrice des signaux calciques, impliquée dans les réponses aux stress de l'environnement chez Arabidopsis thaliana. Ecole doctorale Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB). Toulouse. Université Toulouse. 2010;III:112. [Google Scholar]
- 14.Perochon A, Dieterle S, Pouzet C, Aldon D, Galaud JP, Ranty B. Interaction of a plant pseudo-response regulator with a calmodulin-like protein. Biochem Biophys Res Commun. 2010;398:747–51. doi: 10.1016/j.bbrc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Popescu SC, Popescu GV, Bachan S, Zhang Z, Seay M, Gerstein M, et al. Differential binding of calmodulin-related proteins to their targets revealed through high-density Arabidopsis protein microarrays. Proc Natl Acad Sci U S A. 2007;104:4730–5. doi: 10.1073/pnas.0611615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesarwani M, Yoo J, Dong X. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol. 2007;144:336–46. doi: 10.1104/pp.106.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray SL, Ingle RA, Petersen LN, Denby KJ. Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol Plant Microbe Interact. 2007;20:1431–8. doi: 10.1094/MPMI-20-11-1431. [DOI] [PubMed] [Google Scholar]
- 18.Lee MH, Kim B, Song SK, Heo JO, Yu NI, Lee SA, et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol Biol. 2008;67:659–70. doi: 10.1007/s11103-008-9345-1. [DOI] [PubMed] [Google Scholar]
- 19.Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999;18:111–9. doi: 10.1046/j.1365-313X.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 20.Heo JO, Chang KS, Kim IA, Lee MH, Lee SA, Song SK, et al. Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc Natl Acad Sci U S A. 2011;108:2166–71. doi: 10.1073/pnas.1012215108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang ZL, Ogawa M, Fleet CM, Zentella R, Hu J, Heo JO, et al. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108:2160–5. doi: 10.1073/pnas.1012232108. [DOI] [PMC free article] [PubMed] [Google Scholar]