Abstract
The recognition of stromules as sporadically extended stroma filled tubules from all kinds of plastids constitutes one of the major insights that resulted from the direct application of green fluorescent protein aided imaging of living plant cells. Observations of dynamic green fluorescent stromules strongly suggested that plastids frequently interact with each other while photo-bleaching of interconnected plastids indicated that proteins can move within the stroma filled tubules. These observations readily fit into the prevailing concept of the endosymbiogenic origins of plastids and provided stromules the status of conduits for inter-plastid communication and macromolecule transfer. However, experimental evidence obtained recently through the use of photoconvertible protein labeled stromules strongly supports plastid independence rather than their interconnectivity. Additional information on stress conditions inducing stromules and observations on their alignment with other organelles suggests that the major role of stromules is to increase the interactive surface of a plastid with the rest of the cytoplasm.
Keywords: stromules, plastids, Photoconvertible protein, EosFP, fluorescent protein, confocal microscopy, live imaging
Observations of tubular extensions from chlorophyll containing as well as achlorophyllous plastids can be traced back to nearly 125-y-old literature.1-5 Time-lapse movies using differential interference contrast microscopy suggested that the sporadically extending and retracting tubules could result in plastid interactions.6 However, it was the discovery of the green fluorescent protein (GFP) and its subsequent targeting to the plastid stroma that clearly highlighted fluorescent tubules and resulted in the coining of a simple descriptive term ‘stromule’ by Kohler and Hanson.4,5 Further, GFP photo-bleaching showed that there is a flow and exchange of macromolecules between interconnected plastids.4,7,8 Since their re-discovery numerous conditions leading to stromule induction have been reported in original publications and discussed in several reviews.5-14 The authoritative review by Hanson and Sattarzadeh12 specifically appraised the status of stromules and provided a balanced discussion on the different possible roles that stromules might play within the cell. Most importantly this review clearly states that “most plastids are not connected at any one time, although over the course of a day, it is possible that many plastids within a cell establish transient contacts through stromules”12. Nevertheless, an oversimplification of the original observations on stromules has resulted in leading text books presenting stromules as conduits involved in macromolecule exchange between plastids.15 Further many plant biologists have probably not critically assessed the experimental design reported in original publications3-8 and appear to think of stromules as tubular interconnections between plastids.9,16
Thus the basic experimental design that had utilized already connected plastids to establish the idea of plastid interconnectivity remained unchallenged until recently. It is noteworthy that the primary assumption of plastid connectivity via stroma filled tubules was accepted without actually observing the fusion of participating plastids. Interestingly, nearly 15 y after the use of GFP for establishing stromules as plastidic extensions yet another class of fluorescent proteins has been used to provide fresh insights on plastids and stromules.
New fluorescent proteins such as Eos possess the property of photo-convertibilty and thus provide a tremendous advantage over GFP and other single colored FPs.17 Organelles targeted by EosFP-fusion proteins fluoresce green, like those highlighted by GFP. However, a short exposure to violet-blue light causes a major change in the peptide backbone next to the EosFP chromophore and causes its green fluorescence to change irreversibly to red.18 Thus while single-colored fluorescent proteins do not allow discrimination between different plastids, EosFP targeted to the stroma created the possibility of highlighting each plastid in a different color (Fig. 1A). More important, whereas photobleaching experiments are rarely followed up beyond the fluorescence recovery stage the differential coloring achieved through irreversible color change allows individual plastids and their behavior to be tracked over long durations and to detect potential fusion events. This technical advance in fluorescent protein aided research allowed us to reassess the postulated inter-plastid connectivity and protein exchange.15
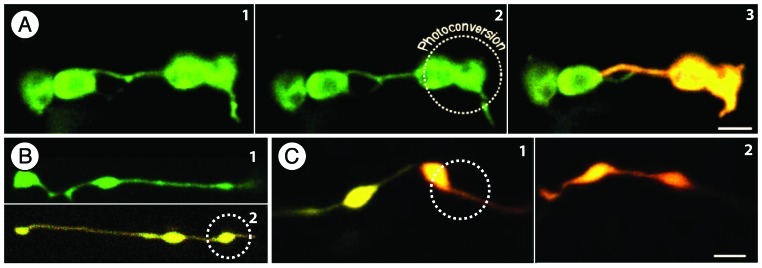
Figure 1. Green to red photoconvertible monomeric Eos fluorescent protein targeted to the plastid stroma allows differential coloring of plastids and has resulted in new insights on stromules. (A) Observing epidermal plastids highlighted through the green non-photoconverted form of tpFNR:mEosFP15 strongly suggests plastid inter-connectivity (1) The dotted circle in (2) indicates the dividing plastid that is illuminated for a few seconds with violet-blue light for achieving irreversible green to red photo-conversion of mEosFP. (3) Photo-conversion results in differential coloring of the plastids and facilitates investigations on stromule interactions and possible exchange of proteins. In this case (3) the red mEosFP remains exclusively in the photoconverted plastid disproving the previous assumption of plastid interconnectivity (1); (B) Etioplasts in dark grown seedlings are pleomorphic and often appear stretched whereby they can be easily misinterpreted as two or more inter-connected plastids. (1) non-photoconverted leucoplast in a root cell in A. thaliana. (2) Photoconverted etioplast in A. thaliana hypocotyl cell with three bulbous dilations along the stretched plastid (circle marking the area illuminated by violet-blue light for photoconversion); (C) Elongated or tubular plastids represent single double membrane bound compartment. Photoconversion on one end of the plastid (1), results in a rapid intermixing of the green and red forms of mEosFP to provide a uniform orange color (2). Such fluorescent protein flow within the plastid compartment is similar to that which was achieved in earlier studies4,7, through the photo-bleaching of GFP in one part of a tubular plastid. Size bars = 5 µm.
The strategy utilizing stroma targeted green to red photoconvertible monomeric Eos fluorescent protein (tpFNR:mEosFP15), for differential coloring of plastids resulted in the following salient observations. Whereas plastids highlighted in green color by mEosFP prior to its photo-conversion appeared to interact through their stromules (Fig. 1A, 1 and 2), differential coloring showed that each plastid and its stromule maintains a clear boundary (Fig. 1A, 3). Similar observations made earlier on stromules from independent plastids that remained in close proximity without exchanging photobleached GFP had led to the conclusion that exchange of plastid contents was prevented since stromules had actually not fused to one another.5 While suggesting that the photobleaching technique can be used to distinguish interconnected plastids from independent plastids the authors had also emphasized that long-term time-lapse studies were needed for confirming whether there is a point during development when independent plastids do connect through their stromules.5 In our recent study clear demarcation between plastids was created through photoconvertible fluorescent protein aided differential coloring of individual plastids. More importantly their visualization over long durations became possible due to the irreversible nature of photoconverted red form of mEos. We were thus able to extend photobleaching based observations spanning a few minutes at the most to several hours.15 Differentially colored plastids did not fuse at any point during our observations and non fusion of plastids was further confirmed by observations on cells of the plastid-division-impaired arc6 mutant with 2–5 plastids only.15 Most importantly, the differential coloring technique when applied to mitochondria, another postulated endosymbiogenic organelle, resulted in a clear exchange and merging of green and red fluorescent forms of mEosFP within a matter of minutes.15
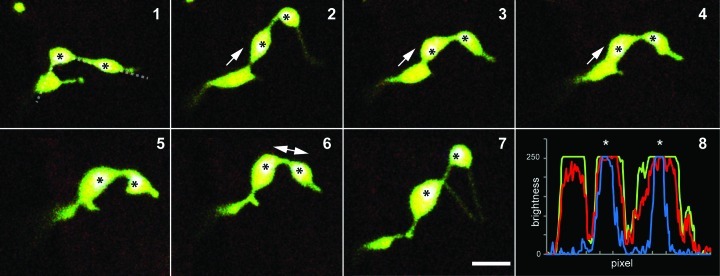
Figure 2. Non-green plastids like etioplasts and leucoplasts lack large rigid internal structures and this allows their shape to be more flexible than mesophyll chloroplasts. The morphological flexibility and the formation of a constricted isthmus in late stages of plastid division frequently gives the impression of two or more plastids being connected by stromules. Because most of these plastids are missing grana organization, the identification of the main plastid body in pleomorphic tubules is difficult. Seven frames (1–7) from a 5 min time series depicting a plastid in a mature A. thaliana root epidermal cell show plastid pleomorphy. At the beginning (1), three bulbous regions are connected by tubules, suggesting plastids interconnected by stromules. However the chlorophyll signal, which can be detected in plastids in mature roots of in vitro, light grown plants, is observed in only two of the plastid shaped bulbous regions (marked by '*'). The RGB-intensity plot (see 8; blue line denotes chlorophyll fluorescence) confirms the observation of two plastids only in the group of bulbous dilations. During the time lapse series the dilation without chlorophyll signal fused with one of the chlorophyll signal-containing bulbs (2–4), indicating that both bulbs were indeed parts of the same plastid. (8) The RGB-intensity plots along the dotted line in (1) illustrate the even distribution of photoconverted red and non-photoconverted green mEosFP (visible in the merged images 1–7 as yellowish color) and clearly proves the presence or absence of chlorophyll in the bulbs (overlay of blue chlorophyll signals with green and red result in white sectors ‘*’). Prominent movements of bulbs are indicated by arrows. Size bar = 5µm.
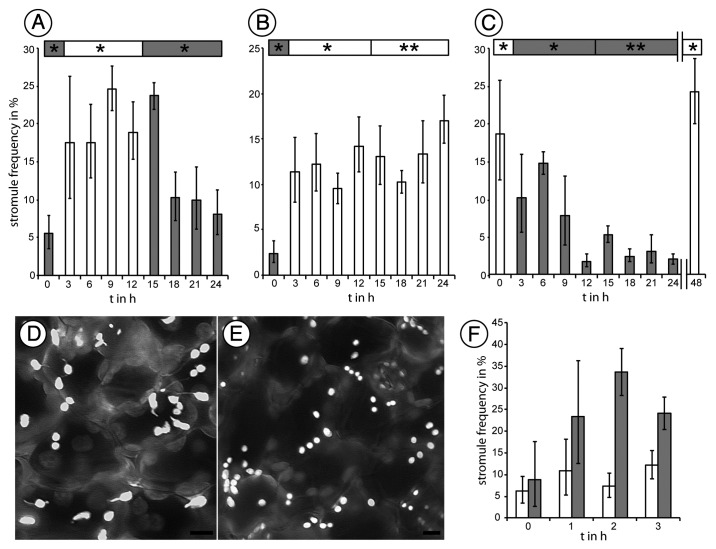
Figure 3. Stromule frequency in epidermal cells of A. thaliana plants expressing FNR-EGFP is dependent upon illumination. The presence of light favors the formation of stromules where in darkness stromule frequency declines slowly until a base level of about 5% is reached. Nevertheless, stromules can be induced by sucrose application in dark-adapted leaves. (A-C) 24-h (h) experiments showing average stromule frequency and the respective 5% confidence intervals. Probes were taken every three hours. The normal 12h/12h day light cycle phases is marked ‘*’ ; extended phases are marked ‘**’. (D) Gray-scale images of epidermal plastids at the end of a normal day showing plastids with stromules, (E) Plastids at the end of a normal night are mainly without stromules. (F) Average stromule frequency and respective 5% confidence intervals for 40 mM sucrose treatment (gray), a prominent inducer of stromules in light adapted leaves, and water treated control (white). Size bar = 5 µm.
Since stromules from independent plastids did not appear to fuse or allow fluorescent protein to be exchanged our next critical question concerned the identity and nature of the ‘interconnected plastids’ observed in earlier studies. An appraisal of literature4,7,11, showed that the so-called ‘interconnected’ plastids were nearly always observed in dark grown plants. It is particularly important to note that dark grown plants display etioplasts with an elongated, tubular, pleomorphic form,15 (Fig. 1B, 1 and 2). Interestingly the initial study that had established the presence of stromules in plant cells had also made its key photobleaching based observations on tubular leucoplasts from root tissue.4 In our recent photo-convertible fluorescent protein aided studies of plastids in hypocotyl and roots of light and dark grown plants we identified two possible mechanisms whereby plastid morphology can convey the impression of two or more interconnected plastids.
First while chloroplasts with well developed grana and starch grains assume a turgid spheroidal shape, etioplasts in dark-grown tissue and leucoplasts in roots possess loose and flexible internal membranes and possibly relaxed outer membrane envelopes. The tubular form of a single elongated plastid with bulbous regions can appear like two or more interconnected plastids (Fig. 1B and C). In complete agreement with earlier observations4,5,7,12 photo-bleaching or photo-conversion performed in any portion of tubular plastids results in a quick redistribution of fluorescent stroma targeted protein (Fig. 1B). Further, observations on clusters of tubular plastids showed that protein redistribution takes place within a unit plastid and is delimited by the double, inner and outer, membrane envelopes. It was concluded that despite appearances, a stroma filled region linking two or more bulbous areas in a tubule is not likely to represent interconnected plastids.15 By definition, as long as an organelle is bounded by two membrane envelopes and contains plastidic features such as pro-lamellar bodies or thylakoid stacks and stroma it should be considered as a single plastid. This qualification is open to amendment if, in the future, it can be shown that two previously independent plastids in a normal non-senescent cell actually come together and fuse to share their stromal contents.
The second possibility involved plastids in late stages of division. Dividing plastids remain connected via an isthmus, which is barely visible in closely appressed chloroplasts. However this short stromal bridge is readily visible as a connecting tubule when daughter plastids are pulled apart or when non-green plastids undergo elongation while dividing (Fig. 2, 6 and 7). This possibility had been previously discussed but not explored experimentally.11 Our observations on the plastid division mutants arc5 and arc6 strongly supported these ideas.15 As pointed out earlier although the isthmus connects two distinct plastid bodies with their complement of internal membranes it is important to note that the two bodies have not yet separated and thus in reality still constitute a single double membrane bound unit.
Our observations in wild type and plastid division mutants strongly suggest that in etiolated hypocotyl cells and in root tissue, a significant population of plastids exhibit pleomorphy due to both phenomena occurring at the same time (Fig. 2).
Whereas our recent findings confirmed the independent nature of plastids suggested earlier,5,7 they strongly suggest that stromules might not be involved in interplastid exchange of proteins in the range of 25 to 27 kDa or more. Whether other smaller proteins are exchanged between plastids with or without the involvement of stromules still remains an open question. However, another recent study,16 was undertaken to determine if plastid DNA or plastid ribosomes are able to enter stromules and thus aid in transfer of genetic information between plastids. Although it is known that plastids do not exchange genetic information, except very rarely, in the case of protoplast fusion,12 the new study also showed that plastid ribosomes do not routinely move into stromules in tobacco and Arabidopsis.16
Our findings on stromules made through the use of photoconvertible mEosFP provided a better picture than was available through the use of single colored fluorescent proteins. Accordingly we have been able to suggest a minor modification to the definition of ‘stromule’ as ‘ a stroma-filled tubule extended by an independent plastid ’ to differentiate them from stroma filled tubules that form an isthmus between pleomorphic elongated-dividing plastids.15
Transfer of materials among plastids does not appear to be the primary function of stromules.12 However, the length of single stromules, often several times the length of the main plastid body strongly suggests that they might be involved in facilitating transfer between compartments.12 Indeed stromules often exhibit branching in close alignment with ER-tubules.19,20 Organelles such as mitochondria and peroxisomes are routinely observed near plastids and often display a more than coincidental localization along stromules.6,21,22 Moreover, observations of increased stromule induction frequency in response to exogenous feeding of sucrose or glucose13 and during abscisic acid (ABA) mediated physiological stress14 suggest that these tubular structures might be formed in response to physiological changes in the cell. One of the prominent changes in the physiological state of a plant cell occurs due to changing sugar levels during the diurnal cycle. How is stromule formation affected by these changes? Our recent investigations on this aspect are presented.
We assessed stromule frequency over 24 h in soil grown Arabidopsis plants expressing tpFNR-GFP that highlights stromules.19 Plants were grown initially under short day conditions (8 h light/16 h darkness) for 6 weeks to obtain well developed rosette leaves before being shifted to a 12 h light / 12 h night rhythm for 7 cycles. Leaves from these plants were harvested every three hours for observing stromules in upper epidermal plastids as described earlier.13 During the dark period leaves were harvested under a green safe light. In general, stromule frequency was found to be approximately 5% at the end of a dark period, just before exposure to light. However, 3 h after exposure to light the stromule frequency rose significantly and approached 18%. The 18 to 25% stromule frequency range was maintained throughout the light period and into the first three hours of darkness. However, observations taken 6 h in darkness showed a general decrease in stromule frequencies that finally reverted to the 5–10% range by the end of the second night (Fig. 3A). In order to check whether stromule induction was totally dependent upon the light-dark cues or was under a circadian control, experiments were performed under extended periods of light and darkness. Whereas the extended light period allowed a higher stromule frequency to be maintained (Fig. 3B) the extended darkness sustained a low basal level of stromule frequency (Fig. 3C). Switching a plant subjected to a prolonged dark period back to light allowed stromules to be extended again. Interestingly, the addition of 40 mM sucrose, a known inducer of stromule formation in light adapted plants,13 mimicked the effect of light exposure when applied to dark adapted leaves (Fig. 3F). Control treatments with water only maintained a constant stromule frequency (Fig. 3F).
From these experiments we concluded that stromule extension and retraction are strongly influenced by the light-dark regimes and are possibly brought about through alterations in plastid and cytosolic sugar levels. Support for our findings comes from Arabidopsis mutants in mechano-sensitive ion channel (MscS)–like (MSL) proteins.23,24 The observations on MSL mutants suggest that plastids are under hypo-osmotic stress resulting in relaxed envelope membranes during normal plant growth and require MSL2 and MSL3 for dynamic responses including the formation of stromules.
The highly commendable studies performed earlier1-12 had firmly established the presence of stromules as plastidic extensions. The recent findings reaffirm the uniqueness of plastids and strongly suggest that stromules do not act as conduits for inter-plastid communication but as important extensions that increase plastid interactions with the rest of the cytoplasm (Fig. 4). The observations on regulation of stromule formation by different abiotic and biotic cues pave the way for further detailed experiments on these exciting but still enigmatic organelle extensions within the plant cell.
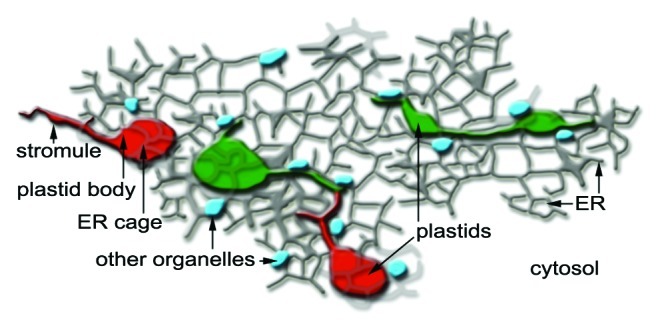
Figure 4. The new findings using stroma targeted photoconvertible EosFP change our idea of stromules as being interplastid conduits and instead present them as plastidic extensions for more effective communication and interaction with other cellular components such as the ER, small organelles such as mitochondria and peroxisomes (depicted in blue) and the cytosol in general. Notably, the new insight presents plastids and their stromules as pleomorphic but unique organelles and not subunits of a single homogenous plastid population. The uniqueness of each plastid might eventually be responsible for the tremendously flexible and versatile nature of plant cells.
Acknowledgments
We thank Joerg Wiedenmann for the gift of mEosFP and gratefully acknowledge the review and suggestions of Maureen Hanson. Funding to the JM lab is by the Natural Sciences and Engineering Research Council of Canada (NSERC); the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation, Ontario (OMRI).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21342
References
- 1.Haberlandt G. Die chlorophyllkorper der selaginellen. Flora. 1888;71:291–308. [Google Scholar]
- 2.Wildman SG, Hongladarom T, Honda SI. Chloroplasts and mitochondria in living plant cells: Cinephotomicrographic studies. Science. 1962;138:434–6. doi: 10.1126/science.138.3538.434. [DOI] [PubMed] [Google Scholar]
- 3.Menzel D. An interconnected plastidom in Acetabularia: Implications for the mechanism of chloroplast motility. Protoplasma. 1994;179:166–71. doi: 10.1007/BF01403955. [DOI] [Google Scholar]
- 4.Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. Exchange of protein molecules through connections between higher plant plastids. Science. 1997;276:2039–42. doi: 10.1126/science.276.5321.2039. [DOI] [PubMed] [Google Scholar]
- 5.Köhler RH, Hanson MR. Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci. 2000;113:81–9. doi: 10.1242/jcs.113.1.81. [DOI] [PubMed] [Google Scholar]
- 6.Gunning BE. Plastid stromules: video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma. 2005;225:33–42. doi: 10.1007/s00709-004-0073-3. [DOI] [PubMed] [Google Scholar]
- 7.Köhler RH, Schwille P, Webb WW, Hanson MR. Active protein transport through plastid tubules: velocity quantified by fluorescence correlation spectroscopy. J Cell Sci. 2000;113:3921–30. doi: 10.1242/jcs.113.22.3921. [DOI] [PubMed] [Google Scholar]
- 8.Kwok EY, Hanson MR. GFP-labelled Rubisco and aspartate aminotransferase are present in plastid stromules and traffic between plastids. J Exp Bot. 2004;55:595–604. doi: 10.1093/jxb/erh062. [DOI] [PubMed] [Google Scholar]
- 9.Gray JC, Sullivan JA, Hibberd JM, Hansen MR. Stromules: Mobile protrusions and interconnections between plastids. Plant Biol. 2001;3:223–33. doi: 10.1055/s-2001-15204. [DOI] [Google Scholar]
- 10.Pyke KA, Howells CA. Plastid and stromule morphogenesis in tomato. Ann Bot (Lond) 2002;90:559–66. doi: 10.1093/aob/mcf235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwok EY, Hanson MR. Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep. 2004;23:188–95. doi: 10.1007/s00299-004-0824-9. [DOI] [PubMed] [Google Scholar]
- 12.Hanson MR, Sattarzadeh A. Stromules: recent insights into a long neglected feature of plastid morphology and function. Plant Physiol. 2011;155:1486–92. doi: 10.1104/pp.110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schattat MH, Klösgen RB. Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biol. 2011;11:115. doi: 10.1186/1471-2229-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray JC, Hansen MR, Shaw DJ, Graham K, Dale R, Smallman P, et al. Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 2012;69:387–98. doi: 10.1111/j.1365-313X.2011.04800.x. [DOI] [PubMed] [Google Scholar]
- 15.Schattat MH, Griffiths S, Mathur N, Barton K, Wozny MR, Dunn N, et al. Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell. 2012;24:1465–77. doi: 10.1105/tpc.111.095398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newell CA, Natesan SK, Sullivan JA, Jouhet J, Kavanagh TA, Gray JC. Exclusion of plastid nucleoids and ribosomes from stromules in tobacco and Arabidopsis. Plant J. 2012;69:399–410. doi: 10.1111/j.1365-313X.2011.04798.x. [DOI] [PubMed] [Google Scholar]
- 17.Mathur J, Radhamony R, Sinclair AM, Donoso A, Dunn N, Roach E, et al. mEosFP-based green-to-red photoconvertible subcellular probes for plants. Plant Physiol. 2010;154:1573–87. doi: 10.1104/pp.110.165431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Röcker C, Salih A, et al. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc Natl Acad Sci USA. 2004;101:15905–10. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schattat MH, Barton K, Baudisch B, Klösgen RB, Mathur J. Plastid stromule branching coincides with contiguous endoplasmic reticulum dynamics. Plant Physiol. 2011;155:1667–77. doi: 10.1104/pp.110.170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schattat MH, Barton K, Mathur J. Correlated behavior implicates stromules in increasing the interactive surface between plastids and ER tubules. Plant Signal Behav. 2011;6:715–8. doi: 10.4161/psb.6.5.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwok EY, Hanson MR. In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. BMC Plant Biol. 2004;4:2. doi: 10.1186/1471-2229-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sage TL, Sage RF. The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant Cell Physiol. 2009;50:756–72. doi: 10.1093/pcp/pcp033. [DOI] [PubMed] [Google Scholar]
- 23.Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 24.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol. 2012;22:408–13. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]