Abstract
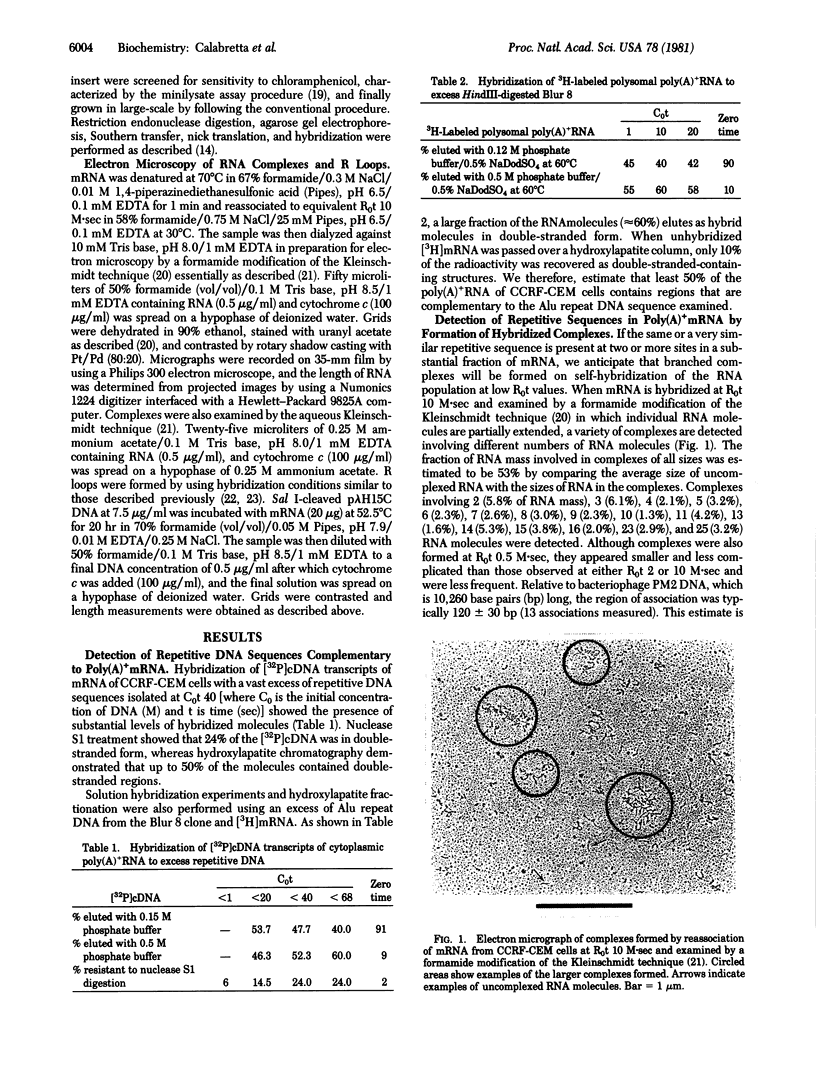
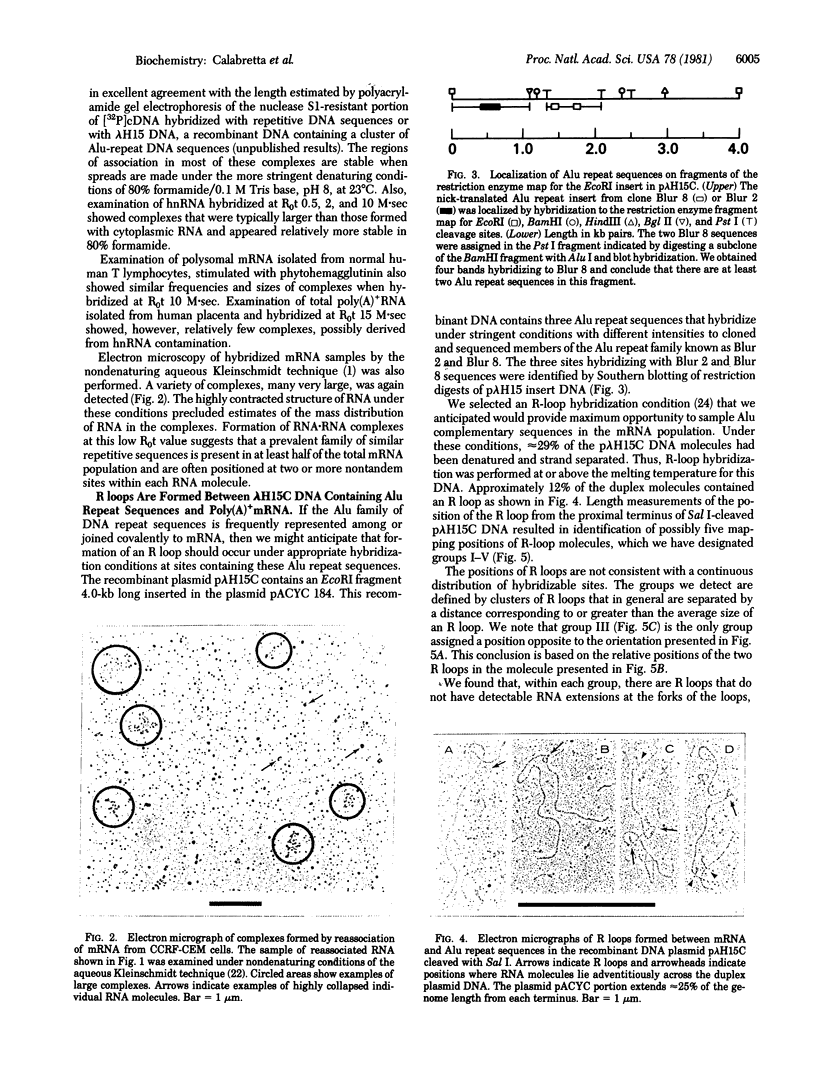
Approximately one-half of the polysomal poly(A)+RNA from CCRF-CEM human lymphoblastoid cells associates at low R0t (10 M.sec) [where R0 is the initial concentration of RNA (M) and t is time (sec)] to form branched complexes detectable by electron microscopy. The complexes typically involve 2-16 molecules associated over double-stranded regions 120 +/- 30 base pairs long. Formation of such complexes suggests that poly(A)+RNA contains repeated-sequence elements that are highly represented in the mRNA population. Hybridization of polysomal poly(A)+RNA with a recombinant human DNA plasmid, p lambda H15C, which is shown to contain at least three regions complementary to two different members of the Alu family of DNA repeat sequences, showed a total of five regions where R loops are formed. The hybridized regions comprising these groups are 260 +/- 180, 240 +/- 170, 150 +/- 70, 180 +/- 60, and 180 +/- 80 base pairs long. The relative frequencies of R loops formed at these different sites indicate that sequences in this recombinant DNA are represented in the mRNA population at different frequencies. The hybridizing sequence of the RNA molecules is located near one terminus in 13% of the R loops and internally in 53% of the R loops. Surprisingly, 35% of the R loops apparently involve RNA molecules hybridized over their entire length of only 200 +/- 110 base pairs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Dhar R., Khoury G. A small RNA induced late in simian virus 40 infection can associate with early viral mRNAs. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1379–1383. doi: 10.1073/pnas.77.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. D., Britten R. J., Davidson E. H. Message sequences and short repetitive sequences are interspersed in sea urchin egg poly(A)+ RNAs. Nature. 1980 Sep 11;287(5778):111–117. doi: 10.1038/287111a0. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Dina D., Meza I., Crippa M. Relative positions of the "repetitive", "unique" and poly(A) fragments of mRNA. Nature. 1974 Apr 5;248(448):486–490. doi: 10.1038/248486a0. [DOI] [PubMed] [Google Scholar]
- Dott P. J., Chuang C. R., Saunders G. F. Inverted repetitive sequences in the human genome. Biochemistry. 1976 Sep 7;15(18):4120–4125. doi: 10.1021/bi00663a032. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Specific nucleotide sequences in HeLa cell inverted repeated DNA: enrichment for sequences found in double-stranded regions of heterogeneous nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):591–601. doi: 10.1016/0022-2836(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Evans R., Wilson M., Salditt-Georgieff M., Darnell J. E. Oligonucleotides in heterogeneous nuclear RNA: similarity of inverted repeats and RNA from repetitious DNA sites. Biochemistry. 1978 Jul 11;17(14):2776–2783. doi: 10.1021/bi00607a012. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. A family of short, interspersed repeat sequences at the 5' end of a set of Dictyostelium single-copy mRNAs. Cell. 1979 Apr;16(4):787–796. doi: 10.1016/0092-8674(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Klein W. H., Murphy W., Attardi G., Britten R. J., Davidson E. H. Distribution of repetitive and nonrepetivite sequence transcripts in HeLa mRNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1785–1789. doi: 10.1073/pnas.71.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Binding of adenovirus VA RNA to mRNA: a possible role in splicing? Nature. 1980 Jun 19;285(5766):575–577. doi: 10.1038/285575a0. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Jelinek W. Determination of nucleotide sequences from double-stranded regions of HeLa cell nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):571–589. doi: 10.1016/0022-2836(77)90103-6. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Shirakawa S., Saunders P. P., Arrighi F. E., Hsu T. C. Populations of repeated DNA sequences in the human genome. J Mol Biol. 1972 Feb 14;63(3):323–334. doi: 10.1016/0022-2836(72)90430-5. [DOI] [PubMed] [Google Scholar]
- Tashima M., Calabretta B., Torelli G., Scofield M., Maizel A., Saunders G. F. Presence of a highly repetitive and widely dispersed DNA sequence in the human genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1508–1512. doi: 10.1073/pnas.78.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]






