Abstract
The potato tuber constitutes a model system for the study of dormancy release and sprouting, suggested to be regulated by endogenous plant hormones and their balance inside the tuber. During dormancy, potato tubers cannot be induced to sprout without some form of stress or exogenous hormone treatment. When dormancy is released, sprouting of the apical bud may be inhibited by sprout control agents or cold temperature. Dominance of the growing apical bud over other lateral buds decreases during storage and is one of the earliest morphophysiological indicators of the tuber's physiological age. Three main types of loss of apical dominance (AD) affect sprouting shape. Hallmarks of programmed cell death (PCD) have been identified in the tuber apical bud meristem (TAB-meristem) during normal growth, and are more extensive when AD is lost following extended cold storage or chemical stress. Nevertheless, the role of hormonal regulation in TAB-meristem PCD remains unclear.
Keywords: apical dominance, bud activation, dormancy release, physiological age, potato tuber, sprouting, stress response
Introduction
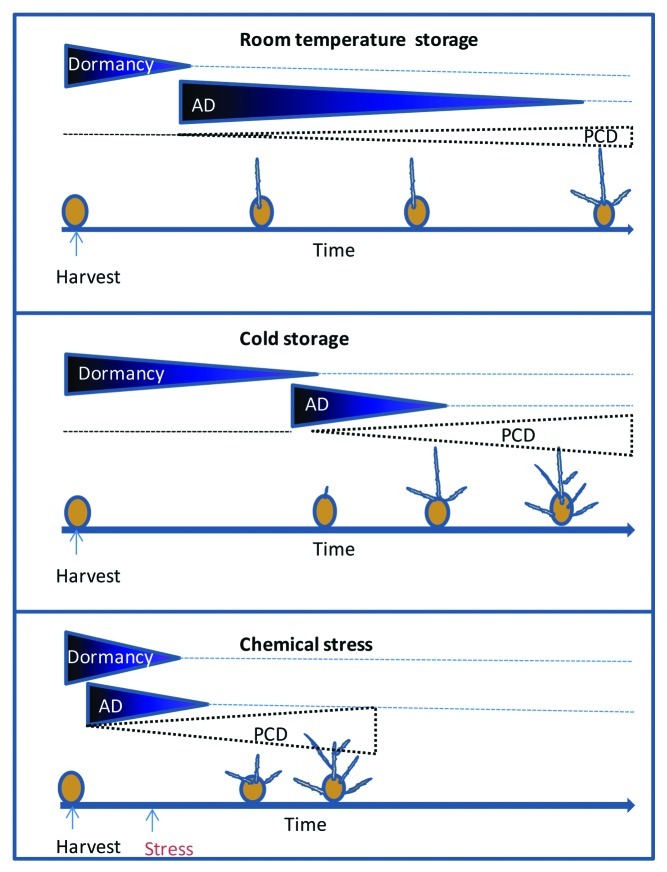
The potato (Solanum tuberosum L.) tuber is a swollen underground stem formed by swelling of the subapical underground stolons.1 As the tuber elongates, a growing number of lateral bud meristems (termed eyes) are formed in a spiral arrangement on its surface.2 After harvest, tuber buds are generally dormant and will not sprout or grow, even if the tubers are placed under optimal conditions for sprouting (i.e., warm temperature, darkness, high humidity). The dormancy observed in postharvest potato tubers is defined as endodormancy,3 and is due to an unknown endogenous signal(s) that mediates suppression of meristem growth.4 Dormancy is thought to be a physiological adaptation to intermittent periods of environmental limitations and is therefore a survival mechanism that prevents sprouting when tubers would be exposed to extreme temperatures.5 The duration of the endodormancy period is primarily dependent on the genotype, but other factors, such as growth conditions of the crop and storage conditions after tuber harvest, are also important.6,7 Following a transition period of between 1 and 15 weeks depending on the storage conditions and variety, dormancy is broken and apical buds start to grow.7 Typically one eye/sprout becoming dominant and inhibiting the growth of the other eyes that are paradormant (meristem arrested by external environmental factors).5 Tubers stored at room temperature will sprout weeks before those stored in the cold, with a single long bud (Fig. 1). Tuber sprouting is usually initiated from its apical bud, located opposite the tuber-stolon connection site. Although the postharvest potato tuber is used as a model system for the study of metabolic processes associated with dormancy release, sprouting and aging, very few studies have been done on apical dominance (AD) during these processes.8-10
Figure 1. Schematic representation of dormancy release and loss of apical dominance (AD) as a result of potato tuber storage at room temperature or in the cold, or following a specific chemical stress.
Hormonal Regulation of Tuber Bud Sprouting
The level of each bud's autonomy in terms of timing of dormancy release and sprouting, and its interactions with other buds on the same tuber, are still unclear. Endogenous plant hormones and their relative balance within the tuber are suggested to regulate endodormancy, bud activation and sprouting.6,11-15 Ethylene and abscisic acid have been associated with the onset and maintenance of tuber dormancy,16 and molecular analysis has indicated that the expression of genes associated with the catabolic metabolism of abscisic acid correlates with dormancy release of bud meristems in potato tubers.17-20 In correlation, ABA content is highest immediately after harvest when meristem dormancy is deepest, and it falls gradually during storage as dormancy weakens.21 in spite of that, continuous exposure to diniconazole and 8'-acetylene-ABA during microtuber development had no effects on subsequent sprouting at any time point or significantly increased the rate of microtuber sprouting, respectively. Suggesting that, although a decrease in ABA content is a hallmark of tuber dormancy progression, the decline in ABA levels is not a prior condition for dormancy release.22
Gibberellins (GAs) are inducers of bud activation and elongation after dormancy is released, but their endogenous levels are not associated with maintenance of dormancy.11,13 Interestingly, at the time of initial sprouting, internal levels of these bioactive GAs were lower than those found in deeply dormant tubers.5 The endogenous contents of GA19, GA20, and GA1 were relatively high immediately after harvest, declined during storage, and rose to the highest levels during the period of robust sprout growth.11 Hartman et al.13 showed that transgenic potato plants with modified GA biosynthesis—expressing Arabidopsis GA 20-oxidase under the control of the chimeric STLS1/CaMV35 promoter—exhibit early tuber sprouting. These results showed that endogenous GA is able to terminate tuber dormancy and promote sprout outgrowth.
Biologically active cytokinins (CKs) increase over time in dormant potato tissues, suggesting a role for this class of hormones in bud activation.13,14,23 Expression of isopentenyltransferase from Agrobacterium tumefaciens in potato tubers to enhance endogenous CK levels, or cytokinin oxidase/dehydrogenase1 (CKX) to reduce endogenous CK content, produced an earlier sprouting phenotype compared with the wild type or a prolonged dormancy period, respectively.13 This result supports an essential role for CKs in bud activation and shows that GA is not sufficient to break dormancy in the absence of CK.
There is evidence that the most abundant naturally occurring auxin, indole acetic acid (IAA), is at its highest level at the early stages of tuber dormancy, and later decreases in the buds during storage.12,24,25 Feeding experiments have indicated that changes in IAA biosynthesis rate are a major cause of auxin variation in buds.12 In dormant buds from freshly harvested tubers, the free hormone was found to accumulate mostly in the apical meristem, leaf and lateral bud primordia, as well as differentiating vascular tissues underlying the apical meristem, whereas at the end of the storage period, only lateral bud primordia from growing buds displayed appreciable auxin levels.12 Since AD is gradually lost during storage, auxin might be the link between bud activation and its AD.
AD in Potato Tubers
AD in potato tubers results in control of the apical bud over lateral bud outgrowth. It is similar to the AD condition exerted by the shoot tip in many different plants (for review see ref.26-29). Cline30 suggested that apical dominance and its release may be devided into four developmental stages: lateral bud formation (stage I), imposition of inhibition (apical dominanc) (stage II), initiation of lateral bud outgrowth following decapitation (stage III), and subsequent elongation and development of lateral bud into branch (stage IV). He suggested that there is some overlap between the four stages and the degree of inhibition imposed in stage II may vary between species.30 The current view suggests that shoot AD and branching regulation involve three long-range hormonal signals: auxin, which is synthesized mainly in young expanding leaves then moves down the plant in the polar transport stream, and strigolactone and CKs, synthesized in both the root and shoot, which move up the plant, most likely in the transpiration stream. Auxin clearly plays a role in AD, i.e., suppression of lateral buds activation by the apical meristem, but the mechanism by which the auxin signal is perceived in the lateral bud is subject to debate.26
Michener8 showed that when the intact potato tuber begins to grow after dormancy is released, one or more apical buds grow, but the lateral buds usually do not. If, however, lateral buds and apical buds are excised and grown separately, both start to grow at the same time. Moreover, in non-dormant tubers, any first-growing, large bud usually inhibits the growth of late-growing, smaller ones.8 Teper-Bamnolker et al.31 observed three main types of AD loss in stored potato: loss of dominance of the apical buds over those situated more basipetally on the tuber (“type I”); loss of dominance of the main bud in any given eye over the subtending axillary buds within the same eye (“type II”), and loss of dominance of the developing sprouts over their own branching, meaning that side stems do not emerge from the base of the sprout as in type II (“type III”).
Type I loss of dominance has been shown to exhibit classical stem-like behavior, but the developing apical bud suppresses only mature or dormancy released buds. Removing the apical bud induces early sprouting of all other mature buds in the same tuber. After 30, 60 and 90 d in cold storage, an average of 1, 2 and 9 buds sprouted, respectively,31 suggesting the need for each bud to reach maturity and autonomous dormancy release before it is controlled by the tuber apical bud meristem (TAB-meristem). Cline30 distinguish between initiation of axillary bud growth and subsequent axillary shoot elongation, which may be under the control of different hormone factors, as shown lately by Hartmann et al.13 Removal of a lateral meristem complex or wounding between buds did not impact AD or sprouting rate.31 These experiments emphasize the importance of TAB-meristem presence and viability in the control of lateral bud meristem growth, before sprouting is observed.
AD and Tuber Physiological Age
The physiological age of the seed tuber is the physiological stage that influences its productive capacity.32 The physiological status of a seed tuber at any time is determined by genotype, chronological age, and environmental conditions from tuber initiation until new plant emergence (reviewed by Caldiz33). Struik32 suggested that the summed temperature during storage is the predominant factor affecting physiological aging, although its effect is moderated by light conditions and genetic factors. The physiological age of seed tubers affects future crop performance, i.e., stem emergence rate, percentage of emergence, number of emerged stems per mother tuber, time to tuber initiation, crop vigor and growth, dry matter distribution and tuber yield.34-37
Sprout type is one of the earliest morphophysiological indicators of a seed tuber's physiological age. Krijthe38 described four stages of sprouting shape in storage after dormancy is released: (i) AD where only one sprout develops, (ii) additional multiple buds sprouting as a result of reduced AD, (iii) branching of the sprouting stems, and (iv) in the aging mother tubers, sprout replacement by daughter tubers.
Effect of Sprouting Control on AD
Previous studies have shown that immediately after harvest, during their dormant period, potato tubers cannot be induced to sprout without some form of stress or exogenous hormone treatment.13,14,39 On a large commercial scale, Rindite (a mixture of ethylene chlorhydrin, ethylene dichloride and carbon tetrachloride),40 bromoethane (BE),41 CS242,43 and GA344 have been used to break tuber seed dormancy. Michener8 found that in dormant tubers treated with ethylene chlorhydrin, much of the auxin disappears. The auxin reappears within 2 or 3 d after treatment termination. Michener8 also observed loss of AD after the chemical treatment, and its restoration by application of IAA to the apex of the tubers. He concluded that auxin inhibits bud growth in the dormant tuber and that removal of the auxin by the action of ethylene chlorhydrin allows growth to proceed.
The phytotoxic chemical BE shortens the natural dormancy period from 2–4 mo to approximately 10 d.45-47 Campbell et al.45 observed that transcript profiles in BE-induced cessation of dormancy are similar to those observed in natural dormancy release, suggesting that both follow a similar biological pattern during this transition. Thus, BE treatment can be used to compress and synchronize release from the dormant period, which is an advantage from an experimental standpoint.45 Teper-Bamnolker et al.31 showed that BE application induces early sprouting in freshly harvested 'Nicola' and 'Désirée' tubers, as well as loss of AD. Buds surrounding the apical buds tended to grow faster than those located in more distant segments of the tuber. Loss of type I AD as a result of BE treatment was followed by loss of type III dominance, expressed as excessive branching of the growing shoots.31 Teper-Bamnolker et al.48 also showed that very low doses of the sprout inhibitor R-carvone can also induce early sprouting and loss of AD. Whereas high doses of this inhibitor were shown to damage cellular membranes in the apical meristem, no such damage was detected when the sprout-inducing low dose was used, suggesting a signaling effect.48 At both R-carvone doses, the final result was loss of all types of AD when the tuber sprouted leading to a bush-like pattern of growing buds.
The mode of action of phytotoxic chemicals in inducing dormancy release and altering apical bud dominance is poorly understood. Teper-Bamnolker et al.31 proposed programmed cell death (PCD) in the TAB-meristem as one of the mechanisms regulating AD. Hallmarks of PCD were identified in the TAB-meristems during normal growth, and these were more extensive when AD was lost following either extended cold storage or BE treatment (Fig. 1). Hallmarks included DNA fragmentation, induced gene expression of vacuolar processing enzyme 1 (VPE1) and elevated VPE activity.31 Treatment of tubers with BE and then VPE inhibitor induced faster growth and AD recovery in detached and nondetached apical buds, respectively, suggesting that PCD is associated with weakening of tuber AD, allowing early sprouting of mature lateral buds.31
Cold storage is the main tool used worldwide to delay sprouting of stored tubers. When the tuber is exposed to cool temperatures during its dormancy period, the number of sprouting buds after dormancy is released increases with time of exposure. In other words, an increase in the number of weeks of exposure to cool temperatures reduces AD.32 Fauconnier et al.49 found that AD can last for up to approximately 60 d in storage in cvs. Bintje and Désirée. Between 60 and 240 d of storage, sprout number per tuber increased linearly with time due to loss of AD. Low temperature (4°C as compared with 12°C) reduces sprouting capacity and AD, and increases the number of stems when the tubers eventually do sprout.50
To date, none of the sprout control agents studied in potato have been shown to delay loss of AD. Dyson and Digby51 suggested that calcium is necessary to maintain AD of the sprout and prevent some of the changes attributed to physiological aging. Calcium application delays the loss of AD, probably by preventing the subapical necrosis typical to sprouting of potato tubers in dark storage.
Conclusions and Perspectives
Potato tubers exhibit AD behavior that is very similar to that of other stems. Apical bud dominance may serve as a marker for tuber physiological age. However, it can be altered by a number of abiotic stresses, including storage temperature and chemical sprouting control agents. Some of these factors have been shown to induce PCD in the TAB-meristem of the potato tuber.31 PCD plays an important role in various stages of plant development, such as embryogenesis, self-incompatibility, xylogenesis and senescence, and in response to biotic or abiotic stresses.52-59 However, the role of hormonal regulation in TAB-meristem PCD is still unclear. The enzyme VPE1 could be a key factor in TAB-meristem development and dominance, as use of a specific VPE inhibitor restored growth and dominance of the apical bud, though this needs to be further proven in transgenic potato tubers.
Glossary
Abbreviations:
- AD
apical dominance
- BE
bromoethane
- CK
cytokinin
- PCD
programmed cell death
- TAB-meristem
tuber apical bud meristem
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21324
References
- 1.Harris PM. The potato crop: the scientific basis for improvement 1992: Chapman and Hall. [Google Scholar]
- 2.Goodwin P. The control and branch growth on potato tubers: I. Anatomy of buds in relation to dormancy and correlative inhibition. J Exp Bot. 1967;18:78–86. doi: 10.1093/jxb/18.1.78. [DOI] [Google Scholar]
- 3.Lang GA, Early JD, Martin GC, Darnell RL. Endo, para-and ecodormancy: physiological terminology and classification for dormancy research. Hort Sci. 1987;22:371–7. [Google Scholar]
- 4.Suttle JC. Physiological regulation of potato tuber dormancy. Am J Potato Res. 2004;81:253–62. doi: 10.1007/BF02871767. [DOI] [Google Scholar]
- 5.Suttle JC. Dormancy and Sprouting. In: Vreugdenhil, D, ed. Potato Physiology and Biotechnology. Advances and perspectives, edition no 1; Amsterdam: Elsevier, 2007: 287-305. [Google Scholar]
- 6.Turnbull CGN, Hanke DE. The control of bud dormancy in potato tubers. Planta. 1985;165:359–65. doi: 10.1007/BF00392233. [DOI] [PubMed] [Google Scholar]
- 7.Wiltshire JJJ, Cobb AH. A review of the physiology of potato tuber dormancy. Ann Appl Biol. 1996;129:553–69. doi: 10.1111/j.1744-7348.1996.tb05776.x. [DOI] [Google Scholar]
- 8.Michener HD. Dormancy and apical dominance in potato tubers. Am J Bot. 1942;20:558–68. doi: 10.2307/2437105. [DOI] [Google Scholar]
- 9.Kumar G, Knowles NR. Involvement of auxin in the loss of apical dominance and plant growth potential accompanying aging of potato seed tubers. Can J Bot. 1993;71:541–50. doi: 10.1139/b93-060. [DOI] [Google Scholar]
- 10.Holmes JC, Lang RW, Singh AK. The effect of five growth regulators on apical dominance in potato seed tubers and on subsequent tuber production. Potato Res. 1970;13:342–52. doi: 10.1007/BF02358279. [DOI] [Google Scholar]
- 11.Suttle JC. Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical assessment. J Plant Physiol. 2004;161:157–64. doi: 10.1078/0176-1617-01222. [DOI] [PubMed] [Google Scholar]
- 12.Sorce C, Lombardi L, Giorgetti L, Parisi B, Ranalli P, Lorenzi R. Indoleacetic acid concentration and metabolism changes during bud development in tubers of two potato (Solanum tuberosum) cultivars. J Plant Physiol. 2009;166:1023–33. doi: 10.1016/j.jplph.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann A, Senning M, Hedden P, Sonnewald U, Sonnewald S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011;155:776–96. doi: 10.1104/pp.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suttle JC. Ethylene is not involved in hormone-and bromoethane-induced dormancy break in Russet Burbank minitubers. Am J Potato Res. 2009;86:278–85. doi: 10.1007/s12230-009-9081-3. [DOI] [Google Scholar]
- 15.Ji ZL, Wang SY. Reduction of abscisic acid content and induction of sprouting in potato, Solanum tuberosum L., by thidiazuron. J Plant Growth Regul. 1988;7:37–44. doi: 10.1007/BF02054160. [DOI] [Google Scholar]
- 16.Suttle JC, Hultstrand JF. Role of endogenous abscisic acid in potato microtuber dormancy. Plant Physiol. 1994;105:891–6. doi: 10.1104/pp.105.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simko I, McMurry S, Yang HM, Manschot A, Davies PJ, Ewing EE. Evidence from polygene mapping for a causal relationship between potato tuber dormancy and abscisic acid content. Plant Physiol. 1997;115:1453–9. doi: 10.1104/pp.115.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Destefano-Beltrán L, Knauber D, Huckle L, Suttle JC. Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol Biol. 2006;61:687–97. doi: 10.1007/s11103-006-0042-7. [DOI] [PubMed] [Google Scholar]
- 19.Ewing EE, Simko I, Omer EA, Davies PJ. Polygene mapping as a tool to study the physiology of potato tuberization and dormancy. Am J Potato Res. 2004;81:281–9. doi: 10.1007/BF02871770. [DOI] [Google Scholar]
- 20.Campbell MA, Gleichsner A, Alsbury R, Horvath D, Suttle J. The sprout inhibitors chlorpropham and 1,4-dimethylnaphthalene elicit different transcriptional profiles and do not suppress growth through a prolongation of the dormant state. Plant Mol Biol. 2010;73:181–9. doi: 10.1007/s11103-010-9607-6. [DOI] [PubMed] [Google Scholar]
- 21.Suttle JC. Postharvest changes in endogenous ABA levels and ABA metabolism in relation to dormancy in potato tubers. Physiol Plant. 1995;95:233–40. doi: 10.1111/j.1399-3054.1995.tb00832.x. [DOI] [Google Scholar]
- 22.Suttle JC, Abrams SR, De Stefano-Beltrán L, Huckle LL. Chemical inhibition of potato ABA-8′-hydroxylase activity alters in vitro and in vivo ABA metabolism and endogenous ABA levels but does not affect potato microtuber dormancy duration. J Exp Bot. 2012 doi: 10.1093/jxb/ers146. [DOI] [PubMed] [Google Scholar]
- 23.Suttle JC. Involvement of ethylene in potato microtuber dormancy. Plant Physiol. 1998;118:843–8. doi: 10.1104/pp.118.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorce C, Lorenzi R, Ceccarelli N, Ranalli P. Changes in free and conjugated IAA during dormancy and sprouting of potato tubers. Funct Plant Biol. 2000;27:371–7. [Google Scholar]
- 25.Sukhova LS. I. MachÁČkovÁ, J. Eder, N.D. Bibik, and N.P. Korableva, Changes in the levels of free IAA and cytokinins in potato tubers during dormancy and sprouting. Biol Plant. 1993;35:387–91. doi: 10.1007/BF02928514. [DOI] [Google Scholar]
- 26.Dun EA, Ferguson BJ, Beveridge CA. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiol. 2006;142:812–9. doi: 10.1104/pp.106.086868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyser O. The control of shoot branching: an example of plant information processing. Plant Cell Environ. 2009;32:694–703. doi: 10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillips IDJ. Apical dominance. Annu Rev Plant Physiol. 1975;26:341–67. doi: 10.1146/annurev.pp.26.060175.002013. [DOI] [Google Scholar]
- 29.Cline MG. Apical dominance. Bot Rev. 1991;57:318–58. doi: 10.1007/BF02858771. [DOI] [Google Scholar]
- 30.Cline MG. Concepts and terminology of apical dominance. Am J Bot. 1997;84:1064–1064. doi: 10.2307/2446149. [DOI] [PubMed] [Google Scholar]
- 31.Teper-Bamnolker P, Buskila Y, Lopesco Y, Ben-Dor S, Saad I, Holdengreber V, et al. Release of apical dominance in potato tuber is accompanied by programmed cell death in the apical bud meristem. Plant Physiol. 2012;158:2053–67. doi: 10.1104/pp.112.194076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struik P. The canon of potato science: 40. Physiological age of seed tubers. Potato Res. 2007;50:375–7. doi: 10.1007/s11540-008-9069-2. [DOI] [Google Scholar]
- 33.Caldiz DO. Physiological age research during the second half of the twentieth century. Potato Res. 2009;52:295–304. doi: 10.1007/s11540-009-9143-4. [DOI] [Google Scholar]
- 34.O'Brien P, Allen E, Bean J, Griffith R, Jones SA, Jones J. Accumulated day-degrees as a measure of physiological age and the relationships with growth and yield in early potato varieties. J Agric Sci. 1983;101:613–31. doi: 10.1017/S002185960003865X. [DOI] [Google Scholar]
- 35.Vakis N. Influence of physiological ageing of seed potatoes on yield and earliness. Potato Res. 1986;29:417–25. doi: 10.1007/BF02359973. [DOI] [Google Scholar]
- 36.Van Loon C. Effect of physiological age on growth vigour of seed potatoes of two cultivars. 4. Influence of storage period and storage temperature on growth and yield in the field. Potato Res. 1987;30:441–50. doi: 10.1007/BF02361921. [DOI] [Google Scholar]
- 37.Moll A. The effects of physiological ageing of seed tubers on growth characteristics of eight potato cultivars tested under controlled conditions. Potato Res. 1994;37:11–20. doi: 10.1007/BF02360427. [DOI] [Google Scholar]
- 38.Krijthe N. Observations on the sprouting of seed potatoes. Potato Res. 1962;5:316–33. [Google Scholar]
- 39.Struik PC, Wiersema SG. Seed potato technology, ed. W. Pers. 1999, Wageningen: CSIRO. 383. [Google Scholar]
- 40.Rehman F, Lee SK, Kim HS, Jeon JH, Park J, Joung H. Dormancy breaking and effects on tuber yield of potato subjected to various chemicals and growth regulators under greenhouse conditions. J Biol Sci. 2001;1:818–20. doi: 10.3923/jbs.2001.818.820. [DOI] [Google Scholar]
- 41.Coleman WK. Large scale application of bromoethane for breaking potato tuber dormancy. Am J Potato Res. 1984;61:587–9. doi: 10.1007/BF02852968. [DOI] [Google Scholar]
- 42.Meijers CP. Effect of carbon-disulphide on the dormancy and sprouting of seed-potatoes. Potato Res. 1972;15:160–5. doi: 10.1007/BF02355962. [DOI] [Google Scholar]
- 43.Salimi K, Tavakkol Afshari R, Hosseini MB, Struik PC. Effects of gibberellic acid and carbon disulphide on sprouting of potato minitubers. Sci Hortic (Amsterdam) 2010;124:14–8. doi: 10.1016/j.scienta.2009.12.026. [DOI] [Google Scholar]
- 44.Rappaport L, Lippert LF, Timm H. Sprouting, plant growth, and tuber production as affected by chemical treatment of white potato seed pieces. Am J Potato Res. 1957;34:254–60. doi: 10.1007/BF02855192. [DOI] [Google Scholar]
- 45.Campbell M, Segear E, Beers L, Knauber D, Suttle J. Dormancy in potato tuber meristems: chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct Integr Genomics. 2008;8:317–28. doi: 10.1007/s10142-008-0079-6. [DOI] [PubMed] [Google Scholar]
- 46.Alexopoulos AA, Aivalakis G, Akoumianakis KA, Passam HC. Bromoethane induces dormancy breakage and metabolic changes in tubers derived from true potato seed. Postharvest Biol Technol. 2009;54:165–71. doi: 10.1016/j.postharvbio.2009.07.004. [DOI] [Google Scholar]
- 47.Destefano-Beltrán L, Knauber D, Huckle L, Suttle J. Chemically forced dormancy termination mimics natural dormancy progression in potato tuber meristems by reducing ABA content and modifying expression of genes involved in regulating ABA synthesis and metabolism. J Exp Bot. 2006;57:2879–86. doi: 10.1093/jxb/erl050. [DOI] [PubMed] [Google Scholar]
- 48.Teper-Bamnolker P, Dudai N, Fischer R, Belausov E, Zemach H, Shoseyov O, et al. Mint essential oil can induce or inhibit potato sprouting by differential alteration of apical meristem. Planta. 2010;232:179–86. doi: 10.1007/s00425-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 49.Fauconnier ML, Rojas Beltrán J, Delcarte J, Dejaeghere F, Marlier M, Jardin P. Lipoxygenase pathway and membrane permeability and composition during storage of potato tubers (Solanum tuberosum L. cv Bintje and Desiree) in different conditions. Plant Biol. 2002;4:77–85. doi: 10.1055/s-2002-20439. [DOI] [Google Scholar]
- 50.Hartmans KJ, Van Loon C. Effect of physiological age on growth vigour of seed potatoes of two cultivars. I. Influence of storage period and temperature on sprouting characteristics. Potato Res. 1987;30:397–410. doi: 10.1007/BF02361918. [DOI] [Google Scholar]
- 51.Dyson P, Digby J. Effects of calcium on sprout growth and sub-apical necrosis in Majestic potatoes. Potato Res. 1975;18:290–305. doi: 10.1007/BF02361732. [DOI] [Google Scholar]
- 52.Lam E. Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol. 2004;5:305–15. doi: 10.1038/nrm1358. [DOI] [PubMed] [Google Scholar]
- 53.Lam E. Programmed cell death in plants: orchestrating an intrinsic suicide program within walls. Crit Rev Plant Sci. 2008;27:413–23. doi: 10.1080/07352680802467744. [DOI] [Google Scholar]
- 54.Fukuda H. Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol. 2004;5:379–91. doi: 10.1038/nrm1364. [DOI] [PubMed] [Google Scholar]
- 55.Bozhkov PV, Filonova LH, Suarez MF. Programmed cell death in plant embryogenesis. Curr Top Dev Biol. 2005;67:135–79. doi: 10.1016/S0070-2153(05)67004-4. [DOI] [PubMed] [Google Scholar]
- 56.Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–90. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Della Mea M, De Filippis F, Genovesi V, Serafini Fracassini D, Del Duca S. The acropetal wave of developmental cell death of tobacco corolla is preceded by activation of transglutaminase in different cell compartments. Plant Physiol. 2007;144:1211–22. doi: 10.1104/pp.106.092072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turner S, Gallois P, Brown D. Tracheary element differentiation. Annu Rev Plant Biol. 2007;58:407–33. doi: 10.1146/annurev.arplant.57.032905.105236. [DOI] [PubMed] [Google Scholar]
- 59.Bonneau L, Ge Y, Drury GE, Gallois P. What happened to plant caspases? J Exp Bot. 2008;59:491–9. doi: 10.1093/jxb/erm352. [DOI] [PubMed] [Google Scholar]