Abstract
Some studies showed that anesthetics reduce the response of physical stimuli in Mimosa pudica and in Venus Flytrap (Dionaea muscipula), peculiar plants that have the ability to respond to touch stimuli. In this research we tested the effects of ketamine, lidocaine, diethyl ether, and amlodipine on the movements of Mimosa pudica and Venus Flytrap. With a literature review, we tried to bring elements to theorize about the interaction of these substances with these plants. The angular displacement in Mimosa´s petiole and in Dionaea leaves is what was measured to compare the drugs group with control groups.
Keywords: Dionaea muscipula, Mimosa pudica, anesthetics, ether, lidocaine
Introduction
Some studies showed that anesthetics reduce the response of physical stimuli in Mimosa Pudica1-4 and in Dionaea muscipula (Venus Flytrap),2 peculiar plants that have the ability to respond to touch stimuli. In 1878, Claude Bernard observed that ether had effects on M. pudica, and found that this substance rendered the plant insensitive after being exposed for 25 min.1 In 1875, Charles Darwin, while investigating anesthetics effects on the botanical carnivore’s leaf-closing (D. muscipula), tried chloroform and then ether vapor. He observed that sensibility of this plant was altered by the presence of ether.5 Okazaki2 tested volatile anesthetic agents such as methoxyflurane, chloroform, halothane, enflurane and sevoflurane im M. pudica, and proposed that the response to anesthetic agents in plants maybe similar to that in animals. Applying different volatile agents it was determined that plant action vary with the anesthetic potency. It was proposed that In terms of reversible anesthesia, animals seem to equal plants in order of potency of anesthetics, and the minimum immobilizing concentration. This author suggests the existence of a certain common mechanism in anesthesia between animals and plants. Milne and Beamish3 showed that inhalational and local anesthetics reduce tactile and thermal responses in Mimosa pudica, using in this experiment halothane and lidocaine. Plants were also treated with agents known to disrupt either K+ efflux (tetraethylammoniumchloride), Na+, K+-adenosine triphosphatase or Ca+- influx (verapamil) to determine the ionic species involved in the tactile response of M. pudica. All of this ionic blockers employed produced a slower response movement, as well as growth retardation.6,7 Ion channel blockers tetraethylammonium chloride and Ba2+ as well as uncouplers carbonylcyanide-3-chlorophenylhydrazone (CCCP), carbonylcyanide-4-tri〉uoromethoxyphenyl hydrazone, pentachlorophenol, and 2, 4-dinitrophenol increase the time of trap closure in venus flytrap and require a signi□cantly larger electrical charge to close the trap in the experiments using electrical stimuli.8 Yao9 demonstrated the effects of actin and calcium-related reagents on the bending angle of Mimosa pudica petioles. It was demonstrated a decrease in angle movement of the petioles related with some substances (latrunculin A; phalloidin; LaCl3; nifedipine and Ruthenium Red). Ethylene, discussed in the contemporary plant sciences as stress hormone, is also a very powerful anesthetic.10-12 Moreover, several anesthetics were reported to overcoming dormancy in seeds13 and to inhibit cytoplasmic streaming.14
Plants can react to mechanical stimuli with the use of mechano-sensitive channels but only certain plants with rapid and highly noticeable touch-stimulus response, such as Mimosa Pudica and Venus 〉ytrap. In both species of plants, the mechanisms involved in plant’s response are related to specialized cells that have the capacity of change rapidly it conformation, where the flux of water and ions occurs through the membranes of these cells. The motor cells of the midrib of D. muscipula and those found in the primary pulvinus of M. pudica probably act in a parallel manner.9
In this research we tested the effects of ketamine, lidocaine, diethyl ether, and amlodipine on the movements of Mimosa Pudica and Dionaea muscipula. With a literature review, we tried to bring elements to theorize about the interaction of these substances with these plants. A question that can be initially made is: are plants a possible model to study anesthetics and other drugs used for animals?
Results
The angular displacement in Mimosa and in Venus is what was measured to compare the drugs group with control groups. Assumed p-value = 0,01, amlodipine showed have no significant effects vs. control in mimosa and Venus flytrap (p > 0,10) with 118 degrees of freedom. In the same way Ketamine had no significant effects vs. control in mimosa and Venus flytrap (p > 0,10). Lidocaine had significant effect vs. control group in mimosa causing strong immobilization of the petioles (p < 0,001) and spontaneous closing of leaflets, but not in Venus Flytrap (0,02 > p > 0,01). Ether had an evident effect in Mimosa and Venus Flytrap (p < 0,001) causing almost total immobilization. (Fig. 1)
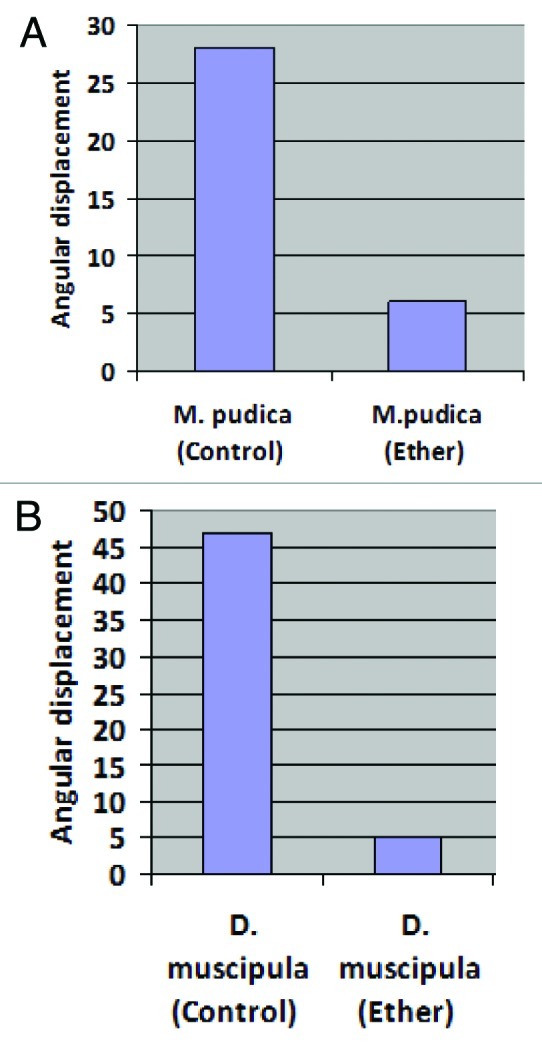
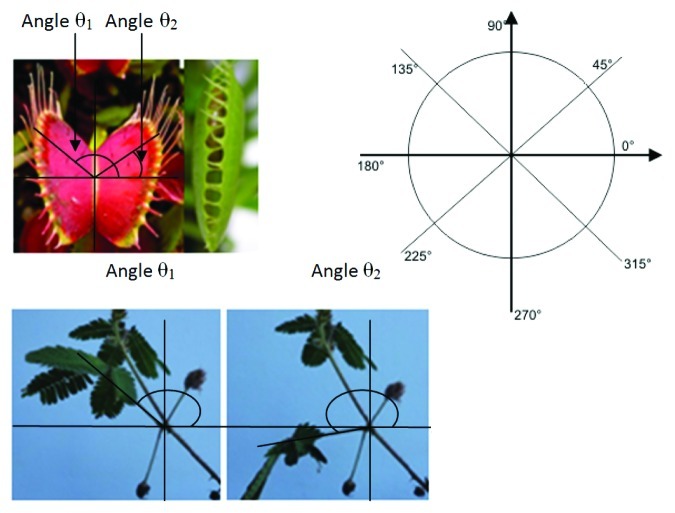
Figure 1.Dionaea muscipula (on the left) and Mimosa pudica (on the right). Normal angular displacement in D. muscipula (Δθ) given by equation: [(θ1 - 90°) + (90° - θ2) before stimulation] - [(θ1 - 90°) + (90° - θ2) after stimulation] = ± 47° and in Mimosa Pudica (Δθ) the result of subtracting: (θ2 – θ1 = ± 28°).The illustrations show the plants before (left) and after mechanical stimulation (right), demonstrating the angular displacement. Circle angles from 0 to 360 degrees to calculate the positions at the end.
Both, the ether and lidocaine, showed clearly toxicity observed in both plants depending of drug doses. Two Mimosa plants had serious dehydration after lidocaine and one Mimosa had the leaflets dehydrated after the exposure with more then 1ml of ether 35%. All Venus Flytrap plants had some leaves affected after few days with lidocaine use (dehydration and death portions of plant). Ether did not show toxicity in Venus Flytrap plants. It shows that the reversibility of the “state of anesthesia” is a critical point, although it is observable after 12 h (standard deviation = 6h) in mimosas with 40 d of life time (ether). In Venus flytrap the return of the normal reaction after ether exposure happened after 40 min (standard deviation = 30 min) (ether).
Discussion
There are some methodologies to determine the effects of certain substances on those kinds of plants. Two of them are remembered here. In a microscopy analysis, the patch-clamp technique allows the study of single or multiple ion channels in cells. In a macroscopic analysis, it is possible to observe the reaction of plants treated with some substances. In this macroscopic view it is important to know common patterns of this power to move of these plants and much of these patterns have been studied in some works related to Mimosa pudica and Dionaea muscipula.8,9
The drugs in this study were chosen based on previous works that obtained immobilization of these sensitive plants (anesthetics and calcium channel blockers). Each drug dose, was chosen without a certain criterion in case of ketamine and amlodipine, and based on previous works in case of ether and lidocaine.2,3,5
Some initial questions may be collocated: the aqueous drugs actually reached a site of action, and what concentration was achieved? All drugs are somewhat a stressor to cells and beyond the concentrations that bind to specific proteins to mediate specific action and they can alter the function of multiple systems. Are some effects mediated by these broader actions? In plants, these anesthetic agents might bind to protein(s) and because of their lipid solubility, may mediate actions by altering membrane lipid. Are these proteins related to any animal protein? The importance of drug doses is suggested by the toxicity found with the ether and with lidocaine. Both drugs produced dehydration, an effect possible due to the plants being subjected to a great osmotic stress. Is the immobilization of the plants caused by this osmotic stress on the “motor cells” ? Is this immobilization related with anesthesia?
In case of the anesthetics, there are several theories that attempt to explain their mechanism of action. These theories are based on the macroscopic, microscopic (cellular) and molecular level. Certainly all contribute in some way to the elucidation of the phenomenon, however, no explains them in isolation. The unitary theory defends the action of anesthetics in various parts of the central nervous system in humans, where they act in molecular affinity and lipid solubility. The lipid theory comes from Meyer and Overton that observed the correlation between the potency of anesthetics and their olive oil solubility. The protein theories often assume specific receptors related to the action of anesthetics.15 Many receptor, ion channel and enzymes were associated in this action (nicotinic acetylcholine receptor, NMDA receptor, non-NMDA glutamate receptor, GABAA receptor, GABAB receptor, glicine receptor, peptide receptor, G-protein-linked systems,α2-adrenergic receptor, Na+ channel, K+ channel, Cl- channel, protein Kinase C, phospholipase C, inositide turnover, mitochondrial electron transport, transport ATPases, luciferases, etc.).16 If we believe that these plant movements depend on propagation of an action potentials through specialized cells (in analogy with a neurons cell),8,9,17 the immobilization by some drugs let us think that exist some similarity in the blockage of action potentials in plants and in animals. However, this similarity must be demonstrated yet.
Claude Bernard, in an essay on the mechanism of anesthesia, observing the effects of aqueous solutions of chloroform or diethyl ether and their vapor on an animal muscle model, noted that this biological tissue under the exposure of these substances, became unresponsive to electrical stimuli, rigid, and it transparency was decreased. When the anesthetics were removed by washing or evaporation, the muscle returned to the normal transparency and responded to electrical stimuli. With this observation, this author envisioned, in modern terminology, the concept that anesthesia is a reversible equilibrium state of protein colloid in which the conformation and the state of the hydration changed. According with Ueda, this wisdom probably encompasses the whole present concept of anesthetic-protein interactions.16
Saltveit18 demonstrate that exposure to vapors of the anesthetics halothane and methoxyflurane reduced chilling injury in cucumber hypocotyl segments, cucumber cotyledon discs and tomato pericarp discs. The author proposes that the relative effectiveness of the two anesthetics in reducing chilling injury is “similar to their relative effectiveness in inducing anesthesia in animals and their relative lipid solubilities.” The response of the plant tissues to methoxyflurane and halothane, which increase membrane fluidity, and to high pressures, which decrease membrane fluidity, are according with the hypothesis that cold-induced phase transition of membranes, could be responsible for chilling injury.18 Nevertheless the author suggests that other cellular components may also be affected, saying that low temperatures, high pressures and anesthetics can alter protein conformation, affect ion channels, depolymerize microtubules and cause the release of calcium from membrane lipids.18
Our interest in this study is to focus the attention in the possible relation of some chosen substances here, with the motor cells of the midrib of Venus Flytrap and the primary pulvinus of Mimosa pudica.
In relation with osmoregulation in pulvinar flexor cells, this movements are consequence of divergent volume and turgor changes in two oppositely positioned parts of this cells. The called “Osmotic Motor” is power-driven by a plasma membrane proton ATPase, which take KCl fluxes and, consequently, water, crossways the pulvinus into swelling cells and out of shrinking cells.19 Luminosity signals and signals from the endogenous biological clock (involved in circadian rhythm that causes the movement of leaflets during the 24 h of a day) congregate on the channels through which these fluxes happen.19 Ion channels are alleged to be the conduits for the entry and efflux of K+ and the efflux of Cl−19 and Water channels (aquaporins) are alleged to serve as water conduits from side to side the pulvinar motor cell membranes (γ-TIP aquaporin in Mimosa for example).20 One of the present models for the osmotic volume changes of pulvinar cells, does not diverge in principle from that established for the stomata guard cells.21 In a distinction to guard cells, in the integral pulvinus the solute and water fluxes may occur to some extent as well via plasmodesmata interconnecting the pulvinar motor cells. The forces for pulvinar cell shrinking for the period of the very fast seismonastic reaction of Mimosa might be different in some ways (seismonastic and thigmonastic movements). Leaflets collapse in seconds, and not in tens of minutes, as in rhythmic movements. This peculiar quick movement is thought to result from a abrupt sucrose unloading from phloem addicted to the extensor apoplast, rising the osmotic drive for water efflux from cells22 and/or ultrafiltration of water out of the cells due to sudden squeezing achievement exerted by activated cytoskeletal elements.23 The very fast movement of the leaves of Venus fly trap, occurring in an 100 ms range – is another case of different process: according to Forterre24 the osmotic motor performs a relatively trivial function: only that of shifting the setting point of the elastically unsteady leaf, already inherently hanging for snapping. Kasamo25 investigating the inhibition of tonoplast and plasma membrane H+-ATPase by local anesthetics (dibucaine, lidocaine, tetracaine and procaine), proposed that local anesthetics may act directly on the ATPase moiety without lipid mediation. Dibucaine inhibited tonoplast and plasma membrane ATPases more than the others local anesthetics.
Cytosolic Ca2+ has been the essential focus in most studies of signaling in the pulvinus, with attempts to confirm it as part of the phosphatidylinositol signaling pathway. The possible target effectors of Ca2+ may be actin, calmodulin and annexins. Applying effectors of Ca2+, such as calmodulin or, EGTA, or calcium mobilization antagonists, to pulvini interfered with their movement rhythms as well as with their severe movement responses to illumination.26,27
Ethylene is notable for its hormonal actions in plants at concentrations as low as MAC × 10−6 and have also anesthetics characteristics.10 Powell10 tested the hypothesis that its anesthetic and hormonal properties of ethylene might be connected, by comparing its effects with those of halothane on Vicia faba and other species of plants. In this study halothane and ethylene are shown to have comparable properties at or near clinical anesthetic concentrations, for example in the reduction of mitotic index. Rost29 investigated the effect of exogenous treatment with ethylene gas in the meristem of roots of Pisum sativum. In this work it was observed a transitory block in cell cycle progression in the meristem during this treatment.
In this research we tested the effects of ketamine, lidocaine, diethyl ether, and amlodipine on the movements of Mimosa pudica and D. muscipula, thinking in the questions: do the processes involved in plants employ any of the protein/signaling pathways that would be blocked by amlodipine (L-type calcium channels), lidocaine (sodium channels) or ketamine (NMDA receptors)? Are the mechanisms of actions of inhaled anesthetics on the plant cells similar to the mechanisms of actions on mammal cells? These act on the ion channels, on cell membrane, or in both locals?
These drug effects, especially in the case of ketamine, lidocaine and amlodipine are highly specific for these channels and the blocking of aquaporins and other plant channels needs to be demonstrated yet.
In this study, we focus the attention in some different anesthetics (local and general) and in one calcium channel blocker, to look for some insight about the mechanisms of anesthetics action and motor cells peculiarities. Ether was the drug that had the more evident effect in M. pudica and D. muscipula causing almost total immobilization of plants. With this kind of investigation we cannot define specific receptor for the action of anesthetics or propose profound theories about motor cells interaction. Because studies using anesthetics in these species of plants are few, with this research we aim to contribute a lithe more in this area. Future studies could bring more elements to understand why some anesthetics can immobilize plants movements.
Materials and Methods
Plants of Mimosa Pudica were grown in vases in soil in an average temperature of 25°C up to 45 d of life time. Adults Venus flytrap plants were acquired in a specialized store. Fifteen mimosa plants and Fifteen Venus flytrap plants were used in the experiments. Measurements of the petiole bending (mimosa) and leaves closing (Venus) were performed with and angle transfer device. Normal angular displacement (Δθ) for Mimosa (average = 28°, standard deviation = 7°) and Venus (average = 47°, standard deviation = 6°), was obtained by the equation: θ2 after stimulation – θ1 before stimulation = ± 28° (in M. pudica) and by the equation [(θ1 - 90°) + (90° - θ2) before stimulation] - [(θ1 - 90°) + (90° - θ2) after stimulation] = ± 47° (Fig. 2). The average angular displacement of the control group (that was treated with10ml of distilled water applied on the roots of the plants) were compared with the average angular displacement of the drugs groups (amlodipine 5mg +10ml of distilled water, Ketamine 2ml +8ml of distilled water, Lidocaine 10% 1ml + flush of 9ml of distilled water, and ether 35% - 1ml applied in piece cotton). The stimuli to activate the falling of the petiole in Mimosa was mechanical touch using a monofilament with 10 g Semmes-Weinstein. In Venus Flytrap monofilament with 10 g Semmes-Weinstein was used to Trigger hair irritation and cause trap movement (1 or 2 hair stimulated). The experiments were performed during the morning period, with controlled lighting with a 60 W compact fluorescent lamp. After the experiments, time of recovery of the plants was timed. The substances were removed by washing the plants after the time of analysis. Integrity of the individuals was observed in a qualitative manner. 60 tests were performed for which drug and for the two species of plants (60 control group vs. 60 drug group). T-test was used to analyze difference between two means in relation to the variation in the data.
Figure 2. The graphs show the angular displacement in both plants. A) M. pudica: in control group de average angular change was 28° (standard deviation = 7°). In ether group the average angular change was 6° (standard deviation = 7°). B) D. muscipula: the average angular change in control group was 47° (standard deviation = 6°) and 5° in ether group (standard deviation = 6°).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21000
References
- 1.Bernard C. Lectures on Phenomena of Life Common to Animals and Plants. 1th ed. Paris: J.-B. Ballliere and Son, 1878. [Google Scholar]
- 2.Okazaki N, Takai K, Sato T. [Immobilization of a sensitive plant, Mimosa pudica L., by volatile anesthetics] Masui. 1993;42:1190–3. [PubMed] [Google Scholar]
- 3.Milne A, Beamish T. Inhalational and local anesthetics reduce tactile and thermal responses in mimosa pudica. Can J Anaesth. 1999;46:287–9. doi: 10.1007/BF03012612. [DOI] [PubMed] [Google Scholar]
- 4.Wallace RH. Studies on the sensitivity of Mimosa pudica. I. The effect of certain animal anesthetics upon sleep movements. Am J Bot. 1931;18:102–11. doi: 10.2307/2435933. [DOI] [Google Scholar]
- 5.Bause GS. Anesthesiology reflections - Darwin Etherizes Venus Flytraps. Anesthesiology 2009;111:3:497.
- 6.Kumar N. Mimosa pudica l. a sensitive plant. International Journal of Pharmacy and Pharmaceutical Sciences. 2009;1:50–1. [Google Scholar]
- 7.Saxe H, Rajagopal R. Effect of vanadate on bean leaf movement, stomatal conductance, barley leaf unrolling, respiration, and phosphatase activity. Plant Physiol. 1981;68:880–4. doi: 10.1104/pp.68.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao H, Xu Q, Yuan M. Actin dynamics mediates the changes of calcium level during the pulvinus movement of Mimosa pudica. Plant Signal Behav. 2008;3:954–60. doi: 10.4161/psb.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell JN, Grant CJ, Robinson SM, Radford SG. A comparison with halothane of the hormonal and anaesthetic properties of ethylene in plants. Br J Anaesth. 1973;45:682–90. doi: 10.1093/bja/45.7.682. [DOI] [PubMed] [Google Scholar]
- 11.Urban BW, Bleckwenn M. Concepts and correlations relevant to general anaesthesia. Br J Anaesth. 2002;89:3–16. doi: 10.1093/bja/aef164. [DOI] [PubMed] [Google Scholar]
- 12.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 13.Taylorson RB, Hendricks SB. Overcoming dormancy in seeds with ethanol and other anesthetics. Planta. 1979;145:507–10. doi: 10.1007/BF00380106. [DOI] [PubMed] [Google Scholar]
- 14.Ewart AJ. On the Physics and Physiology of the Protoplasmic Streaming in Plants. Oxford, 1903. [Google Scholar]
- 15.Saraiva RA. Inhalation Anesthetics. Rev Bras Anestesiol. 1994;44:43–52. [Google Scholar]
- 16.Halsey MJ. Mechanism of General Anesthesia, In: Edmond I, Eger II. Anesthetic Uptake and Action. Baltimore. Williams Wilkins, 1974;45-76. [Google Scholar]
- 17.DiPalma JR, McMichael R, DiPalma M. Touch receptor of venous flytrap, Dionaea muscipula. Science. 1966;152:539–40. doi: 10.1126/science.152.3721.539. [DOI] [PubMed] [Google Scholar]
- 18.Saltveit ME. Effect of High-Pressure Gas Atmospheres and Anaesthetics on Chilling Injury of Plants. J Exp Bot. 1993;44:1361–8. doi: 10.1093/jxb/44.8.1361. [DOI] [Google Scholar]
- 19.Moran N. Osmoregulation of leaf motor cells. FEBS Lett. 2007;581:2337–47. doi: 10.1016/j.febslet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Uehlein N, Kaldenhoff R. Aquaporins and plant leaf movements. Ann Bot. 2008;101:1–4. doi: 10.1093/aob/mcm278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder JI, Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989;14:187–92. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 22.Fromm J, Eschrich W. Transport processes in stimulated and non-stimulated leaves of Mim osa pudica. II. Energesis and transmission of seismic stimulation. Trees Struct Funct. 1988;2:18–24. doi: 10.1007/BF01196340. [DOI] [Google Scholar]
- 23.Toriyama H, Jaffe MJ. Migration of calcium and its role in the regulation of seismonasty in the motor cells of Mimosa pudica L. Plant Physiol. 1972;49:72–81. doi: 10.1104/pp.49.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–5. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 25.Kasamo K. Inhibition of Tonoplast and Plasma Membrane H+-ATPase Activity in Rice (Oryza sativa L.) Culture Cells by Local Anesthetics. Plant Cell Physiol. 1988;29:215–21. [Google Scholar]
- 26.Kayali S, Agosti RD. Effect of EGTA on the diurnal leaf movement of Phaseolus vulgaris. Plant Physiol. 1997;35:922–915. [Google Scholar]
- 27.Gomez LA, Moysset L, Simon E. Effects of calmodulin inhibitors and blue light on rhythmic movement of Robinia pseudoacacia leaflets. Photochem Photobiol. 1999;69:722–7. doi: 10.1111/j.1751-1097.1999.tb03353.x. [DOI] [Google Scholar]
- 28.Powell JN, Grant CJ, Robinson SM, Radford SG. A comparison with halothane of the hormonal and anaesthetic properties of ethylene in plants. Br J Anaesth. 1973;45:682–90. doi: 10.1093/bja/45.7.682. [DOI] [PubMed] [Google Scholar]
- 29.Rost TL, Jones T, Robbins JA. The role of ethylene in the control of cell division in cultured pea root tips: a mechanism to explain the excision effect. Protoplasma. 1986;130:68–72. doi: 10.1007/BF01283332. [DOI] [Google Scholar]