Abstract
Hydrogen peroxide (H2O2) is a reactive oxygen species that signals between cells, and H2O2 signaling is essential for diverse cellular processes, including stress response, defense against pathogens, and the regulation of programmed cell death in plants. Although plasma membrane intrinsic proteins (PIPs) have been known to transport H2O2 across cell membranes, the permeability of each family member of PIPs toward H2O2 has not yet been determined in most plant species. In a recent study, we showed that certain isoforms of Arabidopsis thaliana AtPIPs, including AtPIP2;2, AtPIP2;4, AtPIP2;5, and AtPIP2;7, are permeable for H2O2 in yeast cells. Since the expression of PIPs is differently modulated in Arabidopsis by abiotic stress or H2O2 treatment, it is important to investigate the integrated regulation of aquaporin expression and their physiological significance in H2O2 transport and plant response to diverse abiotic stresses.
Keywords: aquaporin, hydrogen peroxide, major intrinsic protein, stress, water channel
The functional roles of hydrogen peroxide (H2O2) as a signaling and regulatory molecule have been implicated in many cellular processes, including photosynthesis, photorespiration, stomatal movement, cell cycle, senescence, and stress response.1 The effect of H2O2 in these cellular processes requires a rapid transport of the intercellular messenger molecule across the plasma membrane. Although H2O2 can diffuse across cell membranes, native membranes represent significant barriers against H2O2, and many membranes are poorly permeable to H2O2.2 Because efficient transport of H2O2 across plasma membranes can be greatly increased by specific channel proteins, it is important to determine which channel proteins can facilitate H2O2 transport across plasma membranes.
The major intrinsic proteins (MIPs), commonly referred to as aquaporins, have been determined to facilitate the transport not only of water but also other substrates, including glycerol, ammonia, urea, boric acid, carbon dioxide, nitric oxide, and lactic acid.3,4 Although several studies have demonstrated that certain aquaporins facilitate the diffusion of H2O2 across biological membranes,5,6 reports demonstrating the permeability of plasma membrane intrinsic proteins (PIPs) toward H2O2 are severely limited. In a recent study, we showed that certain isoforms of Arabidopsis thaliana AtPIPs, including AtPIP2;2, AtPIP2;4, AtPIP2;5, and AtPIP2;7, are permeable for H2O2 in yeast cells.7
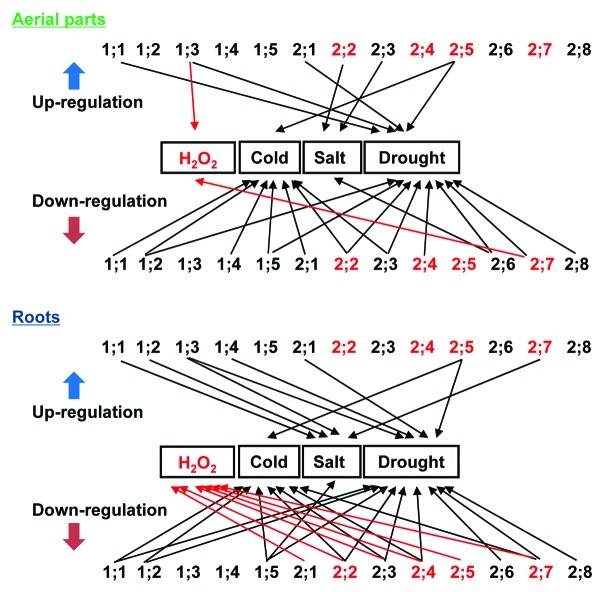
With the increasing understanding of the capacity of aquaporins to facilitate the transport of H2O2 across plasma membranes and the involvement of H2O2 in plant response to abiotic stresses, it is of interest to investigate the physiological significance of aquaporin-mediated H2O2 transport in plants under stress conditions. It has been suggested that abiotic and biotic stress-related stimuli mediated an H2O2-induced internalization of PIPs to downregulate root water transport.8 It has been reported that exogenously applied H2O2 to the roots of cucumber, wheat, maize, and bean affected the hydraulic conductivity under normal or stress conditions.9-12 The comprehensive expression patterns of AtPIPs have been analyzed in Arabidopsis under environmental stresses, including cold, salt, and drought.13 In a recent study, we showed that hydrogen peroxide treatment did not affect the endogenous expression levels of AtPIP1 in the leaves and roots of Arabidopsis, whereas AtPIP2 genes, including AtPIP2;2, AtPIP2;3, AtPIP2;4, AtPIP2;5, and AtPIP2;7, in the roots were downregulated by H2O2 treatment.7 The integrated regulation of AtPIPs expression by H2O2 or abiotic stresses is summarized in Figure 1. The expression of AtPIP2;2, AtPIP2;4, AtPIP2;5, and AtPIP2;7, which are permeable for H2O2 in yeast cells, was differently modulated by abiotic stresses. In particular, the expression of AtPIP2;2, AtPIP2;4, and AtPIP2;7 was downregulated by both H2O2 and drought stress (Fig. 1). The concomitant regulation in the expression of certain aquaporin family members by H2O2 and abiotic stresses suggests that these specific members of aquaporin may play an important role in H2O2-mediated plant response to changing environmental conditions. Further researches should focus on determining the physiological consequence of aquaporin-mediated H2O2 transport in plant adaptation to diverse abiotic stresses.
Figure 1. Summary of the involvement of the Arabidopsis AtPIPs in H2O2 and abiotic stress responses. The expression patterns reported in Hooijmaijers and Jang7,13 were used to group the AtPIP genes based on their H2O2 or stress responses, and the arrows indicate the genes that are upregulated or downregulated by the indicated treatments in the aerial parts and roots of Arabidopsis plants. The numbers 1;1, 1;2, 1;3, etc., in the figure represent AtPIP1;1, AtPIP1;2, AtPIP1;3, etc., respectively, and the red colors represent the AtPIPs that are permeable for H2O2 in yeast cells.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen21 Program (PJ00820303), Rural Development Administration, Republic of Korea.
Glossary
Abbreviations:
- MIP
major intrinsic protein
- PIP
plasma membrane intrinsic protein
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21178
References
- 1.Quan LJ, Zhang B, Shi WW, Li HY. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol. 2008;50:2–18. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 2.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta 2006; 1758:994-1003. [DOI] [PubMed]
- 3.Wu B, Beitz E. Aquaporins with selectivity for unconventional permeants. Cell Mol Life Sci. 2007;64:2413–21. doi: 10.1007/s00018-007-7163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurel C, Verdoucq L, Luu D-T, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 5.Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–92. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 6.Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J. 2008;414:53–61. doi: 10.1042/BJ20080287. [DOI] [PubMed] [Google Scholar]
- 7.Hooijmaijers C, Rhee JY, Kwak KJ, Chung GC, Horie T, Katsuhara M, et al. Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J Plant Res. 2012;125:147–53. doi: 10.1007/s10265-011-0413-2. [DOI] [PubMed] [Google Scholar]
- 8.Boursiac Y, Boudet J, Postaire O, Luu D-T, Tournaire-Roux C, Maurel C. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 2008;56:207–18. doi: 10.1111/j.1365-313X.2008.03594.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Singh AP, Chung GC. Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decreases in water transport. J Exp Bot. 2004;55:1733–41. doi: 10.1093/jxb/erh189. [DOI] [PubMed] [Google Scholar]
- 10.Aroca R, Amodeo G, Fernández-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005;137:341–53. doi: 10.1104/pp.104.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benabdellah K, Ruiz-Lozano J-M, Aroca R. Hydrogen peroxide effects on root hydraulic properties and plasma membrane aquaporin regulation in Phaseolus vulgaris. Plant Mol Biol. 2009;70:647–61. doi: 10.1007/s11103-009-9497-7. [DOI] [PubMed] [Google Scholar]
- 12.Ehlert C, Maurel C, Tardieu F, Simonneau T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 2009;150:1093–104. doi: 10.1104/pp.108.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang JY, Kim DG, Kim YO, Kim JS, Kang H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol Biol. 2004;54:713–25. doi: 10.1023/B:PLAN.0000040900.61345.a6. [DOI] [PubMed] [Google Scholar]