Abstract
Low levels of ultraviolet (UV)-radiation alter the morphology of plants. UV-B exposure can lead to shorter petioles and shorter, narrower and/or thicker leaf blades. The resulting decrease in leaf area has been associated with inhibitory UV-B effects on biomass accumulation. In Arabidopsis, UV-B effects on leaf area have variously been attributed to altered cell division, cell expansion or combinations of these two processes. A dedicated UV-B sensory system, crosstalk between flavonoids and auxins, endoreduplication and generic Stress Induced Morphogenic Responses (SIMR) have all been proposed to contribute to the UV-B phenotype. Here, we propose that UV-mediated morphogenesis, rather than being controlled by a single regulatory pathway, is controlled by a regulatory blur involving multiple compensatory molecular and physiological feedback interactions.
Keywords: Ultraviolet-B, morphogenesis, UVR8, Stress, flavonoids, endoreduplication
In the 1950s Brodführer1 showed that ambient levels of UV (UV)-radiation alter plant morphology. For example, UV altered inflorescence elongation and branching in Arabidopsis thaliana. Interest in such UV effects increased substantially2-4 following measurements in the 1980s of stratospheric ozone depletion, and increases in UV-B (280–315 nm) in the biosphere.5 The consensus of several decades of UV-B research is that natural UV-B levels do not cause stress in acclimated plants. However, subtle inhibitory UV-B effects on biomass accumulation are correlated with morphological alterations such as reductions in leaf expansion.4 Thus, understanding the functional role and mechanism underlying UV-induced morphogenesis is of substantial interest.
UV-Acclimated Phenotype
Impacts of UV-B radiation on leaf morphology are well described and include shorter, narrower and/or thicker leaves, shorter petioles, leaf curling, and alterations in leaf shape.6-9 UV-B also impacts on stem development by inhibiting hypocotyl and stem elongation, by increasing tillering or axillary branching, and this can alter both the root-shoot ratio and the structure of the inflorescence.6 In Arabidopsis, UV initially causes a relatively strong decrease in expansion along the longitudinal leaf axis, resulting in a decreased length/width ratio. When these leaves grow older, the length/width ratio is restored due to stronger inhibition of expansion along the transverse axis, emphasizing that this is a well regulated process.10
Functional Relevance
There is little consensus about a functional role for UV-driven morphogenesis. Some authors have argued that morphogenic alterations diminish UV-B exposure. For example, leaves of short, bushy plants are more likely to be shaded and less exposed to UV-B within a canopy.11 UV-B levels under a canopy can be decreased by 98–99%.12 As a trade-off, morphogenic changes decrease interception of photosynthetic radiation and this can potentially lead to shifts in the competitive balance between species.11 Yet, the possibility that UV-B driven morphogenesis has a function other than UV-protection should also be considered. Plants possess a specific UV-photoreceptor and signaling pathway,13,14 and it is possible that plants use UV-B as a proxy for factors such as high intensity visible radiation, heat, or drought which are typically associated with high UV-B.
Underlying Cellular and Molecular Mechanisms
Reductions in leaf area have variously been attributed to inhibition of cell division,15 cell expansion,10 or combinations of these two processes. Yet, increased cell expansion,7 and cell division16 have also been reported. It is not clear why such diversity in UV-responses occurs, and the question arises whether a single underlying process can accommodate all observations, or whether different processes operate under, for example, different experimental conditions? Based on a literature survey, we have identified four potential players in UV-B mediated morphogenesis.
(1) A UV-B sensory pathway
Photoreceptors play a key role in controlling morphology. A UV-B specific photoreceptor, UV RESISTANCE LOCUS 8 (UVR8) is a co-regulator of UV-protection, controlling expression of genes involved in flavonoid biosynthesis, DNA-repair, and anti-oxidative defense.13,14,17 Components of the UVR8-signaling cascade are associated with morphological responses, including CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), ELONGATED HYPOCOTYL 5 (HY5) and REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2.14 Indeed, the uvr8 mutant does not display UV-mediated inhibition of hypocotyl growth,18 although the uvr8 mutant grown under supplemental UV-B remains smaller than the wild-type.8,18 Interestingly, it has been argued that UVR8 does not induce “dwarfism,” but rather stimulates cell expansion to compensate for UV-B induced decreases in whole leaf growth.8
(2) Crosstalk between flavonoids and auxin
Flavonoids have both anti-oxidant and UV-screening properties and typically accumulate in UV-B exposed tissues and organelles.19 Flavonoid-aglycones impact on auxin homeostasis by negatively affecting polar transport through efflux carriers,20 and/or by altering auxin catabolism.21 Consistently, some Arabidopsis flavonoid mutants display alterations in auxin distribution and plant morphology.22-24 Hectors et al.9 showed that two Arabidopsis auxin mutants displayed altered flavonoid accumulation, and an altered morphogenic response when exposed to UV-B. This implies that UV-induced morphological responses may well be affected by crosstalk between flavonoid accumulation and auxin homeostasis. Interestingly, the UVR8-pathway controls expression of several flavonoid biosynthesis genes,17 thus crosstalk between flavonoids and auxins may also be linked to UVR8 signaling.
(3) Cell cycle control and endoreduplication
Central to regulating organ size is control of cell division and/or cell expansion.25 Endoreduplication is a determinant of cell size, with higher ploidy levels associated with bigger cells.26 UV-B impedes cell cycle progression,27 decreasing both cell numbers and endoreduplication.26 However, UV-B also downregulates the transcription factor E2Fe/DEL1, which represses onset of endoreduplication, and expression of DNA photolyase PHR1.26 It was proposed that this switch can lead to UV-mediated ploidy-driven expansion growth, compensating for smaller cell numbers.26 Interestingly, the UVR8 mutant has a different endoreduplication profile than the corresponding wild type under UV-B,8 implying that crosstalk between the UVR8 pathway and cell cycle control may impinge on morphogenesis.
(4) Stress induced morphogenic responses
Many different stressors induce alterations in plant morphology. The concept of stress induced morphogenic responses (SIMR) captures the similarities across a range of environmental stressors.28 The key components of SIMR are inhibition of cell elongation, localized stimulation of cell division and alterations in cell differentiation status, and these are hypothesized to arise through common processes such as increased ROS production and altered phytohormone metabolism.28 SIMR-style morphogenesis is likely to be limited to high UV-B levels that result in generation of high levels of ROS. Nevertheless, the low UV-B UVR8 pathway is likely to influence SIMR-style morphogenesis through the upregulation of antioxidant defenses.17
Conclusions
UV-B can inhibit growth through cellular damage27 (Fig. 1). Alternatively, there is evidence that UVR8 either causes dwarfism18 or compensates for inhibition of growth under UV-B.8 It is not clear how the UVR8 pathway affects morphology, this may be through a direct process, or involve crosstalk with other morphogenic processes (Fig. 1). Our survey indicates that UVR8 mediated changes in flavonoid accumulation, endoreduplication and antioxidant scavenging capacity can potentially generate feedback regulation at the biochemical/physiological level, fine-tuning the morphological response, and accommodating the observed diversity of morphogenic effects (Fig. 1). These physiological feedback interactions complement those at the gene-expression level (involving COP1, RUP 1 and RUP2).13,14 Taken together, we speculate that UV-mediated morphogenesis, rather than being controlled by a single regulatory pathway, is controlled by a regulatory blur of compensatory molecular and physiological feedback interactions.
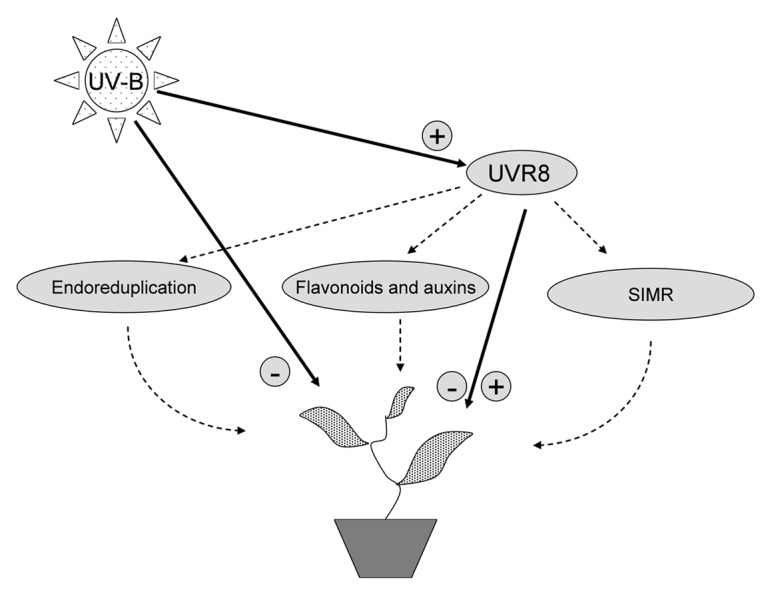
Figure 1. Summary of morphogenic processes in UV-B exposed plants. UV-B can directly inhibit growth through cellular damage (Jiang et al., 2011). Alternatively, dwarfism is mediated through the UVR8 pathway (Favory et al., 2009), or UVR8 compensates for inhibitory effects of UV-B on growth (Wargent et al., 2009b). The UVR8 pathway may directly regulate morphogenesis, or this may involve crosstalk with other processes, including flavonoid accumulation, endoreduplication and antioxidant scavenging. This can potentially generate feedback regulation at the biochemical/physiological level, fine-tuning the morphogenic response, and accommodating the observed diversity of morphogenic effects.
Acknowledgments
The authors were supported by grants from the Research Foundation Flanders (FWO, project G.0382.04N), FWO Research Community (W0.038.04N), Science Foundation Ireland (SFI project 11/RFP.1/EOB/3303) and COST-Action FA0906 (UV4Growth).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21260
References
- 1.Brodführer U. Der Einfluss Einer Abgestuften Dosierung von Ultravioletter Sonnenstrahlung auf das Wachstum der Pflanzen. Planta. 1955;45:1–56. doi: 10.1007/BF01937677. [DOI] [Google Scholar]
- 2.Jordan BR. The effects of ultraviolet-B radiation on plants: a molecular perspective. In: Advances in Botanical Research, Callow JA (ed). 1996; 22:97-162. [Google Scholar]
- 3.Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–5. doi: 10.1016/S1360-1385(98)01215-1. [DOI] [Google Scholar]
- 4.Ballaré CL, Caldwell MM, Flint SD, Robinson SA, Bornman JF. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem Photobiol Sci. 2011;10:226–41. doi: 10.1039/c0pp90035d. [DOI] [PubMed] [Google Scholar]
- 5.Andrady A, Aucamp PJ, Bais AF, Ballaré CL, Björn LO, Bornman JF, et al. United Nations Environment Programme, Environmental Effects Assessment Panel Environmental effects of ozone depletion and its interactions with climate change: progress report, 2009. Photochem Photobiol Sci. 2010;9:275–94. doi: 10.1039/b923342n. [DOI] [PubMed] [Google Scholar]
- 6.Jansen MAK. Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol Plant. 2002;116:423–9. doi: 10.1034/j.1399-3054.2002.1160319.x. [DOI] [Google Scholar]
- 7.Wargent JJ, Moore JP, Roland Ennos A, Paul ND. Ultraviolet radiation as a limiting factor in leaf expansion and development. Photochem Photobiol. 2009;85:279–86. doi: 10.1111/j.1751-1097.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- 8.Wargent JJ, Gegas VC, Jenkins GI, Doonan JH, Paul ND. UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 2009;183:315–26. doi: 10.1111/j.1469-8137.2009.02855.x. [DOI] [PubMed] [Google Scholar]
- 9.Hectors K, van Oevelen S, Guisez Y, Prinsen E, Jansen MAK. The phytohormone auxin is a component of the regulatory system that controls UV-mediated accumulation of flavonoids and UV-induced morphogenesis. Physiol Plant. 2012;145:594–603. doi: 10.1111/j.1399-3054.2012.01590.x. [DOI] [PubMed] [Google Scholar]
- 10.Hectors K, Jacques E, Prinsen E, Guisez Y, Verbelen JP, Jansen MAK, et al. UV radiation reduces epidermal cell expansion in leaves of Arabidopsis thaliana. J Exp Bot. 2010;61:4339–49. doi: 10.1093/jxb/erq235. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PW, Ballaré CL, Caldwell MM. Photomorphogenic effects of UV-B radiation on plants: consequences for light competition. J Plant Physiol. 1996;148:15–20. doi: 10.1016/S0176-1617(96)80288-4. [DOI] [Google Scholar]
- 12.Flint SD, Caldwell MM. Solar UV-B and visible radiation in tropical forest gaps: measurements partitioning direct and diffuse radiation. Glob Change Biol. 1998;4:863–70. doi: 10.1046/j.1365-2486.1998.00191.x. [DOI] [Google Scholar]
- 13.Jenkins GI. Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol. 2009;60:407–31. doi: 10.1146/annurev.arplant.59.032607.092953. [DOI] [PubMed] [Google Scholar]
- 14.Heijde M, Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012;17:230–7. doi: 10.1016/j.tplants.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Lake JA, Field KJ, Davey MP, Beerling DJ, Lomax BH. Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation. Plant Cell Environ. 2009;32:1377–89. doi: 10.1111/j.1365-3040.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 16.Staxen I, Bornman JF. A morphological and cytological study of Petunia hybrida exposed to UV-B radiation. Physiol Plant. 1994;91:735–40. doi: 10.1111/j.1399-3054.1994.tb03013.x. [DOI] [Google Scholar]
- 17.Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, et al. A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci USA. 2005;102:18225–30. doi: 10.1073/pnas.0507187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009;28:591–601. doi: 10.1038/emboj.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–93. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 20.Peer WA, Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 2007;12:556–63. doi: 10.1016/j.tplants.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Zenk MHM, Müller G. In vivo destruction of exogeneously applied indolyl-3-acetic acid as influenced by naturally occurring phenolic acids. Nature. 1963;200:761–3. doi: 10.1038/200761a0. [DOI] [Google Scholar]
- 22.Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, et al. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell. 2007;19:148–62. doi: 10.1105/tpc.106.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringli C, Bigler L, Kuhn BM, Leiber RM, Diet A, Santelia D, et al. The modified flavonol glycosylation profile in the Arabidopsis rol1 mutants results in alterations in plant growth and cell shape formation. Plant Cell. 2008;20:1470–81. doi: 10.1105/tpc.107.053249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimoto-Shirasu K, Roberts K. “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol. 2003;6:544–53. doi: 10.1016/j.pbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Radziejwoski A, Vlieghe K, Lammens T, Berckmans B, Maes S, Jansen MAK, et al. Atypical E2F activity coordinates PHR1 photolyase gene transcription with endoreduplication onset. EMBO J. 2011;30:355–63. doi: 10.1038/emboj.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L, Wang Y, Björn LO, Li S. UV-B-induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips. Planta. 2011;233:831–41. doi: 10.1007/s00425-010-1340-5. [DOI] [PubMed] [Google Scholar]
- 28.Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]


