Abstract
(+)-SKF 10047 (N-allyl-normetazocine) is a prototypic and specific sigma-1 receptor agonist that has been used extensively to study the function of sigma-1 receptors. (+)-SKF 10047 inhibits K+, Na+ and Ca2+ channels via sigma-1 receptor activation. We found that (+)-SKF 10047 inhibited NaV1.2 and NaV1.4 channels independently of sigma-1 receptor activation. (+)-SKF 10047 equally inhibited NaV1.2/1.4 channel currents in HEK293T cells with abundant sigma-1 receptor expression and in COS-7 cells, which barely express sigma-1 receptors. The sigma-1 receptor antagonists BD 1063,BD 1047 and NE-100 did not block the inhibitory effects of (+)-SKF-10047. Blocking of the PKA, PKC and G-protein pathways did not affect (+)-SKF 10047 inhibition of NaV1.2 channel currents. The sigma-1 receptor agonists Dextromethorphan (DM) and1,3-di-o-tolyl-guanidine (DTG) also inhibited NaV1.2 currents through a sigma-1 receptor-independent pathway. The (+)-SKF 10047 inhibition of NaV1.2 currents was use- and frequency-dependent. Point mutations demonstrated the importance of Phe1764 and Tyr1771 in the IV-segment 6 domain of the NaV1.2 channel and Phe1579 in the NaV1.4 channel for (+)-SKF 10047 inhibition. In conclusion, our results suggest that sigma-1 receptor agonists directly inhibit NaV1.2/1.4 channels and that these interactions should be given special attention for future sigma-1 receptor function studies.
Introduction
The sigma receptor was originally described as a novel opioid receptor subtype, but it is now considered to be a unique receptor [1], [2]. Sigma receptors consist of two subtypes: sigma-1 and sigma-2. The sigma-1 receptor was first cloned from guinea pigs in 1996 [3], [4], [5], but the sigma-2 receptor has not been cloned. The sigma-1 receptor is widely expressed in the brain and peripheral organs, and it may be involved in numerous processes, such as Alzheimer's disease, schizophrenia, pain, drug addiction, stroke, cancer, depression and anxiety [2], [6]. The molecular mechanisms of sigma-1 receptor effects in these diseases are not understood. One of the most important molecular actions of sigma-1 receptors is the modulation of various voltage- and ligand-gated ion channels [2], [7], [8].
Voltage-gated sodium channels initiate and propagate action potentials in excitable cells. Nine voltage-gated sodium channel isoforms have been identified in mammals [9], [10]. NaV1.2 is the most abundant sodium channel isoform in the central nervous system comprising approximately 80% of the total rat brain voltage-gated sodium channels [11]–[12], and it controls axonal action potential conduction and neurotransmitter release in presynaptic terminals [13]. NaV1.2 mutations cause inherited febrile seizures and epilepsy [9]. The NaV1.4 channel is the predominant voltage-gated Na+ channel isoform in skeletal muscle [14], and various channel mutations are associated with muscular diseases, including potassium-aggravated myotonia, paramyotonia congenita, hyperkalemic periodic paralysis, hypokalemic periodic paralysis and normokalemic periodic paralysis [15]. The major cardiac voltage-gated Na+ channel is NaV1.5 [16], [17], which is involved in many arrhythmic disorders, such as long-QT syndrome type 3, Brugada syndrome, conduction disease, sinus node dysfunction and atrial standstill [18], [19].
(+)-SKF 10047 is a prototypic and specific sigma-1 receptor agonist that has been extensively used to investigate sigma-1 receptor function. (+)-SKF 10047 inhibits cardiac NaV1.5 channels in HEK293 cells, COS-7 cells and cardiac myocytes [20], [21], but little is known about NaV1.2/NaV1.4 modulation by sigma-1 receptor activation.
We found that (+)-SKF 10047 inhibited NaV1.2 and NaV1.4 channel currents, but these inhibitory effects were independent of sigma-1 receptor activation. (+)-SKF 10047 inhibited NaV1.2/1.4 channel currents equally in HEK293T cells (which have abundant sigma-1 receptor expression) and COS-7 cells (which barely express sigma-1 receptors). The present study is the first report of the direct NaV1.2/1.4 channel current inhibition by sigma-1 receptor agonists, which should be given special attention for investigation of sigma-1 receptor function.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Fudan University (Permit Number: 2007-0002). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Chemicals
H-89, PKAI, BIM I, GTPγS, lidocaine hydrochloride, PRE-084, DTG, BD 1063, DM and BD 1047 were purchased from Sigma Aldrich (Sigma Aldrich, St. Louis, MO). Gö6976, CTX (Cholera toxin), NF 023, NF 449 and NE-100 were purchased from Calbiochem (Calbiochem, Germany). (+)-SKF 10047, pertussis toxin (PTX) were purchased from Tocris (Tocris, UK).
H-89 and PKAI are protein kinase A (PKA) inhibitors. Gö6976 and BIM I are protein kinase C (PKC) inhibitors. GTPγS is a G protein activator. NF 023 and PTX are Gi/o antagonists. NF 449 is a Gs antagonist, and CTX is a Gs activator. BD 1063, BD 1047 and NE-100 are selective sigma-1 receptor antagonists. DTG, PRE-084, (+)-SKF 10047 and DM are selective sigma-1 receptor agonists.
Molecular biology
Site-directed F1764A mutagenesis was achieved in the NaV1.2 Na+ channel clone (the rat brain type IIA Na+ channel clone kindly provided by Alan L. Goldin [22]) using the KOD-Plus mutagenesis system (TOYOBO, Japan). The NaV1.2 mutant Y1771A was kindly provided by Professor William A. Catterall [23]. The NaV1.4 Na+ channel clone was incorporated into the pEGFP-N1 in our lab using the rat NaV1.4 gene mRNA sequence (NM013178) on the NCBI website. Site-directed F1579A and Y1586A mutageneses were achieved in the NaV1.4 Na+ channel using the KOD-Plus mutagenesis system (TOYOBO, Japan). The homo sigma-1R gene (NM_005866) with a flag tag was incorporated into a pCDNA3 vector. The siRNA sequence that corresponded to nucleotides 500–519 of the human sigma-1 receptor open-reading frame (NM005866) was inserted into a pGPU6/GFP/Neo plasmid (GenePharm, Shanghai) to generate vector-based siRNA. All of these constructs were confirmed by sequencing.
SYBR Green-based Real-time RT-PCR was conducted to detect mRNA expression of sigma-1 receptors in COS-7 cells. The primers for sigma-1 receptor: forward primer: 5′ – GCTGCAGTGGGTGTTCGTGAATG -3′ and reverse primer: 5′ – GGTGGAAGGTGCCAGAGATGATGGTA -3′. The mRNA expression of sigma-1 receptors was normalized by the housekeeping gene GAPDH (forward primer: 5′- GAGTCAACGGATTTGGTCGT -3′ ; reverse primer: 5′- AATGAAGGGGTCATTGATGG -3′). The PCR conditions were as follows: 94°C, 5 min; 38 cycles of 94°C, 30 s; 58°C, 30 s; 72°C,30 s; 72°C, 8 min. Cycle threshold (Ct) values and concentrations of samples were calculated using Bio-RAD iCycler software.
Cell culture and transfection
HEK293T cells and COS-7 cells were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). Both cell lines were grown in Dulbecco's modified Eagle's medium (DMEM, GIBICO) supplemented with 10% fetal bovine serum and a 1% antibiotic antimycotic solution in 35-mm Petri dishes (Corning Life Sciences, Lowell, MA). Transient transfections with the NaV1.2, NaV1.4, NaV1.5, flag-sigma-1 receptor, sigma-1 receptor RNAi plasmid and mutant channels were performed using Lipofectamine 2000 reagents (Invitrogen, USA) according to the manufacturer's instructions. The cells were used for patching or other biochemical tests 48 h after transfection.
Cerebellar granule neurons were derived from cerebellum of 7-day-old Sprague–Dawley rat pups as described previously [24]. Isolated cells were then plated onto 35-mm-diameter Petri dishes coated with poly-l-lysine (1 µg/mL) at a density of 2.5×105/cm2. Cultured cells were incubated at 37°C with 5% CO2 in Dulbecco's Modefied Eagle's medium supplemented with 10% fetal calf serum, glutamine (5 mm), insulin (5 µg/mL), KCl (25 mm), and 1% antibiotic/antimycotic solution. All experiments were carried using cerebellar granule neurons at 7–9 days in culture.
Western blot
Cell homogenates were prepared using HEPES-NP40 lysis buffer (20 mM HEPES, 150 mM NaCl, 0.5% NP-40, 10% glycerol, 2 mM EDTA, 100 µM Na3VO4, 50 µM NaF, pH 7.5). The protein samples were resolved using 10% SDS PAGE and transferred to polyvinyldifluoride (PVDF) membranes (Millipore, 0.45 µm) in a transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol, v/v) at 100 V for 1 h. The PVDF membranes were blocked with 10% nonfat dry milk in TBST (TBS containing 0.05% Tween 20) for 1 h at room temperature. The membranes were incubated with the primary antibody, anti-sigma-1 receptor [Rabbit polyclonal antibody (a gift from Dr. Teruo Hayashi, Japan) in TBST with 5% BSA (bovine serum albumin)], overnight at 4°C. The blot was washed 3 times for 10 min in TBST and incubated with a horseradish peroxidase-conjugated secondary antibody (1/20,000) (KangChen Bio-tech) in TBST with 5% nonfat dry milk for 1 h at room temperature. The blots were developed using enhanced chemiluminescence (ECL) reagents from Pierce and the ChemiDoc XRS+imaging system from Bio-Rad.
Electrophysiology
Whole-cell currents in the HEK293T and COS-7 cells were recorded using an Axopatch 200B amplifier (Axon Instruments, Sunnyvale, CA). The bath solution contained (in mM) 145 NaCl, 2.5 KCl, 10 HEPES and 1 MgCl2 (pH adjusted to 7.4 using NaOH). The internal solution contained (in mM) 140 CsCl, 4 KCl, 10 HEPES, and 5 EGTA (pH adjusted to 7.4 using CsOH). The pipettes were created from capillary tubing(BRAND, Wertheim, Germany) and had resistances of 5 to 7 MΩ under these solution conditions. All of the recordings were performed at room temperature. A superimposed Na+ current was evoked by a 30-ms depolarizing pulse from a holding potential of −100 to −20 mV. Steady-state Na+ channel activation was obtained using the following protocol. The cells were held at −100 mV and depolarized in 10-mV steps from −70 to +30 mV at 10-s intervals. The normalized conductance was plotted as a function of the command potential. The conductance was calculated as G Na = I Na/(Vm-Vrev). The data points were fitted using the Boltzmann function: G Na/G Na-max = 1/{1+exp[(V m1/2-V m)/k]}. Steady-state inactivation of Na+ channel was achieved using the following protocol. Five hundred millisecond conditioning pre-pulses ranging from −130 to −20 mV in 10-mV increments were applied prior to the −20 mV test pulse. The peak current amplitudes were normalized to the maximum current and plotted against the pre-pulse potential. The normalized current points were fitted using the Boltzmann function: INa/INa-max = 1/{1+exp[(V m-V m1/2)/k]}+A. The currents were sampled at 10 kHz and filtered at 3 kHz. The currents were corrected online for leak and residual capacitance transients using a P/4 protocol.
Data acquisition and analysis
The data acquisition and analysis were performed with pClamp 8.01 (Axon Instruments) and/or Origin 7.5 software (MicroCal, Northampton, MA). The statistical analysis consisted of unpaired or paired (depending on the circumstances) Student's T tests. Values are given as the means±SEM, and n indicates the number of tested cells. P<0.05 was defined to be a statistically significant difference between groups. Multiple comparisons were analyzed using a one-way analysis of variance (ANOVA) followed by the post-hoc Tukey test. The dose-response curve was fitted by a sigmoidal dose-response equation: I/Imax = 1/(1+10 ∧ (log IC50 – [SKF]) *p), where IC50 is the concentration producing half maximal block, [SKF] is the SKF 10047 concentration, p is the Hill coefficient.
Results
(+)-SKF 10047 inhibits NaV1.2 channel currents in a dose-dependent manner
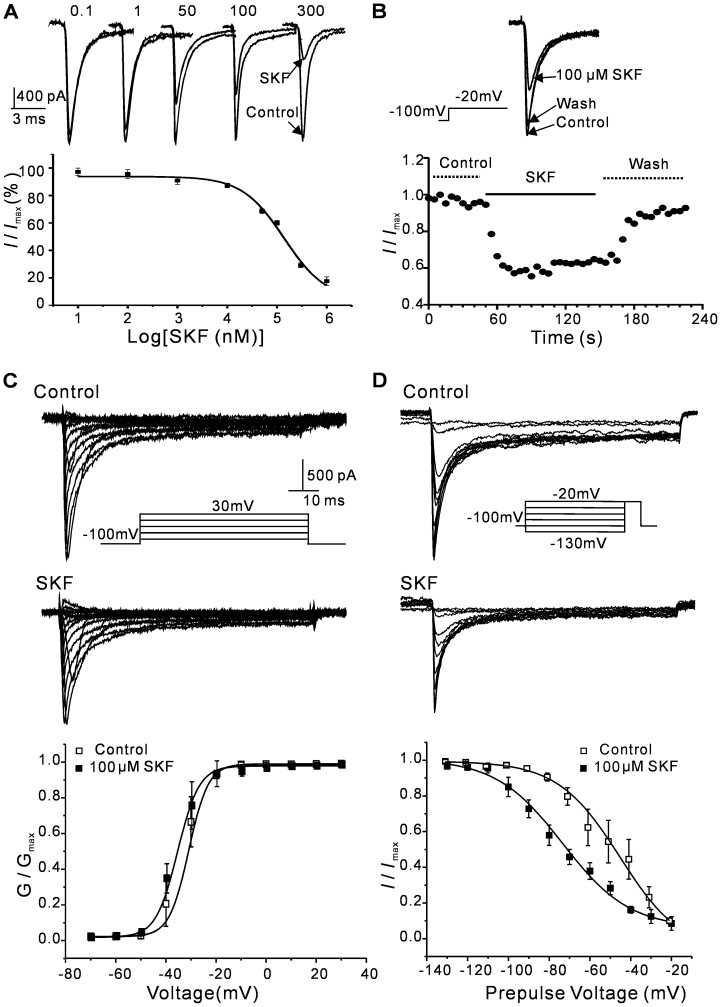
I Na currents were elicited by a 30-ms depolarizing pulse to −20 mV from a holding potential of −100 mV at 10 s intervals. The currents were recorded for 1 min to obtain a stable baseline during blank solution perfusion, and the drug solution was perfused until a stable inhibition level was achieved. (+)-SKF 10047 inhibited the NaV1.2 channel currents in a dose-dependent manner (0.01 µM: 2.8±2.7%, n = 5; 0.1 µM: 5.7±2.1%, n = 5; 1 µM: 12.7±1.5%, n = 6; 50 µM: 31±1.5%, n = 6; 100 µM: 39.5±1.3%, n = 6; 300 µM: 71±1.7%, n = 6; 1000 µM : 82.4±3.1%, n = 6; P<0.05, Fig 1A). The dose-response curve was fitted using equation given in the method, and the IC50 value and Hill coefficient are 140 µM and −1.0 respectively (Fig1 A). The inhibitory effect of (+)-SKF 10047 on I Na began quickly and reached a maximum effect within 90 s. The inhibition was reversible within 1–2 min (Fig 1B).
Figure 1. (+)-SKF 10047 inhibition of the NaV1.2 current in transfected HEK293T cells.
A, representative current traces of NaV1.2 current blocked by various concentrations of (+)-SKF 10047 (0.1∼300 µM, top). The currents were elicited by a test pulse of −20 mV from a holding potential of −100 mV. The dose-response curve was fitted using the equation given in methods. The half-maximal inhibition value was 140 µM and the Hill coefficient was −1.0. (bottom). B, A sample current shows the reversible 100 µM (+)-SKF 10047 inhibition of the NaV1.2 current (top). The time course of the NaV1.2 current inhibition by 100 µM (+)-SKF 10047 (bottom). C, The effect of (+)-SKF 10047 on steady-state activation of NaV1.2 . The current traces show the voltage-dependent NaV1.2 current activation curves in the absence (top) and presence (middle) of (+)-SKF 10047; the normalized data points were fitted using the Boltzmann equation (bottom). (+)-SKF 10047 did not alter the steady-state NaV1.2 activation. P>0.05, n = 5. D, The effect of (+)-SKF 10047 on steady-state inactivation of NaV1.2. The control currents (top) and currents following treatment with 100 µM (+)-SKF 10047 (middle) are shown. The normalized data points were fitted using the Boltzmann function (bottom).
The effects of (+)-SKF 10047 on steady-state NaV1.2 channel inactivation and activation were tested using 100 µM SKF10047. The steady-state activation was determined using a 70-ms depolarizing pulse from a holding potential of −100 mV to potentials between −70 and +60 mV in 10-mV steps at 10-s intervals. The (+)-SKF 10047 did not significantly alter the voltage-dependence of the NaV1.2 channel steady-state activation (control: V 1/2 = −32.9±2.6 mV, n = 5; (+)-SKF 10047: V 1/2 = −33.4±1.4 mV, n = 12; P>0.05, Fig 1C). The steady-state inactivation was examined using a −20 mV test depolarization from the holding potential in 10-mV increments (−130 mV to −20 mV; 2 s). (+)-SKF 10047 significantly shifted the steady-state NaV1.2 inactivation by approximately −27 mV towards negative potentials (control: V 1/2 = −50.1±8.0 mV, n = 5; SKF: V 1/2 = −77.1±3.9 mV, n = 5; P<0.05, Fig 1D).
(+)-SKF 10047 inhibits NaV1.2/1.4 channel currents through a sigma-1 receptor-independent pathway
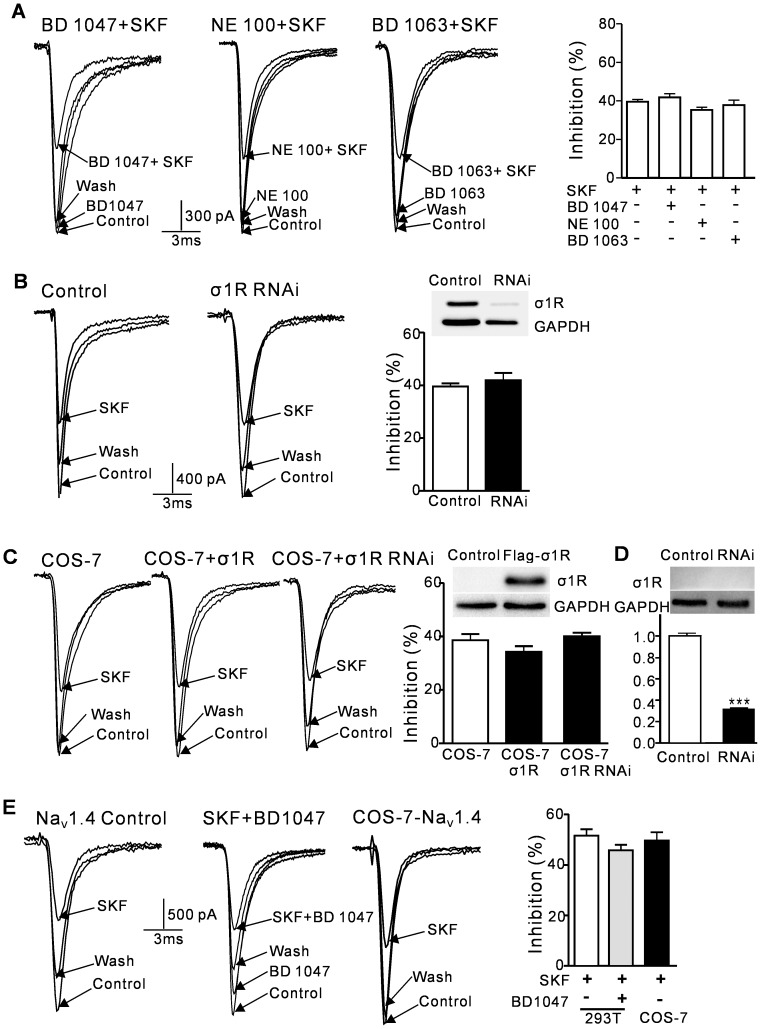
We performed three different experiments to investigate (+)-SKF 10047 inhibition of NaV1.2 channels by sigma-1 receptor activation. First, the selective sigma-1 receptor antagonists BD 1047, NE-100 and BD 1063 were used to block the inhibitory effect of (+)-SKF-10047. The effects of (+)-SKF 10047 (100 µM) on I Na were not altered in the presence of BD 1047 (2 µM), NE-100 (5 µM) or BD 1063 (2 µM) (Fig 2A). The average (+)-SKF 10047 I Na inhibitions were 41.8±2.0% (n = 6) , 35.2±1.6% (n = 5), 37.8±2.6% (n = 5) in the presence of BD 1047, NE-100 and BD 1063, respectively, which were not significantly different from the effect of (+)-SKF 10047 alone (39.5±1.3%, n = 6; P>0.05, Fig 2A). Second, the siRNA technique was used to block the inhibitory effect of (+)-SKF-10047. A sigma-1 receptor siRNA construct was designed to target nucleotides 500–519 in the human sigma-1 receptor gene, as has been previously reported [21]. The sigma-1 receptor protein expression in the HEK293T cells was reduced by 58.7% after transfection with this siRNA construct (Fig 2B). The inhibitory effects of (+)-SKF 10047 on the HEK293T cells with the control vector and the HEK293T cells with the sigma-1 receptor RNAi vector were similar (control: 39.5±1.25%, n = 6; RNAi: 41.9±2.8%, n = 5; P>0.05, Fig 2B). The sigma-1 receptor was barely expressed in the COS-7 cells (Fig 2C, D), which is consistent with previous studies [21], [25]. Third, the inhibitory effect of (+)-SKF 10047 on COS-7 cells was investigated. No significant differences between the HEK293T and COS-7 cells were observed (HEK293T: 39.5±1.3%; COS-7: 38.5±2.4%, n = 6; P>0.05). Sigma-1 receptor overexpression in the COS-7 cells did not alter the inhibitory effect of (+)-SKF 10047 on I Na (COS-7: 38.5±2.3%, n = 6; COS-7 with sigma-1 receptor overexpression: 33.1±2.8%, n = 7; P>0.05, Fig 2C). In order to further confirm that (+)-SKF 10047 inhibition of NaV1.2 currents is independent of sigma-1 receptor activation, we also carried out sigma-1 receptor RNAi experiment in COS-7 cells. Because the sigma-1 receptor expression was hardly detectable by western blot, the quantitative real-time PCR was used to measure the mRNA expression level (Fig2D). The mRNA expression level was reduced 70% by RNAi. Knockdown of the sigma-1 receptor expression in the COS-7 cells did not alter the inhibitory effect of (+)-SKF 10047 on I Na (COS-7: 38.5±2.3%, n = 6; COS-7 with sigma-1 receptors RNAi: 41.2±1.4%, n = 6; P>0.05, Fig 2C).
Figure 2. The inhibitory effects of (+)-SKF 10047 on NaV1.2 and NaV1.4 are independent of sigma-1 receptor activation.
A, The sample currents for BD 1047, NE-100 and BD 1063 inhibition of NaV1.2 in HEK293T cells with and without (+)-SKF 10047. The sigma-1 receptor antagonists BD 1047, NE-100 and BD 1063 did not prevent (+)-SKF 10047 -evoked inhibition of NaV1.2 in the HEK293T cells (right). B, The effect of (+)-SKF 10047 on NaV1.2 in the HEK293T cells was not reduced by sigma-1 receptor shRNA plasmid coexpression. The sigma-1 receptor protein levels were reduced by 58.7% in the Western blot. C, SKF10047 inhibition of NaV1.2 currents was similar in the COS-7 cells with/without sigma-1 receptor overexpression and knockdown. The Western blot of the sigma-1 receptor expression level (top right). D, The protein expression of sigma-1 receptors was hardly detectable in the Western blot (top). The normalized mRNA expression of sigma-1 receptors in COS-7 cells, and the sigma-1 receptor mRNA expression was reduced by 70% (bottom). E, The I Na for NaV1.4 in the HEK293T cells was also reversibly inhibited by SKF, and it was not blocked by BD 1047. The SKF10047 inhibition of NaV1.4 channel currents was similar in the COS-7 and HEK293T cells.
The impact of (+)-SKF 10047 on other Na+ channels was also investigated. The inhibitory effect of (+)-SKF 10047 on NaV1.4 was examined in the HEK293T and COS-7 cells. No significant differences between the HEK293T cells transfected with NaV1.4 (51.5±2.5%, n = 5) and the COS-7 cells transfected with NaV1.4 (49.9±3.3% n = 5; P>0.05) were observed. The sigma-1 receptor antagonist BD 1047 was used to investigate the role of sigma-1 receptor activation in (+)-SKF 10047 inhibition of NaV1.4. BD 1047 did not alter the (+)-SKF 10047-evoked inhibition of NaV1.4 (47.7±2.2%, n = 4, P>0.05 compared to (+)-SKF 10047 alone).
(+)-SKF 10047 inhibition of NaV1.2 currents is independent of the PKA, PKC, and G protein pathway
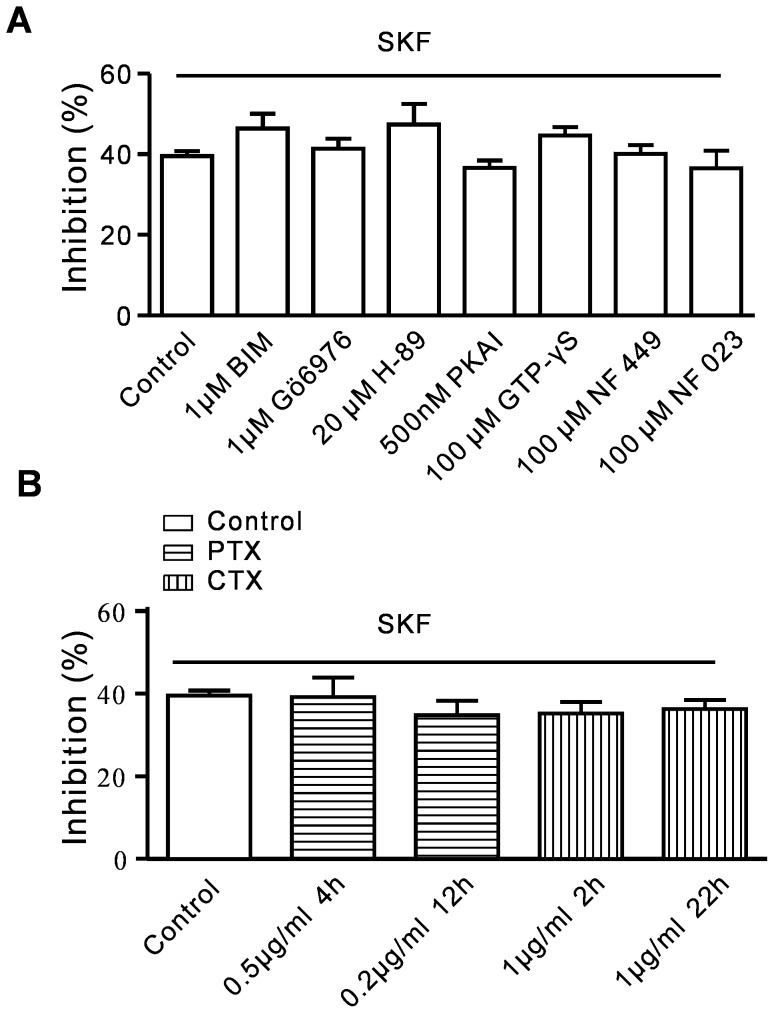
The data above suggested that (+)-SKF 10047 inhibited NaV1.2 currents either directly or via signaling pathways that were independent of sigma-1 receptors. NaV1.2 is inhibited by G-protein, PKC and PKA pathway activation [26]. Therefore, we investigated the roles of the PKA, PKC and G-protein pathways in (+)-SKF-10047-evoked inhibition of NaV1.2 currents. When the PKA inhibitors H-89 (20 µM) and PKAI (500 nM) were present in the internal solution, (+)-SKF 10047 inhibited I Na by 47.4±5.0% (n = 5) and 36.6±1.8% (n = 6), respectively. However, this inhibition was not significantly different with that of 100 µM (+)-SKF 10047 alone (39.5±1.3, n = 6; P>0.05) (Fig 3A). The (+)-SKF 10047 inhibitory effects in the presence of the PKC inhibitors BIM I (1 µM) and Gö6976 (1 µM) were not significantly different from that of 100 µM (+)-SKF 10047 alone (BIM I: 46.4±3.6%, n = 5; Gö6976: 41.3±2.5%, n = 5; P>0.05, Fig 3A).
Figure 3. Effect of (+)-SKF 10047 on NaV1.2 in the presence of G protein and PKC or PKA signaling pathway inhibitors.
A, Histograms of the means±SEM of the peak current inhibition percentages recorded from the NaV1.2-transfected HEK293T cells. The inhibitory effect of (+)-SKF 10047 on NaV1.2 was not altered by the presence of PKA inhibitors (H-89 and PKAi), PKC inhibitors (BIM I and Gö6976), a G protein agonist (GTPγS), a Gs antagonist (NF 449) or a Gi/o antagonist (NF 023) in the pipette solution. P>0.05, compared to SKF-1007 alone. B, Pre-incubating the HEK293T cells with CTX (a Gs activator) or PTX (a Gi/o inhibitor) for different lengths of time and at different concentrations did not alter the inhibitory effects of (+)-SKF 10047 on NaV1.2. (P>0.05, compared to the untreated cells).
The following experiments were performed to investigate the role of G proteins in (+)-SKF 10047 inhibition of NaV1.2 channel currents. CTX (a Gs activator) and PTX (a Gi/o inhibitor) were added to the culture medium at different concentrations and for various lengths of time. The cells were incubated with 1 µg/ml CTX for 2 and 22 h and with 200 ng/ml or 500 ng/ml PTX for 4 or 12 h, respectively. The inhibitory effects of 100 µM (+)-SKF 10047 on NaV1.2 channel currents were not altered in the presence of these toxins. The average I Na inhibitions were 35.2±2.8% (1 µg/ml CTX for 2 h, n = 5), 36.2±2.3% (1 µg/ml CTX for 22 h, n = 5), 39.2±4.8% (200 ng/ml PTX for 4 h, n = 5) and 34.8±3.4% (500 ng/ml PTX for 12 h, n = 5), which were not significantly different with effect of 100 µM (+)-SKF 10047 alone (P>0.05) (Fig 3B). The inhibitory effect of (+)-SKF 10047 was also not significantly altered by the presence of GTPγS (a G-protein activator), NF 023 (a Gi/o antagonist) or NF 449 (a Gs antagonist) in the internal solution (Fig 3A).
These results suggested that (+)-SKF 10047 directly inhibited the NaV1.2 channel currents.
Therefore, we investigated the frequency- and use-dependency of (+)-SKF 10047 NaV1.2 channel current inhibition.
(+)-SKF 10047 inhibits NaV1.2 channel currents in a frequency- and use-dependent manner
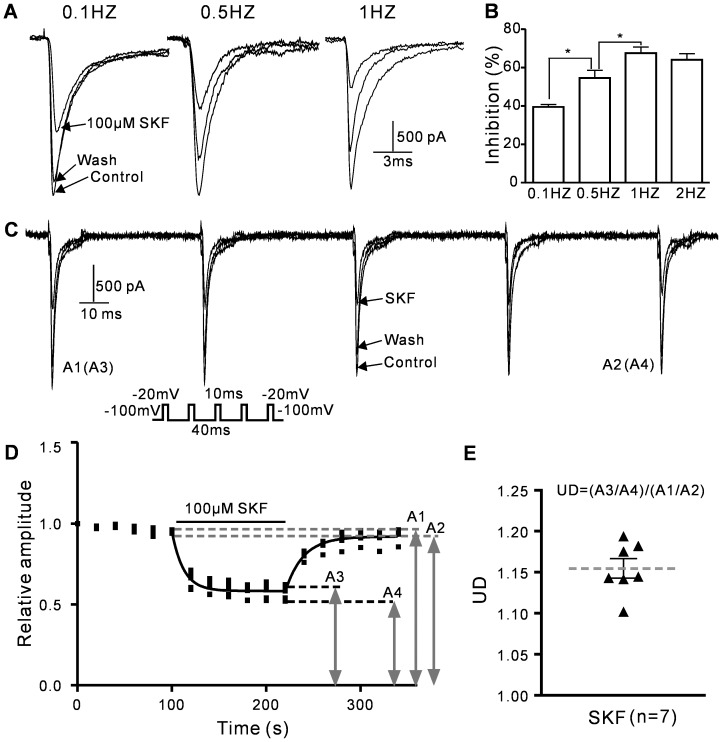
The effect of frequency on (+)-SKF 10047 inhibition of NaV1.2 channel currents was investigated using different frequencies of 30-ms pulses that depolarized the holding potential from −100 to −20 mV. The cells were stimulated for 2 min in the continued presence of 100 µM (+)-SKF-10047. The average NaV1.2 channel current inhibitions for stimulations at 0.1, 0.5, 1 and 2 HZ were 39.5±1.3% (n = 6), 54.6±4.0% (n = 7), 67.6±3.2% (n = 9), and 64.1±3.2% (n = 7),respectively (P<0.05 compared to 0.1 Hz) (Figs 4A and B). These results suggested that (+)-SKF 10047 exerted frequency-dependent effects on the NaV1.2 channel. The use-dependency of the inhibition was evaluated using 5-Hz trains of five pulses that depolarizing the holding potentials from −100 to −10 mV (Fig 4C). The trains were repeated every 20 s. (+)-SKF 10047 (100 µM) was applied after 6 control trains and washed out, and the 6 trains were repeated (Fig 4D). The use-dependence (UD) was determined by the peak amplitudes of the first and last evoked currents of the last train in the presence and absence of (+)-SKF 10047 (UD = (A3/A4)/(A1/A2), Fig 4E). The UD of 7 samples ranged from 1.10 to 1.19 (Fig 4E). These results suggested that the inhibitory effect of (+)-SKF 10047 on the NaV1.2 channel was use-dependent and that the drug may preferentially bind to the depolarized (i.e., open or inactivated) channel.
Figure 4. Frequency- and use-dependent inhibitory effect of (+)-SKF 10047 on I Na.
A, Sample current traces at 0.1, 0.5 and 1 Hz pulse frequencies were obtained in the presence and absence of 100 µM (+)-SKF-10047. B, SKF inhibition of I Na increased with increased pulse frequency. *, P<0.05 compared to 0.1 Hz. C, Sample NaV1.2 current traces for 5 Hz-train depolarizations from −100 to −20 mV were obtained in the presence and absence of 100 µM (+)-SKF-10047. D, The time course of the peak amplitude changes during the train pulses. Use-dependence (UD) = (A3/A4)/(A1/A2). E, The individual UD data points (black triangles) and the UD means±SEM (gray dashed line) under the 5-Hz train pulse condition. The UD ranged from 1.10 to 1.19.
The effect of (+)-SKF 10047 on the NaV1.2 channel is altered by the site-directed mutation of F1764A and Y1771A
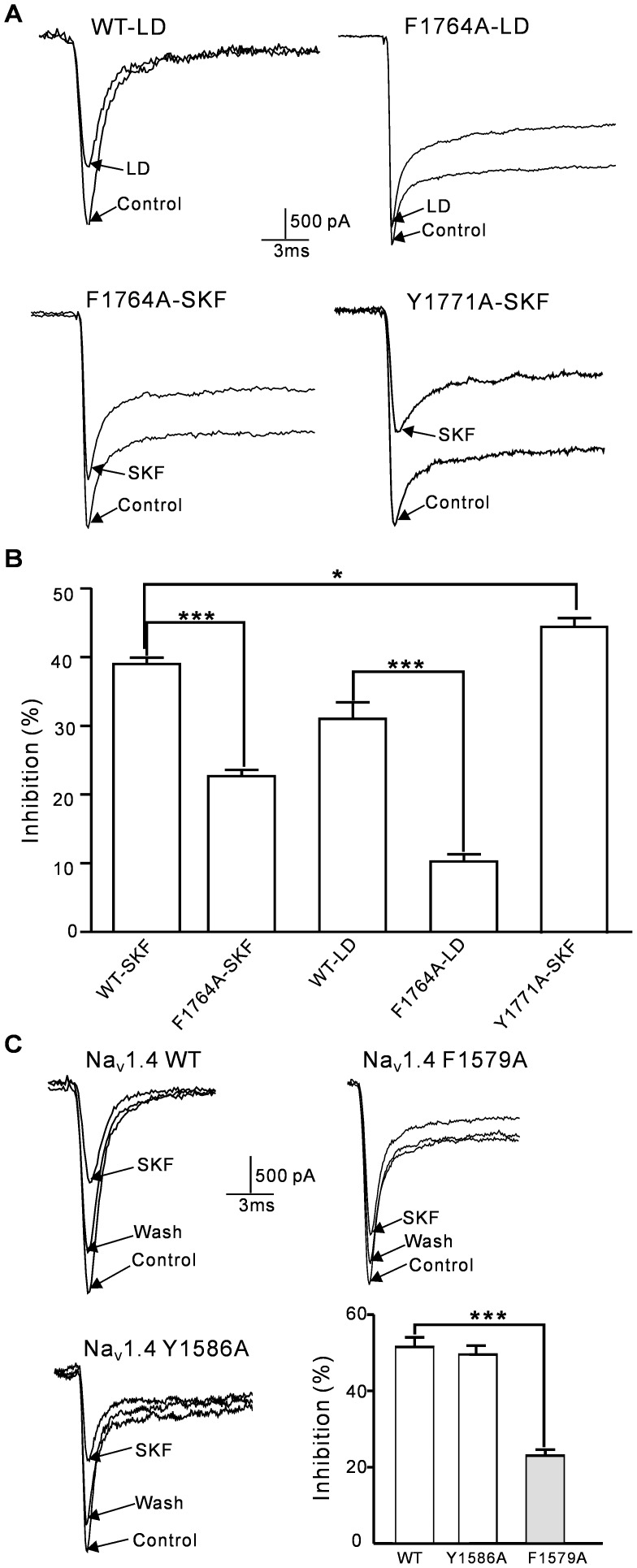
Local anesthetic (LA) drugs, such as lidocaine (LD), may directly inhibit rat NaV1.2 channel currents, and the amino acid residues Phe-1764 and Tyr-1771 in the S6 transmembrane segment of domain IV are critical for this inhibition [23], [27]. LD induced less inhibition in the F1764A mutant NaV1.2 channel than in the wild-type channel (Figs 5A and B).
Figure 5. The effects of (+)-SKF 10047 or lidocaine on wild-type and mutant NaV1.2/NaV1.4 in HEK293T cells.
A, Sample current traces for wild-type and mutant NaV1.2 channels (F1764A or Y1771A) were obtained in the presence and absence of 100 µM (+)-SKF 10047 or 100 µM lidocaine. B, The statistical analyses of I Na inhibition for wild-type and mutant NaV1.2 channels in the presence of 100 µM (+)-SKF 10047 or 100 µM lidocaine. The (+)-SKF 10047 inhibition of the F1764A and Y1771A mutant channel currents was significantly different from the inhibition of the wild-type NaV1.2 current (*, P<0.05; ***, P<0.001). The lidocaine-induced I Na inhibition also differed significantly between the F1764A and wild-type NaV1.2 channels (***, P<0.001, Student's t test). C, Sample current traces for wild-type, F1579A and Y1586A mutant NaV1.4 channels were obtained in the presence and absence of 100 µM (+)-SKF 10047. The mutation of F1579A significantly reduced the (+)-SKF 10047 inhibition of NaV1.4 channel currents (***, P<0.001).
F1764A and Y1771A single-point mutant NaV1.2 channels were investigated in HEK293T cells to assess similarities in the effects of (+)-SKF 10047 and LD on the NaV1.2 channel. The (+)-SKF 10047 inhibition of the F1764A mutant channel currents was significantly less than that of the wild-type channel currents (WT: 39.5±1.3%; F1764A mutant: 25.9±1.6%, n = 9; p<0.001, Fig 5B). Interestingly, the (+)-SKF 10047 inhibitory effect on the Y1771A mutant channel currents was significantly higher than that of the wild-type channel currents (Y1771A mutant: 45.2±1.3%, n = 6, P<0.05 compared to WT). These data suggested the importance of the F1764 and Y1771 residues for (+)-SKF 10047 inhibition of NaV1.2 channel currents.
The effect of (+)-SKF 10047 on the NaV1.4 channel is altered by the site-directed mutation of F1579A
The mutations of F1579A and Y1586A, which correspond to the F1764A and Y1771A in NaV1.2 channel, were introduced into NaV1.4 channels. F1579A and Y1586A single-point mutant NaV1.4 channels were investigated in HEK293T cells. The (+)-SKF 10047 inhibition of the F1579A mutant NaV1.4 channel currents was significantly less than that of the wild-type channel currents (WT: 51.5±2.5%, n = 5; F1579A mutant: 23.0±1.7%, n = 7; p<0.001, Fig 5C). The (+)-SKF 10047 inhibition of NaV1.4 channel currents was not changed by Y1586A mutation. (WT: 51.5±2.5%, n = 5; Y1586A mutant: 49.3±0.5%, n = 14; p>0.05, Fig 5C).
The sigma-1 receptor-selective agonists DM and DTG also reduce rat NaV1.2 currents
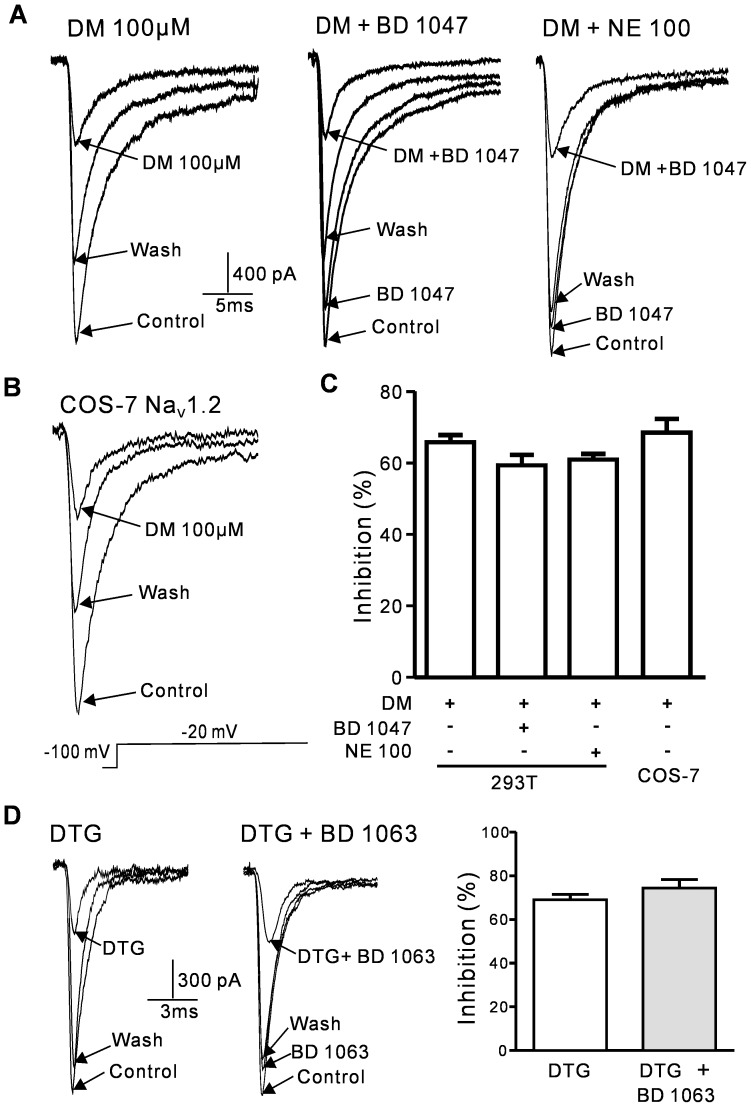
The sigma-1 receptor-selective agonists DM, DTG and PRE-084 were used to assess the inhibitory effects of other sigma-1 receptor ligands on rat NaV1.2 channels. PRE-084 exerted little effect on the NaV1.2 channel currents (data not shown), but DM and DTG reversibly inhibited the NaV1.2 channel currents (Fig 6A, D). The average inhibition from 100 µM DM in the NaV1.2-transfected HEK293T cells was 65.8±2.0% (n = 6). The sigma-1 receptor antagonists BD 1047 and NE-100 were used to investigate the role of sigma-1 receptors in this inhibitory effect. The average DM inhibitions in the presence of 2 µM BD 1047 and 2.5 µM NE-100 were 59.7±2.9% (n = 5) and 61.2±1.6% (n = 5), respectively. These results were not significantly different from those observed with DM alone (Fig 6C). DM inhibition of NaV1.2 channel currents was also investigated in the COS-7 cells. The inhibitory effect of DM on NaV1.2 channel currents in the COS-7 cells was similar to the effect in the HEK293T cells (COS-7: 68.7±3.9%, n = 5; HEK293T: 65.8±2.0%, n = 6; P>0.05; Figs 6B and C). Previous study has been shown that DTG effect (via activation of the sigma-1 receptor) could be blocked by BD 1063 [28]. Our results showed that The DTG inhibition of NaV1.2 channel currents was not altered by sigma-1 receptor antagonist BD 1063 (100 µM DTG: 69.0±2.5%, n = 6; 100 µM DTG+2 µM BD 1063: 71.8±3.6%, n = 4; P>0.05).These results suggested that DM and DTG inhibited NaV1.2 currents via a sigma-1 receptor-independent pathway.
Figure 6. DM and DTG inhibition of NaV1.2 channel currents in HEK293T cells was independent of sigma-1 receptor activation.
A, Sample current traces showing DM inhibition of NaV1.2 channels in the HEK293T cells with and without 2 µM BD 1047 or NE-100. B, Sample current traces showing DM inhibition of NaV1.2 channels in the COS-7 cells. C, Histograms of the means±SEM of the peak current inhibition percentages of DM. The I Na inhibitory effects of DM+2 µM BD 1047 and DM+2.5 µM NE-100 were similar to the effects of 100 µM DM. The DM inhibition of I Na was not significantly different between the HEK293T and COS-7 cells. D, Sample current traces showing DTG inhibition of NaV1.2 channels in the HEK293T cells with and without 2 µM BD 1063. The average percent inhibition of I Na by DTG in the absence and presence of BD 1063 (right).
(+)-SKF 10047 inhibits sodium currents in rat cerebellar granule neurons
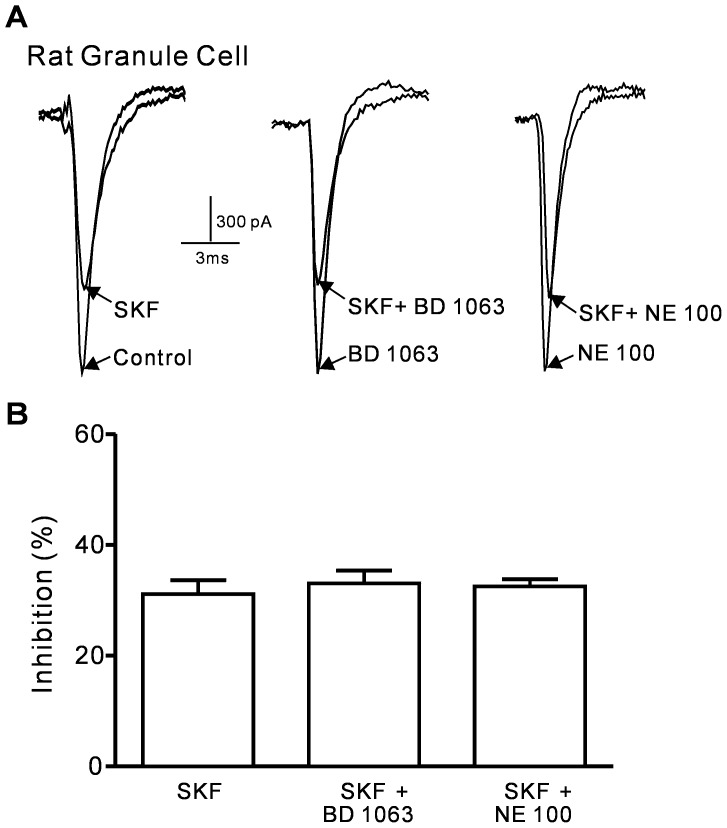
The primary cultured rat cerebellar granule cells, which express mainly NaV1.2 and NaV1.6 channel [29], were used to test whether (+)-SKF 10047 has similar inhibitory effect on native sodium currents. 100 µM (+)-SKF 10047 reversibly inhibited sodium currents in granule cells and the inhibition was not blocked by BD 1063 and NE-100 (SKF: 31.1±2.6%, n = 4; SKF+BD 1063: 33.1±2.3%, n = 5; SKF+NE-100: 32.5±1.3%, n = 4; P>0.05, Fig 7A,B).
Figure 7. (+)-SKF 10047 inhibition of I Na in rat cerebellar granule cells was not affected by sigma-1 receptor antagonist.
A, Sample current traces showing SKF 10047 inhibition of I Na in rat cerebellar granule cells with and without 2 µM BD 1063 and 2.5 µM NE-100. B, Histograms of the means±SEM of the peak current inhibition percentages of SKF 10047. The INa inhibitory effect of SKF 10047 was not altered by BD 1063 or NE-100.
Discussion
(+)-SKF 10047 is a prototypic and specific sigma-1 receptor agonist that has been extensively used to study sigma-1 receptor function. By activation of sigma-1 receptors, (+)-SKF 10047 inhibits N-methyl-D-aspartate (NMDA) receptors in rat retinal ganglion cells [30], voltage-gated cardiac NaV1.5 channels [20], [21], [25], L-type voltage-gated calcium channels [31], various types of voltage-gated K+ channels [32], [33], [34]. (+)-SKF 10047 also inhibits glutamate release in rat cerebral cortex neurons [35]. We found that (+)-SKF 10047 inhibited NaV1.2 and NaV1.4 voltage-gated sodium channels, and this inhibitory effect was not eliminated by the sigma-1 selective antagonists BD 1047 and NE-100. These results suggest that (+)-SKF 10047 inhibition of NaV1.2/1.4 channel currents is independent of sigma-1 receptors.
Sigma-1 receptor ligands likely exert effects that are unrelated to sigma-1 receptors. NDHEA sulfate, a sigma-1 receptor agonist, inhibits persistent sodium currents in the rat medial prefrontal cortex via the Gi protein and the PKC signaling pathways [36]. The sigma-1 receptor ligand PRE-084 amplifies dopamine D1 receptor signaling in the prelimbic cortex [37]. G-protein blockade or activation using Gi/o and Gs inhibitors did not alter the (+)-SKF 10047 -induced inhibition of NaV1.2 channel currents. The inhibitory effect of (+)-SKF 10047 on NaV1.2 channel currents persisted in the presence of PKA and PKC inhibitors (Fig3). These results further suggest that (+)-SKF 10047 inhibition of NaV1.2 channel currents is independent of sigma-1 receptors, G-protein-coupled receptor (GPCRs) activity or phosphokinase pathways.
Most local anesthetics are state-dependent blockers of Na+ channels. The mechanism of this blockade may be the high affinity of these drugs for a site on the opening, inactivated and resting channel [38], [39]. (+)-SKF 10047 inhibited NaV1.2 channels in a state-dependent manner, which is consistent with Na+ channel blockade. (+)-SKF 10047 preferentially interacted with inactivated NaV1.2 channels to produce a significant hyperpolarizing shift in the voltage-dependent inactivation, which reduced channel availability and slowed recovery. The marked effects of (+)-SKF 10047 on channel inactivation are consistent with the high affinity of local anesthetics and anticonvulsants, such as LD, for inactivated channels [23], [27]. (+)-SKF 10047 also produced a use-dependent blockade of the NaV1.2 channel following high-frequency stimulation, which suggested an (+)-SKF 10047 affinity for open or inactivated Na+ channels. This result suggests that (+)-SKF 10047 may directly inhibit NaV1.2 channels.
The direct interactions between local anesthetics and NaV1.2 channels have been thoroughly studied, and Phe-1764 and Tyr-1771 mutations in domain IV of the S6 channel transmembrane segment dramatically reduce NaV1.2 channel current inhibition by local anesthetics, such as lidocaine [23], [27]. We found that an F1764A mutation dramatically slowed the fast inactivation of the NaV1.2 current, which is consistent with a previous report [27]. This mutation also reduced the (+)-SKF 10047 inhibition of NaV1.2 channel currents by 35%. However, the Y1771A mutation did not reduce (+)-SKF 10047 inhibition of NaV1.2 channels (Fig 5). These results suggest that the sites or mechanisms of (+)-SKF 10047 binding to NaV1.2 channels are not identical to those of lidocaine. Previous studies suggested that F1764 and Y1771 are direct LA (local anesthetic) drugs binding sites of sodium channels [27], [40]. Both F1764A and Y1771A mutations reduced the LA affinity for open and fast inactivated channels, but the effect of Y1771A was much less than F1764A [27]. Our data showed that Y1771A increased the inhibition of SKF10047, which may similar to the mutations I1761A, V1776A and N1769A increasing the LA drugs inhibition of NaV1.2 channels [27]. Y1771A may increase closed- state NaV1.2 channel sensitivity to SKF 10047 in an indirect way. Since SKF 10047 binds preferentially to inactivated-state channels, Y1771A may partially change the SKF10047 binding site of close-state NaV1.2 channels towards the inactivated conformation. The specific structural and mechanistic differences that determine the effects of (+)-SKF 10047 on NaV1.2 may involve multiple binding sites. However, this hypothesis requires detailed structure–function investigations. The F1579A mutation (corresponding to F1764 in NaV1.2 channel) significantly reduced the (+)-SKF 10047 inhibition of NaV1.4 channel currents, but the Y1586A mutation showed no effect. This is consistent with the previous report that the affinities of local anesthetic drug binding to NaV1.4 channel are determined primarily by interaction with F1759 [40]. Since it has more restricted conformation than lidocaine and Y1586 is on the bottom of channel pore comparing to F1579, (+)-SKF 10047 may not direct interact with Y1586 site.
Interestingly, (+)-SKF 10047 directly inhibited both NaV1.2 and NaV1.4 channels. These inhibitory effects occurred and were washed out within 1–2 minutes. The IC50 value of NaV1.2 for (+)-SKF 10047 was 140 µM. These results are different from previous reports on (+)-SKF 10047 inhibition of cardiac NaV1.5 channels via sigma-1 receptor activation, which required more than 10 min to occur and wash out and had a much lower IC50 Value (70 µM) [21]. The inhibitory effects of (+)-SKF 10047 on NaV1.5 channels also differed between HEK293 cells with abundant sigma-1 receptor expression and COS-7 cells with little sigma-1 receptor expression [21], [25]. This study compared SKF-1047 inhibition of NaV1.2 and NaV1.4 channels in HEK293T cells and COS-7 cells, and no differences between these two cell types were observed (Fig 2). SKF-10047 directly inhibited the COS-7 cells, which express NaV1.2 and NaV1.4 channels but few sigma-1 receptors. The NaV1.2, NaV1.4, and NaV1.5 α-subunit isoforms have greater than 60% amino acid sequence identity. However, these channels exhibit gating, permeability, and conductance functional differences, which result in tissue-specific physiological functions and subtle difference in their pharmacological properties [10]. The markedly different (+)-SKF 10047 inhibition of NaV1.2/NaV1.4 and NaV1.5 currents may be explained by chemical structure differences because the NaV1.5 protein harbors multiple evolutionary conserved amino acid motifs for N-glycosylation in its extracellular domain. The N-glycosylation affects NaV1.5 channel gating [41] , and maybe also affect SKF 10047 binding to the channel.
(+)-SKF 10047 may inhibit various ion channels via sigma-1 receptor activation, but a direct interaction has seldom been noted. Lamy et al. have recently reported that the sigma-1 agonist DTG directly inhibits small-conductance Ca2+-activated K channels in dopaminergic neurons and HEK-293 cells [42]. In addition, DM directly inhibits brain Na+ channels in Xenopus oocytes [43]. The sigma-1 agonists DTG, (+)-SKF 10047 and DM directly inhibited the NaV1.2 and/or NaV1.4 channel currents in the present study. DM and (+)-SKF 10047 belong to the homologous family of benzomorphan compounds. Therefore, further investigation of the effects of other benzomorphan compounds is required.
The (+)-SKF 10047 inhibitory effect was also tested in primary cultured rat cerebellar granule neurons. The sigma-1 receptor antagonist BD 1063 and NE-100 failed to block the (+)-SKF 10047 inhibition of sodium currents in the granule neurons, which suggested that the inhibition was independent of the sigma-1 receptor activation. However, rat cerebellar granule neurons express both NaV1.2 and NaV1.6 channels [29], the (+)-SKF 10047 effect on NaV1.6 channel currents need further investigation.
In conclusion, our study found that the sigma-1 receptor agonists DTG, (+)-SKF 10047 and DM directly inhibited NaV1.2 or NaV1.4 channels in transfected HEK293T and COS-7 cells. The sigma-1 receptor is involved in many diseases, and the final action of sigma-1 receptor activation is most likely to modulate various ion channels. Therefore, the direct effects of sigma-1 receptor ligands on ion channels should receive special attention.
Acknowledgments
The authors would like thank Dr. Teruo Hayashi for providing the sigma-1 receptor antibody, Dr. Alan L. Goldin for providing NaV1.2 construct and Dr. William A. Catterall providing Y1771A mutant NaV1.2 construct.
Funding Statement
This work was supported by a grant from the National Basic Research Program of China (2011CB503703) and the Shanghai Leading Academic Discipline Project [B111]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE (1976) The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197: 517–532. [PubMed] [Google Scholar]
- 2. Maurice T, Su TP (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, et al. (1996) Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A 93: 8072–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mei J, Pasternak GW (2001) Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem Pharmacol 62: 349–355. [DOI] [PubMed] [Google Scholar]
- 5. Seth P, Fei YJ, Li HW, Huang W, Leibach FH, et al. (1998) Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem 70: 922–931. [DOI] [PubMed] [Google Scholar]
- 6. Cobos EJ, Entrena JM, Nieto FR, Cendan CM, Del Pozo E (2008) Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr Neuropharmacol 6: 344–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aydar E, Palmer CP, Djamgoz MB (2004) Sigma receptors and cancer: possible involvement of ion channels. Cancer Res 64: 5029–5035. [DOI] [PubMed] [Google Scholar]
- 8. Monnet FP (2005) Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol Cell 97: 873–883. [DOI] [PubMed] [Google Scholar]
- 9. Catterall WA, Goldin AL, Waxman SG (2005) International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409. [DOI] [PubMed] [Google Scholar]
- 10. Goldin AL (2001) Resurgence of sodium channel research. Annu Rev Physiol 63: 871–894. [DOI] [PubMed] [Google Scholar]
- 11. Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, et al. (2000) Nomenclature of voltage-gated sodium channels. Neuron 28: 365–368. [DOI] [PubMed] [Google Scholar]
- 12. Gordon D, Merrick D, Auld V, Dunn R, Goldin AL, et al. (1987) Tissue-specific expression of the RI and RII sodium channel subtypes. Proc Natl Acad Sci U S A 84: 8682–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engel D, Jonas P (2005) Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45: 405–417. [DOI] [PubMed] [Google Scholar]
- 14. Trimmer JS, Cooperman SS, Agnew WS, Mandel G (1990) Regulation of muscle sodium channel transcripts during development and in response to denervation. Dev Biol 142: 360–367. [DOI] [PubMed] [Google Scholar]
- 15. Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F (2010) Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Arch 460: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kallen RG, Sheng ZH, Yang J, Chen LQ, Rogart RB, et al. (1990) Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron 4: 233–242. [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Li Z, Shen J, Keating MT (1996) Genomic organization of the human SCN5A gene encoding the cardiac sodium channel. Genomics 34: 9–16. [DOI] [PubMed] [Google Scholar]
- 18. Remme CA, Wilde AA, Bezzina CR (2008) Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc Med 18: 78–87. [DOI] [PubMed] [Google Scholar]
- 19. Ruan Y, Liu N, Priori SG (2009) Sodium channel mutations and arrhythmias. Nat Rev Cardiol 6: 337–348. [DOI] [PubMed] [Google Scholar]
- 20. Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB (2011) Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol 300: C328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johannessen M, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, et al. (2009) Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am J Physiol Cell Physiol 296: C1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith RD, Goldin AL (1997) Phosphorylation at a single site in the rat brain sodium channel is necessary and sufficient for current reduction by protein kinase A. J Neurosci. 17: 6086–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ragsdale DS, McPhee JC, Scheuer T, Catterall WA (1996) Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A 93: 9270–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu CL, Zeng XM, Zhou MH, Shi YT, Cao H, Mei YA (2008) Kv 1.1 is associated with neuronal apoptosis and modulated by protein kinase C in the rat cerebellar granule cell. J Neurochem 106(3): 1125–37. [DOI] [PubMed] [Google Scholar]
- 25. Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, et al. (2009) The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323: 934–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheuer T (2011) Regulation of sodium channel activity by phosphorylation. Semin Cell Dev Biol 22: 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ragsdale DS, McPhee JC, Scheuer T, Catterall WA (1994) Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Katnik C, Cuevas J (2010) Sigma receptor activation inhibits voltage-gated sodium channels in rat intracardiac ganglion neurons. Int J Physiol Pathophysiol Pharmacol 2: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 29. Schaller KL, Caldwell JH (2003) Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum 2: 2–9. [DOI] [PubMed] [Google Scholar]
- 30. Zhang XJ, Liu LL, Jiang SX, Zhong YM, Yang XL (2011) Activation of the zeta receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience 177: 12–22. [DOI] [PubMed] [Google Scholar]
- 31. Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, et al. (2008) Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci 49: 4993–5002. [DOI] [PubMed] [Google Scholar]
- 32. Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, et al. (2000) Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol 526 Pt 3: 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilke RA, Lupardus PJ, Grandy DK, Rubinstein M, Low MJ, et al. (1999) K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J Physiol 517 (Pt 2): 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilke RA, Mehta RP, Lupardus PJ, Chen Y, Ruoho AE, et al. (1999) Sigma receptor photolabeling and sigma receptor-mediated modulation of potassium channels in tumor cells. J Biol Chem 274: 18387–18392. [DOI] [PubMed] [Google Scholar]
- 35. Lu CW, Lin TY, Wang CC, Wang SJ (2012) Sigma-1 receptor agonist, SKF10047, inhibits glutamate release in rat cerebral cortex nerve endings. J Pharmacol Exp Ther. 341: 532–42. [DOI] [PubMed] [Google Scholar]
- 36. Cheng ZX, Lan DM, Wu PY, Zhu YH, Dong Y, et al. (2008) Neurosteroid dehydroepiandrosterone sulphate inhibits persistent sodium currents in rat medial prefrontal cortex via activation of sigma-1 receptors. Exp Neurol 210: 128–136. [DOI] [PubMed] [Google Scholar]
- 37. Fu Y, Zhao Y, Luan W, Dong LY, Dong Y, et al. (2010) Sigma-1 receptors amplify dopamine D1 receptor signaling at presynaptic sites in the prelimbic cortex. Biochim Biophys Acta 1803: 1396–1408. [DOI] [PubMed] [Google Scholar]
- 38. Butterworth JFt, Strichartz GR (1990) Molecular mechanisms of local anesthesia: a review. Anesthesiology 72: 711–734. [DOI] [PubMed] [Google Scholar]
- 39. Castaneda-Castellanos DR, Nikonorov I, Kallen RG, Recio-Pinto E (2002) Lidocaine stabilizes the open state of CNS voltage-dependent sodium channels. Brain Res Mol Brain Res 99: 102–113. [DOI] [PubMed] [Google Scholar]
- 40. Lipkind GM, Fozzard HA (2005) Molecular Modeling of Local Anesthetic Drug Binding by Voltage-Gated Sodium Channels. Mol Pharmacol 68: 1611–1622. [DOI] [PubMed] [Google Scholar]
- 41. Rook MB, Evers MM, Vos MA, Bierhuizen MF Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res 93: 12–23. [DOI] [PubMed] [Google Scholar]
- 42. Lamy C, Scuvee-Moreau J, Dilly S, Liegeois JF, Seutin V (2010) The sigma agonist 1,3-di-o-tolyl-guanidine directly blocks SK channels in dopaminergic neurons and in cell lines. Eur J Pharmacol 641: 23–28. [DOI] [PubMed] [Google Scholar]
- 43. Lee JH, Shin EJ, Jeong SM, Lee BH, Yoon IS, et al. (2007) Effects of dextrorotatory morphinans on brain Na+ channels expressed in Xenopus oocytes. Eur J Pharmacol 564: 7–17. [DOI] [PubMed] [Google Scholar]