Abstract
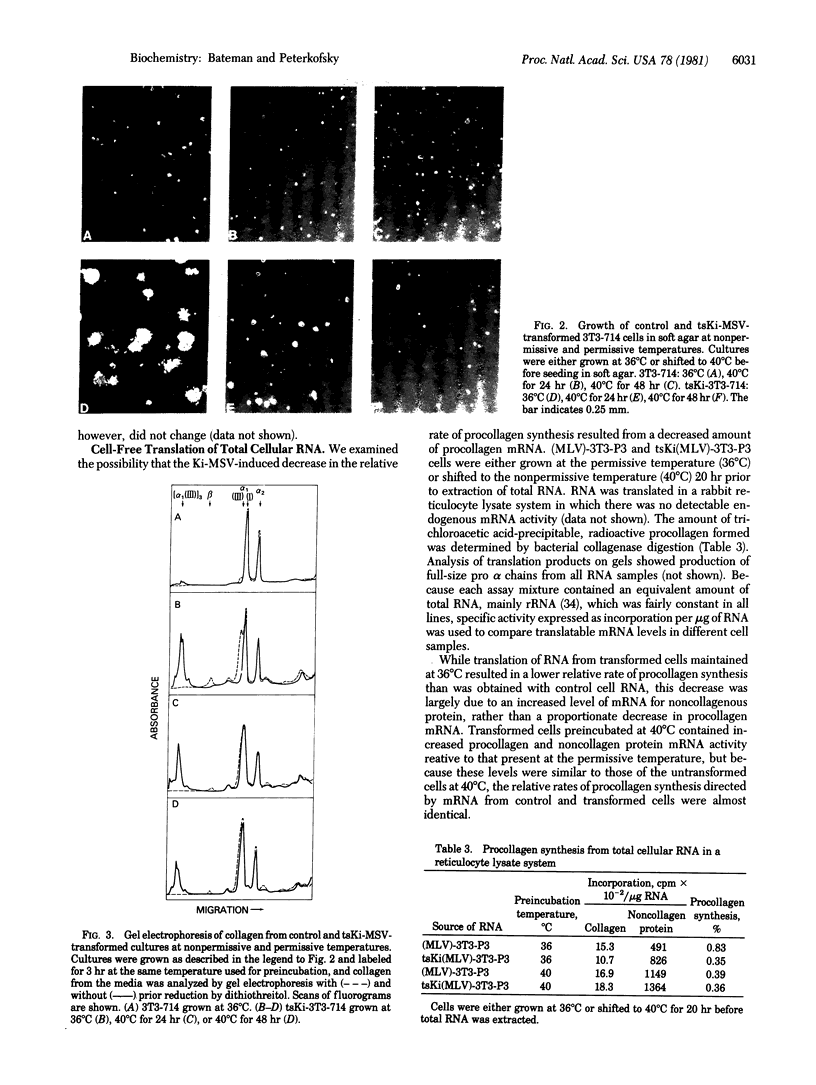
Specific viral transformation rather than cell selection can explain the previously observed increase in the proportion of type III procollagen compared to type I procollagen in BALB 3T3 cells transformed by Kirsten murine sarcoma virus (Ki-MSV). Two subclones of BALB 3T3 A31 were productively infected with with a temperature-sensitive Ki-MSV in the presence of helper murine leukemia virus (MLV), resulting in virtually complete transformation of cultures and eliminating selection of transformed foci. Analysis of radioactive collagen, derived from procollagen by pepsin treatment, showed that both of the tsKi-MSV/MLV-transformed subclones contained a 4-fold greater proportion of type III procollagen than did control MLV-infected cultures. A nonproducer derivative exhibited an even greater change (10-fold), indicating that viral replication was irrelevant. After 48 hr at a nonpermissive temperature, tsKi-MSV-transformed cells retained a high proportion of type III procollagen, suggesting that either this change is not induced by src protein or else there is a slowly reversible or irreversible step involved. Alternatively, type III procollagen mRNA may be long lived. In contrast, the relative rate of procollagen synthesis in transformed cells was clearly regulated by src protein. Translation of mRNA from cells preincubated at permissive or nonpermissive temperatures revealed that the decreased relative rate can be explained by a simultaneous small decrease in the level of procollagen mRNA and a large increase in mRNA for noncollagen proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOOM S., GOLDBERG B., GREEN H. THE LIFETIME OF MESSENGER RNA FOR COLLAGEN AND CELL PROTEIN SYNTHESIS IN AN ESTABLISHED MAMMALIAN CELL LINE. Biochem Biophys Res Commun. 1965 Apr 23;19:317–321. doi: 10.1016/0006-291x(65)90461-4. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Methods of cell transformation by tumor viruses. Methods Cell Biol. 1974;8(0):367–437. doi: 10.1016/s0091-679x(08)60458-6. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brawerman G. The isolation of messenger RNA from mammalian cells. Methods Enzymol. 1974;30:605–612. doi: 10.1016/0076-6879(74)30058-4. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Balian G., Ross R., Bornstein P. Synthesis of types I and III procollagen and collagen by monkey aortic smooth muscle cells in vitro. Biochemistry. 1977 Jul 12;16(14):3243–3249. doi: 10.1021/bi00633a031. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Peterkofsky B., Bateman J. New method for determining the extent of proline hydroxylation by measuring changes in the ratio of [4-3H]:[14C]proline in collagenase digests. Anal Biochem. 1980 Nov 1;108(2):385–393. doi: 10.1016/0003-2697(80)90603-x. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Retention of transformant specific type III collagen in dibutyryl cAMP treated Kirsten sarcoma virus transformed BALB 3T3 cells and in a flat revertant. J Cell Physiol. 1978 Jun;95(3):343–352. doi: 10.1002/jcp.1040950312. [DOI] [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Specific changes in the collagen phenotype of BALB 3T3 cells as a result of transformation by sarcoma viruses or a chemical carcinogen. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2933–2937. doi: 10.1073/pnas.74.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Nagai Y. Separation of the alpha chains of type I and III collagens by SDS-polyacrylamide gel electrophoresis. J Biochem. 1979 Aug;86(2):453–459. doi: 10.1093/oxfordjournals.jbchem.a132544. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kamine J., Rubin H. Coordinate control of collagen synthesis and cell growth in chick embryo fibroblasts and the effect of viral transformation on collagen synthesis. J Cell Physiol. 1977 Jul;92(1):1–11. doi: 10.1002/jcp.1040920102. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang D. R., Weber M. J. Increased membrane transport of 2-deoxyglucose and 3-O-methylglucose is an early event in the transformation of chick embryo fibroblasts by Rous sarcoma virus. J Cell Physiol. 1978 Mar;94(3):315–319. doi: 10.1002/jcp.1040940309. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Parker I., Fitschen W. Procollagen mRNA metabolism during the fibroblast cell cycle and its synthesis in transformed cells. Nucleic Acids Res. 1980 Jun 25;8(12):2823–2833. doi: 10.1093/nar/8.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Bornstein P. Declining procollagen mRNA sequences in chick embryo fibroblasts infected with rous sarcoma virus. Correlation with procollagen synthesis. J Biol Chem. 1979 Jun 25;254(12):4950–4953. [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]