Abstract
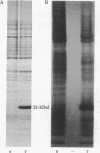
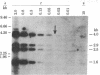
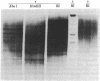
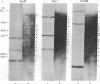
For the eventual purpose of isolating and studying a single animal cell replicon, we have developed a methotrexate-resistant Chinese hamster ovary cell line that has amplified an early-replicating DNA sequence approximately 500 times; this sequence includes the gene coding for dihydrofolate reductase (DHFR; tetrahydrofolate dehydrogenase; 5,6,7,8-tetrahydrofolate:NADP+ oxidoreductase, EC 1.5.1.3). DHFR composes 30% of the cytoplasmic protein in this cell line, and DHFR mRNA represents 25% of the message translatable in vitro. After digestion of genomic DNA from resistant cells with restriction enzymes, a unique set of highly repetitive restriction fragments can be visualized on agarose gels by ethidium bromide staining. These bands are not present in digests of parental DNA. We estimate the total length of the unit repeated sequence to be 135 +/- 15 kilobase pairs. Regardless of the restriction enzyme utilized, a subset of these repetitive fragments hybridizes to radioactive DHFR cDNA. The homogeneously staining regions on mitotic chromosomes in which these amplified sequences are located are shown to be early-replicating, as are the highly repeated restriction fragments themselves. These data suggest that an early replicon can be isolated from this region, and that this entire, normally unique, genomic segment can be cloned and mapped with respect to origins of DNA synthesis and promoters for transcription, as well as other genetic features of interest.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. A novel chromosome abnormality in human neuroblastoma and antifolate-resistant Chinese hamster cell lives in culture. J Natl Cancer Inst. 1976 Sep;57(3):683–695. doi: 10.1093/jnci/57.3.683. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Clark E. M. Satellite DNA in large marker chromosomes of methotrexate-resistant mouse cells. Cell. 1980 Mar;19(3):709–715. doi: 10.1016/s0092-8674(80)80047-x. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Prescott D. M. Shift in buoyant density of DNA during the synthetic period and its relation to euchromatin and heterochromatin in mammalian cells. J Mol Biol. 1971 Aug 28;60(1):151–162. doi: 10.1016/0022-2836(71)90454-2. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J., Berenson R. J., Bertino J. R., Kaufman R. J., Nunberg J. H., Schimke R. T. Correlation of dihydrofolate reductase elevation with gene amplification in a homogeneously staining chromosomal region in L5178Y cells. J Cell Biol. 1979 Nov;83(2 Pt 1):394–402. doi: 10.1083/jcb.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Biedler J. L. Replication pattern of a large homogenously staining chromosome region in antifolate-resistant Chinese hamster cell lines. J Cell Physiol. 1981 Apr;107(1):101–114. doi: 10.1002/jcp.1041070112. [DOI] [PubMed] [Google Scholar]
- Kates J. Detection and utilization of poly(A) sequences in messenger RNA. Methods Cell Biol. 1973;7:53–65. doi: 10.1016/s0091-679x(08)61771-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melera P. W., Lewis J. A., Biedler J. L., Hession C. Antifolate-resistant Chinese hamster cells. Evidence for dihydrofolate reductase gene amplification among independently derived sublines overproducing different dihydrofolate reductases. J Biol Chem. 1980 Jul 25;255(14):7024–7028. [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Johnson L. F. Regulation of dihydrofolate reductase synthesis in an overproducing 3T6 cell line during transition from resting to growing state. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2818–2822. doi: 10.1073/pnas.76.6.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]