Abstract
Interleukin 17 (IL-17) is produced during infection with Listeria monocytogenes and is also an important regulator of tumor development with both pro- and anti-tumorigenic effects. αβ T cells and γδ T cells are among the principle producers of IL-17 in response to infection and other proinflammatory conditions. Listeria-based cancer immunotherapies induce IFNγ directed Th1 dependent tumor regression; however, the role of IL-17 in Listeria based immunotherapy has not been addressed. Therefore, we investigated the ability of attenuated Listeria-based immunotherapy to induce IL-17 producing cells in a model of cervical cancer and the potential impact that these cells have on anti-tumor vaccine efficacy. Here we show that vaccination of tumor bearing mice with Listeria vaccines resulted in elevated levels of intratumoral IL-17 and increased IL-17 production by γδ TCR+ cells, exclusively. IL-17 producing cells were lacking in tumors of γδ T-cell-deficient mice; however, the absence of γδ T cells, including IL-17+ γδ T cells, did not alter tumor progression or abrogate the efficacy of the Listeria-based vaccine indicating that αβ T cells are key for clearance of the tumor. Th1 responses, known to be responsible for anti-tumor Listeria-based vaccine efficacy, appear to be sufficient for tumor regression in γδ T-cell-deficient mice. We conclude that the efficacy of Listeria-based vaccine does not rely on γδ T cells (or IL-17 produced by them) in a TC.1 tumor model; however, Listeria-based immunotherapy can be used to induce IL-17+ γδ T cells that are important for regression observed in alternative cancer models.
Keywords: HPV, IL-17, TC.1 tumor, immunotherapy, listeria, γδ T cells
Introduction
Interleukin 17 (IL-17), a proinflammatory cytokine that is upregulated during immune responses to infection and injury, has been associated with a variety of cancers; however, the role of IL-17 in tumor formation remains unclear. Work reviewed by Murugaiyan et al. reveal both anti-tumor and pro-tumor activities of IL-17 depending on the state of the tumor microenvironment.1 With respect to its tumor promoting potential, IL-17 induces a pro-tumorigenic microenvironment and myeloid-derived suppressor cells2 as well as angiogenesis and, subsequently, tumor growth in vitro tumor models.3 In contrast to its tumor promoting activities, IL-17 has also been linked to tumor regression in immunocompetent mice through a presumed pathway involved in enhanced DC maturation and antigen presentation4 and the loss of the IL-17 signaling pathway negatively affected tumor responses to chemotherapy.5
IL-17 producing T cells have been detected in head and neck cancer, melanoma, prostate cancer and sarcoma models and in the peripheral blood and tumor tissue of patients with ovarian, pancreatic, and renal cell carcinoma.6 While a growing body of literature has addressed the potential roles of IL-17 producing helper and cytotoxic T cells in tumor development, alternative cellular sources of IL-17 and the conditions in which they may be induced, within the context of anti-tumor responses, are becoming more apparent.
IL-17 is not only produced by αβ T cells, but by γδ T cells as well. As noted in a review by Kebelitz and colleagues, γδ T cells, known to exhibit potent cytotoxic effector function, have been isolated from tumor infiltrating lymphocyte (TIL) populations within a variety of cancer types and have become a T-cell subset of interest in efforts to induce anti-tumor responses via the administration of anti-tumor immunotherapeutics.7 The role of γδ T-cell-derived IL-17, within the context of anti-tumor responses, was revealed in recent work that showed that IL-17 producing γδ T cells were required for chemotherapeutic efficacy.5 Ma et al. further show that optimal tumor colonization of IFN-γ-producing CD8+ T cells required tumor infiltrating, IL-17 producing γδ T cells.5 The dependence of alternative, CD8+ T-cell-mediated anti-tumor therapies on γδ T-cell activities remains unknown.
Listeria-based anti-tumor immunotherapeutics rely on antigen specific, CD8+ T cells8 as well as IFNγ directed pro-inflammatory responses9; however, little is known about the role or the sources of IL-17 in Listeria- vaccine induced tumor regression in these models. The appearance of γδ T cells precedes αβ T cells during infection with Listeria10 and appears to act during the early phases of immune responses to a variety of diseases. Recently, γδ T cells were found to be the primary cells producing IL-17A during L. monocytogenes infection.11
Herein we use a mouse tumor model for HPV associated cancer, TC.1, in combination with Listeria based immunotherapy to ask whether IL-17 producing cells play a role in this immunotherapeutic approach. TC.1 is a lung epithelial cell immortalized by HPV-16 E6 and E7 and transformed by pVEJB expressing activated human c-Ha-ras.12 It is an aggressive tumor, syngeneic with the C57Bl/6 mouse. Like human HPV associated tumors, TC-1 constitutively expresses E6 and E7. We use a Listeria-based immunotherapeutic that expresses HPV-16 E7 (Lm-LLO-E7), which was generated with a multi-copy episomal expression system and expresses and secretes a fusion protein that consists of a truncated listerial virulence factor, listeriolysin O, joined at the C-terminus to E7.8 Here we show that this Listeria based vaccine induced γδ T cells to produce IL-17 and IL-17+ γδ T cells were present in the TC.1 tumors of Listeria vaccinated mice. αβ TCR+ cells were not among the IL-17+ tumor cells in vaccinated mice. We further show that Listeria vaccine-dependent tumor regression in the TC.1 mouse tumor model of Lm-LLO-E7 vaccine efficacy was not dependent on the presence of γδ T cells. Thus, we conclude that Listeria based immunotherapy can be used to generate IL-17 producing γδ T-cells that have been shown to facilitate clearance in other cancers.
Results
Listeria based vaccines increase the presence of IL-17 in the TC.1 tumor
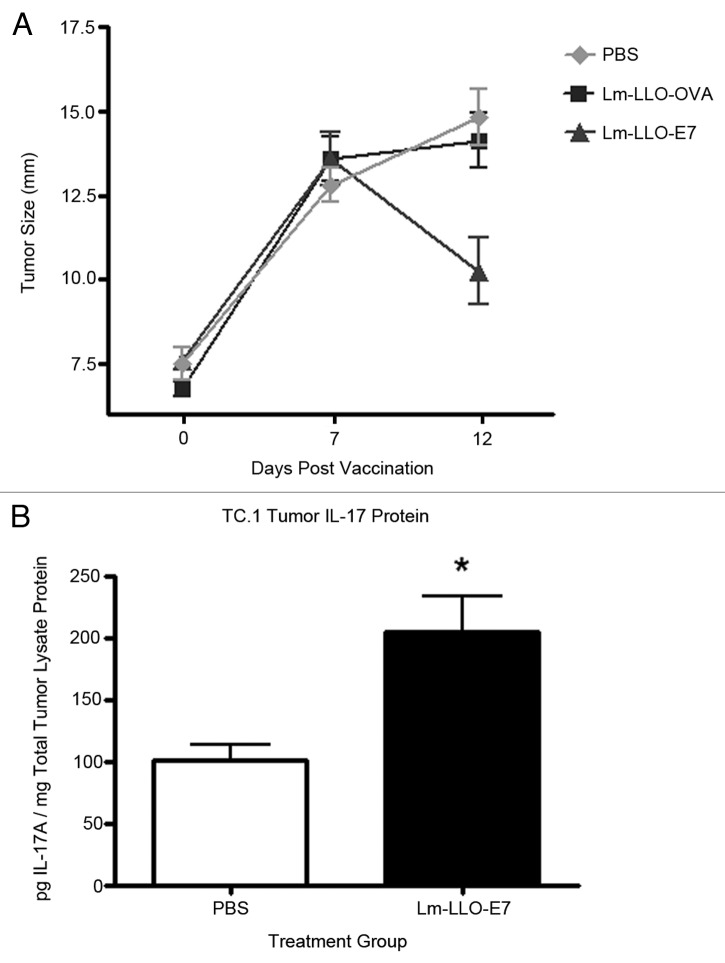
Considering that (1) Listeria infections can induce IL-17 and (2) IL-17 can, under certain conditions, favor tumor regression, we initially evaluated the ability of an attenuated, therapeutic, Listeria-based vaccine to induce IL-17 production in the TC.1 tumor model of Lm-LLO-E7 vaccine induced regression. In this experimental model, significant vaccine induced regression in Lm-LLO-E7 treated, tumor bearing mice is observed by day 12 post-vaccination compared with animals treated with either PBS or a control vaccine (Lm-LLO-OVA) that secretes LLO fused to OVA, an irrelevant antigen (Fig. 1A). Complete tumor regression in Lm-LLO-E7 treated mice occurs by day 21 post vaccination.8 Tissues are harvested within the first week following the first or second administration of the vaccines in order to evaluate responses in tumors poised to regress or actively regressing tumors, respectively. This strategy allows for the isolation and identification of tumor associated immune cell subsets as well as evaluation of cellular and molecular cytokine responses that occur within an existing tumor microenvironment, prior to complete vaccine induced tumor regression. In this study, analysis of tumors from vaccinated mice showed that treatment with Lm-LLO-E7 resulted in elevated levels of IL-17A protein in the tumor lysates of TC.1 bearing mice on day 7 post-vaccination compared with control mice treated with PBS (Fig. 1B).
Figure 1. 2.5 × 105 TC.1 cells were subcutaneously implanted in the flanks of five mice per group. One group of mice was treated with Lm-LLO-E7, one group of mice was treated with a control vaccine (Lm-LLO-OVA) and the third group was treated with PBS when tumors reached 7–8 mm. Mice were treated with a second dose of Lm-LLO-E7, Lm-LLO-OVA or PBS 7 d following the initial dose. Mice were removed from the study 5 d following the second treatment as tumors of Lm-LLO-E7 treated mice were undergoing regression. Mean+/−SEM for each group are shown (A). Tumor lysates were equilibrated based on total protein content. 50 µg of total protein were analyzed, in duplicate, for the presence of IL-17A via ELISA. Values represent pg of IL-17A per mg of total tumor protein. Mean+/−SEM for each group (n = 3) are shown (B).
Vaccination with Lm-LLO-E7 increases the proportion of IL-17 producing γδ T cells in TC.1 tumors
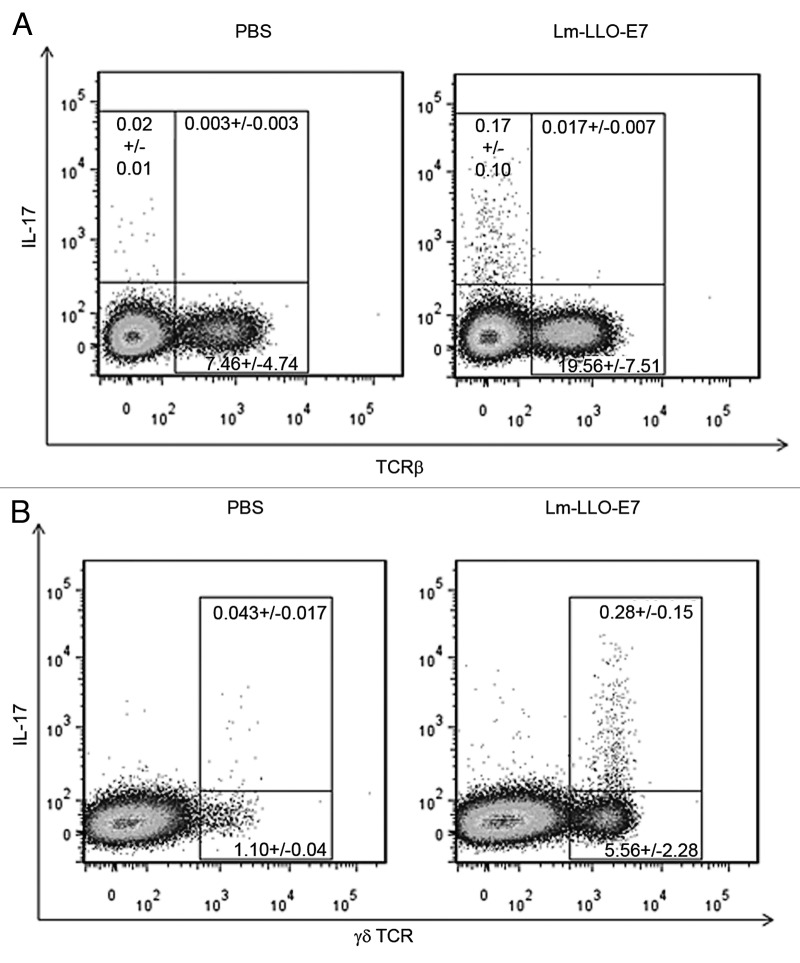
Following our analysis of vaccine induced increases in intratumoral IL-17 protein content, we sought to determine the source of IL-17 in the tumor. We analyzed the cellular responses in spleens and regressing tumors of vaccinated mice at day 5 following the second-vaccination in order to determine whether the elevated levels of IL-17 present in tumors following vaccination were the product of Th17 cells, γδ T cells or both. Analyses of TC.1 tumors, harvested from control and vaccine treated mice, showed that tumor associated, IL-17A producing cells were not αβ TCR+ (Fig. 2A) but expressed γδ TCR (Fig. 2B).
Figure 2. WT mice (3 mice/group) were treated with Lm-LLO-E7 or PBS and tissues harvested on day 12 post-vaccination for further analysis. Lm-LLO-E7 induced IL-17A production in tumor associated γδ T cells (B). Tumor associated αβ T cells did not express IL-17A in either PBS or Lm-LLO-E7 treated mice (A). Representative FACs plots are shown. Values within each gate represent the mean +/− SEM for each cell subset.
Lm-LLO-E7 vaccination does not induce Th17 in the periphery
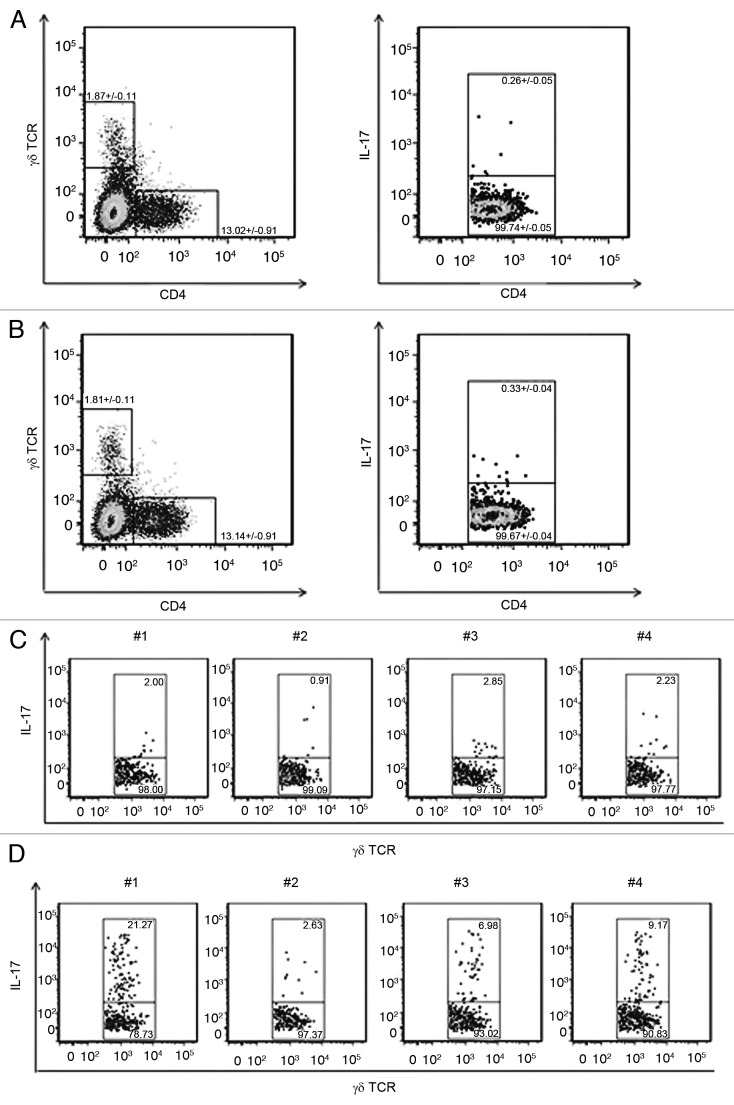
The presence of certain T-cell subsets in tumors following therapeutic treatment can be regulated by locally produced cytokines and chemokines and may not reflect systemic responses. In order to address the possibility that attenuated Listeria vaccines induce Th17 cells that may be excluded from the tumor environment, we performed additional experiments consisting of flowcytometric analyses of splenic cell populations from PBS treated control and Lm-LLO-E7 vaccinated, TC.1 tumor bearing mice. Vaccination did not alter the proportion of CD4+ cells or γδ+ T cells (Fig. 3B, left panel) relative to controls (Fig. 3A, left panel). Although there were no increases in the splenic populations of IL-17+, CD4+ cells following treatment (Fig. 3B, right panel), relative to PBS treated control mice (Fig. 3A, right panel), the percentages of IL-17 producing splenic γδ T cells were higher in Listeria-vaccine treated mice (Fig. 3D), relative to PBS treated mice (Fig. 3C). These data suggest that, similar to wild-type Listeria infections, vaccine strains of Listeria elicit IL-17 producing γδ T cells rather than Th17 cells.
Figure 3. Splenocytes were isolated from 4 tumor bearing, PBS (A, left panel) or Lm-LLO-E7 (B, left panel) treated mice and analyzed by flowcytometry for the presence of IL-17A+ cells. Vaccination induced increases in mean percentage of total γδ+ T cells and CD4+ cells were not statistically significant. Vaccination did not alter the percentages of CD4+, IL-17+ cells or CD4+, IL-17- cells (B, right panel) relative to PBS treated control mice (A, right panel). γδ+ T cells, derived from gates in panels A and B (left panels), were further analyzed for the presence of IL-17 (C, D). Vaccination increased the percentages of γδ+, IL-17+ splenocytes in treated mice (D) relative to PBS treated mice (C). Data from individual mice are shown (C, D).
The efficacy of Lm-LLO-E7 vaccine does not rely on γδ T cells
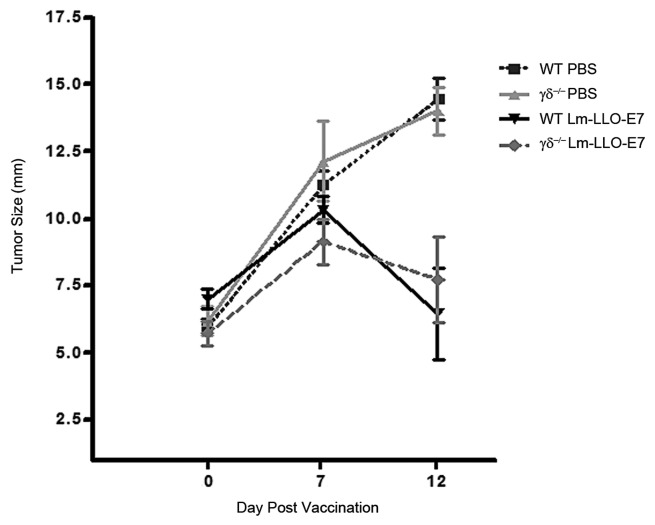
IL-17 producing γδ T cells have been shown to enhance CTL activity during Listeria infections11 and play a role in anti-tumor chemotherapeutic efficacy5; therefore, we examined their role in our immunotherapeutic model by comparing the ability of Lm-LLO-E7 to induce tumor regression in wild-type and in γδ TCR-deficient mice. The lack of γδ T cells in γδ−/− mice did not affect tumor growth in PBS treated control mice compared with WT mice and vaccination of γδ−/− mice with Lm-LLO-E7 induced tumor regression by day 12 post-vaccination (Fig. 4). Analysis of spleens from WT and γδ T-cell-deficient mice (Fig. S1A) confirmed the absence of IL-17+, γδ T cells in Lm-LLO-E7 vaccinated γδTCR deficient mice relative to WT vaccinated mice (Fig. S1B). These data suggest that in a model of Listeria vaccine mediated tumor regression; Lm-LLO-E7 induced, Th1 driven, anti-tumor CTL responses do not require support from, γδ T cells or the IL-17 that they produce in response to therapeutic treatment.
Figure 4. WT and γδ deficient mice (5 mice/group) were treated with Lm-LLO-E7 or PBS twice. Tumors developed at similar rates in all groups of mice and Lm-LLO-E7 induced similar levels of tumor regression in both WT and γδ TCR deficient mice. Mean+/−SEM for remaining mice in each group are shown (n = 4 for WT groups on day 12 post vaccination).
Discussion
The interplay among proinflammatory cytokines can orchestrate favorable host anti-tumor responses under specific circumstances. The timing and the cellular sources of a variety of cytokines have formed the basis of intense research into the development of appropriate therapeutic regimens. Recent focus on the role of IL-17 in tumor development as well as tumor regression has revealed both pro-tumorigenic and anti-tumorigenic properties of IL-17. Additional consideration of the results of these studies suggests that the context in which IL-17 is expressed determines its effect on tumor development. While IL-17 can induce MDSCs,2 is pro-angiogenic and, as a result, pro-tumorigenic,3 IL-17 also enhances CTL activity11 and as reviewed by Murugaiyan et al., inhibits tumor growth through the initiation of cytokine cascades.1 IL-17 in the absence of tumor lytic effector cells may assist in tumor formation; however, IL-17 production may play a role in tumor regression in the presence of vaccine induced, tumor-antigen specific CTLs.
Listeria based vaccines are capable of generating Th1 immune responses, characterized by the production of IFNγ and antigen-specific T cells.8Listeria infection is also known to induce IL-17.11 Using the TC.1 tumor line that expresses HPV-E7, we hypothesized that a live attenuated Listeria-vaccine would also induce IL-17 production and further proposed that a lack of IL-17 would impact vaccine efficacy given its ability to augment CTL activity. Vaccination of tumor bearing mice resulted in an increase in IL-17 in the tumors. In addition, γδ T cells were found to be the source of IL-17 in the tumors of vaccinated mice whereas Th17 cells were absent. These findings are consistent with earlier analyses of Th17 cell development and differentiation in the presence of strong Th1 responses. Harrington and associates demonstrated that IFNγ inhibited Th17 development as a potential mechanism to impede the development of pathogenic cell types that augment autoimmune disease.13 IL-17 producing γδ T cells promote CTL responses against Listeria infection,11 which is also known to require Th1 mediated responses for clearance. In addition, bacterial infection with Mycobacterium induced IL-17 producing γδ T cells rather than CD4+ Th17 cells.14 It was reasoned that an immune response, characterized by a strong IFN-γ response may explain a weak IL-17 response from CD4+ T cells. It was concluded that γδ cells may assume a more dominant role in producing IL-17 during an immune response otherwise dominated by IFN-γ.14 Vaccination with Lm-LLO-E7, an attenuated bacterium expressing both bacterial and viral antigens, has been shown to elicit a strong Th1 cytokine pattern, characterized by IFNγ8, rather than cytokines necessary for Th17 development; specifically IL-6 and TGFβ. Our findings in the current study agree with models in which IL-17 expression is largely restricted to γδ T cells during certain bacterial responses.
Although the Lm-LLO-E7 vaccine was capable of generating IL-17+ γδ T cells, the Th1 response that arises as a result of vaccination appears to be sufficient for Listeria vaccine efficacy, which does not depend on the presence of γδ T cells (Fig. 4). Nevertheless they may augment the activities of anti-tumor T cells in alternative tumor models or therapeutics where IFN-γ may be limiting or in models in which IL-17 has proven to be important for T-cell movement and effector function. For example, recent research has revealed an important role for IL-17 producing γδ T cells in anti-tumor chemotherapeutic efficacy in models of CD8 T-cell-mediated, chemotherapeutic driven tumor regression5 and has demonstrated a link between IL-17 and the production of Th1 chemokines15 that direct effector T-cell migration to the tumor environment. Future research efforts may address the role of IL-17A, induced by Listeria-based vaccines, in other tumor models in which this cytokine may be necessary to optimize vaccine efficacy.
Methods and Methods
Animals and treatment
TC.1 cells (ATCC Manassas, VA) were cultured in RPMI 1640 containing 10% heat inactivated fetal bovine serum (SH30071.03, Thermo Scientific), penicillin/streptomycin (Gibco, 15140), HEPES (Gibco, 15630), 2 mM L-glutamine (Gibco, 25030), 0.05 mM β-mercaptoethanol (Bio-Rad,161–0710), 1 mM sodium pyruvate (University of Pennsylvania Cell Center, Philadelphia, PA), and 2 mM nonessential amino acids (Gibco, 11140) at 37°C with 7% CO2.
2.5 × 105 TC.1 cells (ATCC, CRL-2785), were subcutaneously implanted in the flanks of wild-type female C57BL/6J mice (Jackson Laboratories, 000664) and γδ TCR deficient female C57BL/6J mice (Jackson Laboratories, B6.129P2-Tcrdtm1Mom). Mice were immunized i.p. with 1 × 108 Lm-LLO-OVA, 1 × 108 Lm-LLO-E78 or PBS, when tumors reached 5–8 mm and boosted 1 week later. Tumors were measured as described.8 7 d following the first immunization or 5 d following the second immunization, animals were sacrificed and spleens and tumors were harvested for further evaluation. All mouse experiments were performed according to the regulations of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
IL-17A ELISA
TC.1 tumors were harvested, frozen, cryopulverized and resuspended in tissue lysis buffer16 in the presence of protease inhibitors (Roche Diagnostics, 11 836 170 001). Tumor lysates were freeze-thawed twice, disrupted with a tissue homogenizer and centrifuged to collect lysate supernatant material. Lysate protein content was determined and total protein concentrations were normalized using Bio-Rad protein assay reagent according to the manufacturer’s protocol (Bio-Rad, 500–0006). Samples were analyzed for the presence of IL-17A by ELISA according to the manufacturer’s protocol (eBiosciences, 88–7371–22).
Cell isolation and flowcytometric analysis
Spleens and tumors were dissected from non-vaccinated and Lm-LLO-E7-vaccinated mice and spleens were mechanically dissociated into PBS and further subjected to ACK lysis (Lonza, 10–548E) to remove red blood cells. Tumors were further dissected into 2 mm cubes and subjected to enzyme digestion in digestion buffer (cRPMI containing 2 mg/mL Collagenase P (Roche Diagnostics, 11 249 002 001), 1 mg/mL DNase 1 (Roche Diagnostics, 10 104 159 001), and 50 μg/mL trypsin inhibitor (Sigma, T9253) for 30 min at 37°C. Tumor digests were filtered through a 100 μm mesh filter. Digestion buffer was removed and tumor red blood cells were lysed in ACK lysis buffer. Tumor cells and splenocytes were resuspended in cRPMI and incubated in the presence of 50 ng/mL of PMA (Sigma, P8139) and 750 ng/mL ionomycin (Sigma, I-0634), and Golgi-Stop (BD Biosciences, 554724) for 5 h at 37°C with 7% CO2. Cells were washed and resuspended in FACs buffer (PBS + 0.05% BSA) and incubated with Fc block (eBiosciences, CD16/CD32 Fcγ II/III, clone 93, 16–0161–82) followed by fluorochrome-conjugated primary antibodies (eBiosciences, TCRβ-APC-eFlour-780, clone H57–597, APC-γδ TCR, eBioGL3, 17–5711–82, CD4-Alexa-488, eBio GK1.5, 53–0041–82) . Aqua blue dye (Invitrogen, L34957) was added to each sample to exclude dead cells during flowcytometric acquisition. Samples were washed twice in FACs buffer and resuspended in fixation/permeabilization buffer (eBiosciences, 00–5123–43, 00–5223–56) overnight at 4°C. Cells were washed in permeabilization buffer (eBiosciences, 00–8333–56) and incubated with Fc block in permeabilization buffer, followed by incubation with anti-IL-17A-PE (eBiosciences, IL-17A-PE, eBio17B7, 12–7177–81). Cell acquisition was performed using a flowcytometer (LSRII BD Biosciences) and data processed using FLOWJO software (Tree Star Inc.).
Statistical analysis
Results comparing data obtained from vaccinated and non-vaccinated mice were subjected to ANOVA or one-tailed T tests with significant differences reported as follows: (*) p ≤ 0.05, (**) p ≤ 0.01, (***) p ≤ 0.001. (GraphPad Prism v.5.02, GraphPad Software Inc.).
Supplementary Material
Acknowledgments
This work was supported by CA69632 (YP), T32AI060516–03 (PG), K12GM081259 (PG), and T32CA009140 (PG). The authors are grateful to Dr. Zhen-Kun Pan for technical assistance and Dr. Radhika Goenka for reviewing the manuscript.
Glossary
Abbreviations:
- IL-17
Interleukin 17
- TIL
Tumor infiltrating leukocyte
- PMA
phorbol myristate acetate
- CTL
cytotoxic T lymphocyte
- FACs
Flowcytometry
- PBS
Dulbecco’s Phosphate Buffered Saline
Disclosure of Potential Conflicts of Interest
Yvonne Paterson has a financial interest in Advaxis, Inc., a vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria monocytogenes or listerial products as vaccines. Patrick Guirnalda declares no conflict of interest.
Note
Supplemental materials can be found at: www.landesbioscience.com/journals/oncoimmunology/article/20491
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20491
References
- 1.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–75. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 2.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–8. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 4.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.V99.6.2114. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 7.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 8.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 9.Dominiecki ME, Beatty GL, Pan ZK, Neeson P, Paterson Y. Tumor sensitivity to IFN-gamma is required for successful antigen-specific immunotherapy of a transplantable mouse tumor model for HPV-transformed tumors. Cancer Immunol Immunother. 2005;54:477–88. doi: 10.1007/s00262-004-0610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiromatsu K, Matsuzaki G, Tauchi Y, Yoshikai Y, Nomoto K. Sequential analysis of T cells in the liver during murine listerial infection. J Immunol. 1992;149:568–73. [PubMed] [Google Scholar]
- 11.Xu S, Han Y, Xu X, Bao Y, Zhang M, Cao X. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J Immunol. 2010;185:5879–87. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 12.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 13.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 14.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 15.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res. 2000;60:2136–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.