Abstract
It is widely accepted that generation of tumor specific CD8+ T-cell responses occur via cross-priming; however the source of tumor antigen for this event is unknown. We examined the source and form of tumor antigen required for cross-presentation in the local lymph node (LN) using a syngeneic mouse tumor model expressing a marker antigen. We found that cross-presentation of this model tumor antigen in the LN is dependent on continuous traffic of antigen from the tumor site, but without any detectable migration of tumor resident dendritic cells (DCs). Instead, small numbers of tumor cells metastasize to local LNs where they are exposed to a localized CTL attack, resulting in delivery of tumor antigen into the cross-presentation pathway.
Keywords: cross-presentation, cytotoxic T cell, dendritic cells, lymph nodes, tumor
Introduction
One of the critical questions in tumor immunology is how the host senses the presence of the many potential antigens that are present in each tumor. It was initially thought that the host remained ignorant of the presence of a tumor unless the tumor cells themselves metastasize to the lymph nodes, and thus engage directly with host T-cells.1 In line with this, tumor-specific T cells have been detected in secondary lymphoid organs where tumor cells are present. However, this view has changed over the past few years and it now appears that tumor antigens are efficiently ‘cross presented’ to host T-cells in a process that is almost entirely restricted to the lymph node that drains that tumor.2-5 Migratory dendritic cells (DCs) are the principle cell type responsible for delivering antigens from peripheral tissues to lymph nodes, carrying with them not just antigen, but information regarding the ‘context’ of the antigen, such as the presence of ‘danger’ signals.6-8 DC infiltration of solid tumors is well documented in tumor-bearing animals and patients.9-13 Thus, generation of tumor-specific responses might be expected to involve migration of DCs from the tumor tissue to the tumor draining lymph nodes (TDLN). However, whether this process occurs is uncertain, and indeed several studies have suggested that DC-function and LN migration may be impaired in cancer due to the immunosuppressive nature of the tumor microenvironment.14-19 In such situations the ability of DCs in the TDLN to cross-present tumor antigen may be dependent on the migration of tumor cells themselves.
Sentinel lymph node metastases are well documented in human cancers20-24 and are also found in several murine models of solid tumor.1,5,25-28 It is therefore possible that DCs in the TDLN acquire antigen from metastatic tumor cells for cross-presentation to naïve T cells. In line with this, tumor specific T cells can be detected in secondary lymphoid organs where tumor cells are present.1,26,27,29,30 It has been postulated that generation of effective tumor specific CTL requires small numbers of tumor cells reaching the secondary lymphoid organs early during tumor development,1 thus, strictly extra-lymphatic tumors would be ignored by the host immune system, resulting in tumor outgrowth.1 However, subsequent studies have shown that (1) tumor specific T-cell responses exist in the absence of detectable lymph node metastasis,31,32 and (2) tumors progress despite the presence of tumor cells in secondary lymphoid compartment.5,26,33,34 The majority of these studies examined the ability of tumor cells to directly prime tumor specific CD8+ T cells in secondary lymphoid organs, and not their capacity to act as a source of antigen for cross-presentation.
Normal tumor progression is associated with rapid proliferation of viable tumor cells and differing levels of tumor cell death in the form of apoptosis and necrosis. In addition, tumor cells are known to secrete soluble proteins and antigen carrying exosomes. However the form of tumor antigen that is captured by DCs for cross-presentation has not been fully elucidated. Most of the data describing a source tumor antigen for cross-presentation has been generated by in vitro systems or vaccination experiments, whereby DCs are loaded with different tumor cell preparations and their ability to cross-prime specific T-cell responses are measured in vitro or after in vivo transfer. Such experiments utilize in vitro differentiated DCs in a non-lymph node environment, and while they are important for the development of effective DC immunotherapy protocols, they cannot predict the natural source of tumor antigen for cross-presentation in vivo.
We have previously shown that cell-associated tumor antigen is cross-presented in the local TDLNs by both CD8α+ and CD8α- DCs.35 The ability of CD8α- DC to cross-present tumor antigen led us to speculate that migration of DCs from the tumor site was required for cross-presentation of tumor antigen in the TDLN. We now show that cross-presentation of tumor antigen in the TDLN is dependent on the continuous traffic of antigen from the tumor site, but this occurs in the absence of detectable DC migration. We propose a model where small numbers of tumor cells metastasize to the nodes where they are exposed to localized CTL attack and in this way provide a source of antigen for cross-presentation, providing an additional mechanism for host recognition of the presence of tumor antigens. These observations have implications for immunosurveillance, immunoselection and cancer surgery.
Results
Cross presentation of tumor antigens by lymph node DCs
Our previous studies had shown that antigen presentation in TDLNs is mediated by a bone marrow derived cell36 and that both CD8α+ and CD8α- TDLN DCs were able to cross-present cell-associated tumor antigen to specific CD8+ T cells.35 However, the finding that a putative non-resident CD8α- DC subset can cross present cell associated tumor antigen does not in itself confirm traffic of migratory DCs from the tumor site to the local node, let alone prove them as a source of tumor antigen. Indeed, given that tumor cells can metastasize to lymph nodes, cross-presentation of tumor antigen in the draining node could be explained more directly by the presence of a viable metastatic tumor deposit. To investigate this scenario in more detail we first confirmed that only CD11c+ cells, (DCs), enriched from the TDLN were able to induce proliferation of tumor-specific CD8+ T cells and, importantly, that the CD11c- population, which contained all other cells such as macrophages and tumor cells, did not induce proliferation (Fig. 1A). Then, to determine whether antigen presentation in the TDLN required continuous traffic of antigen from the tumor site, or instead, is sustained by local antigen production, such as metastatic tumor growth, we examined the kinetics of tumor antigen presentation in vivo after surgically removing the tumor mass (and hence the primary source of tumor-derived antigens and tumor-infiltrating DCs; Fig. 1B). Complete tumor resection resulted in the cessation of tumor antigen specific T-cell proliferation in vivo whereas sham surgery had no effect (Pre-op = 40.17% ± 15.95% proliferation compared with day of surgery = 43.22% ± 13.70%, p = 0.266). Reduced but significant proliferation could still be detected at day 7 post-surgery (14.72% ± 6.22%, p = 0.014). The observed kinetics of tumor antigen-presentation confirms that 1) the proliferating tumor-specific T-cells observed in the TDLN are not being activated in the tumor and arriving in the DLN as already-proliferating cells, and 2) strongly argues against the presence of a metastatic tumor deposit and demonstrates that cross-presentation of tumor antigen in the TDLN requires continuous traffic of antigen from the tumor site.
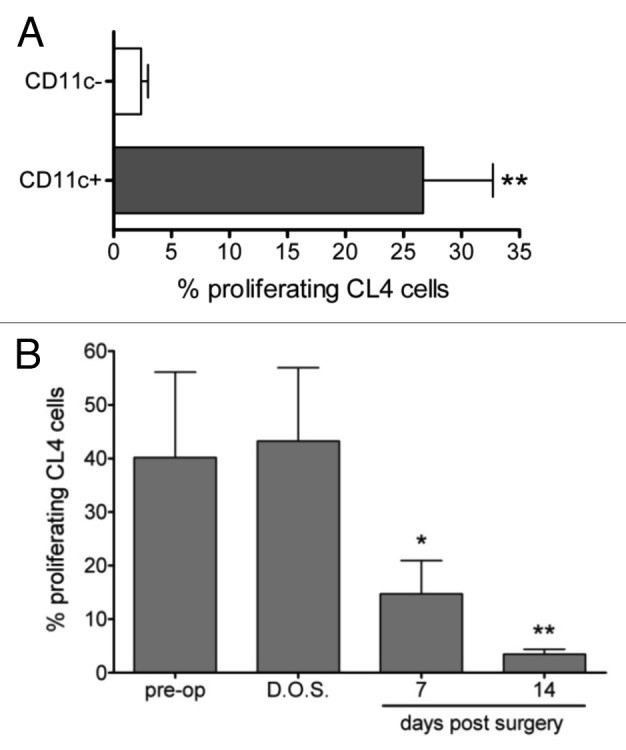
Figure 1. Cross-presentation of tumor antigen in the TDLN is dependent on DCs and persists following tumor removal. A, TDLNs were removed from mice bearing AB1HA mice on day 21 post inoculation and separated into DC-enriched and DC-depleted fractions using CD11c microbeads. DCs were co-cultured with CFSE-labeled CD8+ T cells and analyzed by flow cytometry after 60h. Plot depicts the percentage of gated proliferating CD8+CFSE+ CL4 T cells. Mean ± SEM, Students T test. B, AB1-HA tumors were surgically removed on day 16 post inoculation, and CFSE-labeled CL4-TCR transgenic lymphocytes adoptively transferred at the indicated time points. Percentage of gated proliferating CD8+CFSE+ CL4 T cells plotted against the day of adoptive transfer for mice. Circles represent individual mice. Students T test comparing day of surgery (D.O.S) with indicated time points post-surgery.
Which cell delivers antigen to the TDLN?
As HA is a transmembrane, rather than secreted, protein we reasoned that it must traffic to the TDLN from the tumor site in a cell associated form. This, taken together with (1) cross-presentation of tumor antigen by TDLN DCs and (2) the dependence of in vivo antigen presentation on the presence of a solid growing tumor, suggests a role for migratory DCs in this process. We therefore examined the capacity of tumor infiltrating DCs (TiDC) to traffic to the TDLN by following the fate of injected fluorescent beads. AB1HA tumor bearing mice received an i.t. injection of Fluoresbrite YG beads (1µM; Polysciences). As a control for normal migration of skin DCs, naïve Balb/c received a s.c. injection of the same amount of beads into the equivalent flank. Tumor tissue and draining lymph nodes were collected, stained for CD11c expression and analyzed by flow cytometry. In this setting, the presence of fluorescence in the cellular gate indicates bead capture (Fig. 2A). The beads were captured by TiDCs within 24 h following injection, with greater than 900 bead+CD11c+ cells detected per 105 tumor cell suspension, and captured beads were still detectable 3 d after injection (Fig. 2B). However, we found no evidence for migration of tumor DCs to the TDLN, as there were no bead-positive cells detected in the axillary or inguinal TDLNs at any time point (Fig. 2C). In contrast, skin DCs in naïve Balb/c showed normal migration of DC, with peak numbers of bead+CD11c+ cells present in the DLNs at day 3 post injection (Fig. 2C).
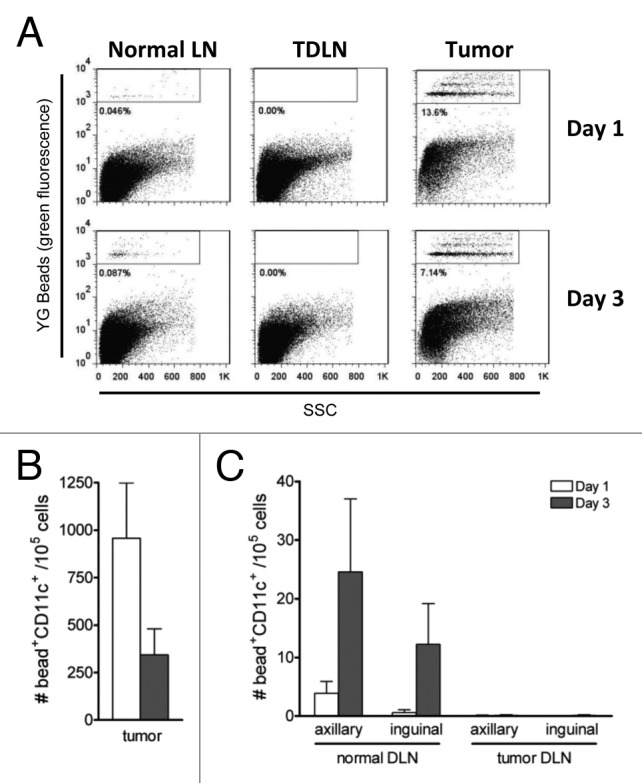
Figure 2. DCs do not migrate from the tumor site. Naïve and AB1-HA bearing mice were injected with 2x107 fluoresbrite carboxy YG microbeads s.c. or i.t. respectively. One and three days later, tissues were harvested, stained with anti-CD11c Ab and analyzed by flow cytometry. A, Representative profiles of tumor, TDLN and normal LN DCs at days 1 and 3 post injection. Bead+ DCs gated as indicated. B, Number of tumor infiltrating bead+ DCs at days 1 and 3 post injection, Four mice per group. C, Number of bead+ DCs in the TDLN (ie. arriving from the s.c. tumor) vs. normal LN (i.e., arriving from tumor-free skin) at days 1 and 3 post injection. Four mice per group.
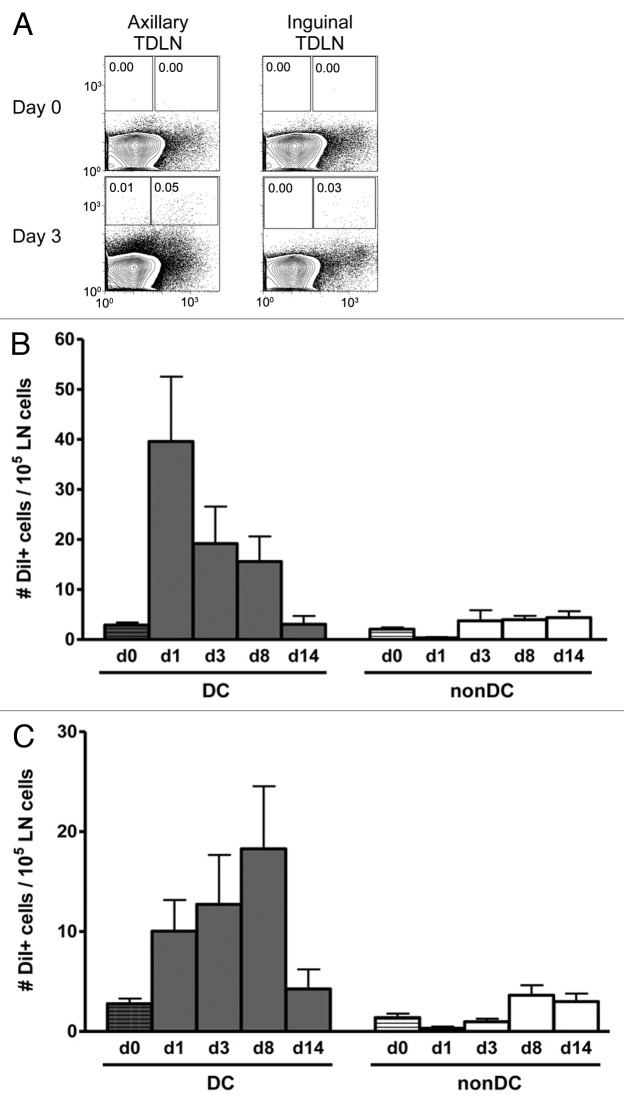
The observed absence of migratory DCs in TDLNs combined with the seemingly paradoxical requirement for continuous transfer of antigen from the tumor environment, led us to revisit the role of metastatic tumor cells as a source of antigen for cross-presentation in the TDLN. AB1-HA tumor cells were labeled with the lipophilic tracer, DiI (Molecular Probes, Invitrogen), and injected s.c. into naïve mice. TDLNs were harvested on days 1, 3, 8 or 14 following injection, stained for CD11c expression and analyzed by flow cytometry for the presence of metastatic DiI-positive tumor cells (Fig. 3). The majority of the DiI signal was associated with CD11c+ cells in the TDLN (Fig. 3A), presumably DCs, as AB1HA tumor cells do not express CD11c (data not shown). The number of DiI+ DCs in the axillary TDLN peaked at day 1 post injection (39.6 DiI+ CD11c+ cells per 105 total LN cells) and steadily declined to background (naïve Balb/c, day 0) by day 14 (Fig. 3B). In contrast, the number of DiI+ DCs in the inguinal TDLN rose steadily over days 1 and 3 to peak at day 8 post injection (18.3 DiI+ CD11c+ cells per 105 total LN cells) (Fig. 3C). Detection of DiI+ nonDCs (DiI+ CD11c-) was less than 4 per 105 total lymph node cells at all time points in both TDLNs. These results indicate that despite the apparent lack of DC migration from the tumor site, tumor antigen is associated with DCs in the TDLN, in the absence of any detectable tumor cell metastasis. This raises the possibility of continuous migration of AB1HA to the TDLN, but with apparent control of metastatic outgrowth.
Figure 3. Tumor antigen is associated with DC in the TDLN. Balb/c mice were injected s.c. with DiI-labeled AB1HA tumor cells. One 3, 8 and 14 d later, axillary and inguinal TDLN were harvested. Following enzymatic digestion, lymph nodes were stained with CD11c specific mAbs and the number of DiI-positive cells determined by flow cytometry. A, Representative profiles of CD11c vs. DiI label in the axillary and inguinal TDLN. Gates show the percentage if DiI+ nonDCs (left) and DiI+ DCs (right). B and C, Number of DiI+ DCs or nonDC at days 0, 1, 3, 8 and 14 post injection in the axillary (B) and inguinal (C) TDLNs. Six mice per group. Mean ± SEM. Data shown is pooled from two individual experiments.
Metastasizing tumor cells as the source of cross presented tumor antigen
Although the results thus far suggest that low numbers of metastasizing tumor cells might be the source of antigen in the local TDLN, PCR analysis of TDLNs to search for occult tumor cells was negative for tumor antigen (HA) expression (Fig. 4A). Therefore, in order to establish the threshold of detection of tumor cells in lymphocyte populations we performed PCR analysis of tumor cells which had been titrated into normal lymph node populations. This showed that the lowest level of detection of tumor cells was 10 tumor cells per 107 lymph node cells (Fig. 4B). This suggested that the number of metastatic tumor cells in the TDLN in our model was below 10:107 and therefore below the reasonable limits of histological and PCR detection. One reason that these low numbers of metastatic tumor cells in TDLNs might not progress is that they are controlled by a local CTL response. In line with this, examination of endogenous CD8+ T cells by the in vivo CTL assay showed that HA-specific CTLs were detected solely in the TDLNs of tumor bearing mice (Fig. 4B). In vivo CTL activity was measured by injecting CFSE-labeled HA peptide-coated and uncoated targets into tumor-bearing mice and measuring their recovery 18h later. The percentage of T-cell killing in the TDLN was significantly greater than that observed in the nonDLN (9.48% ± 4.42 v 4.64% ± 1.14, p = 0.006) or spleen (9.48% ± 4.42 v 4.82% ± 1.87, p = 0.009). To test if this might be restricting the growth of the few metastatic tumor cells. TDLNs were left intact or depleted of CD3+ cells and cultured ex vivo in selective media to determine if an outgrowth of viable AB1HA tumor cells occurred – indeed, re-isolation of viable tumor cells from the TDLN was seen, and was dependent on the removal of CD3+ cells (Fig. 4C). These data suggest that in the absence of DC migration, small numbers of tumor cells may be the source of tumor antigen for cross-presentation in the TDLN. This implies that T cells residing in the TDLN may play a role in controlling metastatic spread.
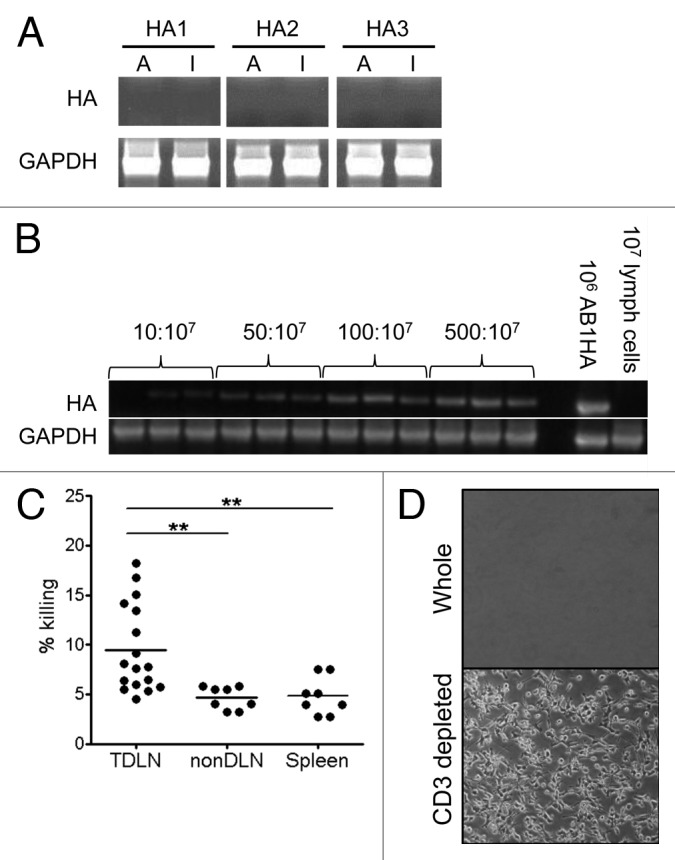
Figure 4. Destruction of occult tumor cells in TDLNs is dependent on T cells. A, PCR analysis of HA (upper) and GAPDH (lower) expression in TDLN of mice bearing day 21 AB1-HA tumors. Representative of 2 individual experiments. B, Limit of detection of AB1HA tumor cells in LN preparations by PCR. C, Amount of HA-specific CD8+ T cell killing in vivo in the TDLN, nonDLN and spleen of AB1-HA bearing mice at day 16 post inoculation. Dots represent individual mice. D, Representative pictures showing AB1-HA tumor cells grown out from cultures of whole TDLN (left) and T cell depleted TDLN (right). Representative of 3 individual experiments.
Discussion
It is widely accepted that generation of tumor specific CD8+ T-cell responses occurs via cross-priming; however the source of tumor antigen for this event is unknown. It has been postulated that the immune system is ignorant of extralymphatic tumors and that generation of an anti-tumor T-cell response is dependent on tumor cell metastasis to secondary lymphoid organs.1,37 Despite this, several studies have shown generation of tumor-specific T cells in the absence of any obvious lymph node metastasis.31,32 In the present study it is shown that cross-presentation of HA tumor antigen in the TDLN is dependent on the continuous traffic of antigen from the tumor site, in the absence of DC migration. Based on the data we propose that cross-presentation of HA tumor antigen is mediated by DCs in the TDLN following acquisition of antigen from small numbers of metastatic tumor cells.
This result is in contrast with dogma suggesting that DCs migrate from peripheral tissues and deliver antigen to resident DCs for cross-presentation. This view has been predominantly generated from models of viral infection whereby tissue resident DCs capture the invading pathogen and following interaction with pathogen associated signals, such as PAMPs, are programmed for migration and terminal differentiation.38-40 While our observations show that CD11c+ DCs in TDLNs are responsible for the cross presentation of tumor antigens, and antigen presentation kinetics following surgical resection of the tumor were consistent with the in vivo life span of DCs in lymph nodes, this model may not properly represent the generation of tumor-specific T-cell responses in the steady-state for several reasons. First, tumor growth is associated with a lack of pro-inflammatory signals and pathogen by-products that may interact with tumor-resident DCs resulting in their activation and subsequent migration to the TDLN.41 Second, tumors produce a variety of immunosuppressive factors that inhibit DC maturation and differentiation, leading to the recruitment and accumulation of immature DCs at the tumor site.41,42 Third, several studies have shown that DC migration is impaired in the presence of a solid growing tumor.14,16,17,19 In line with this fluorescent bead tracking experiments showed that DCs infiltrating AB1-HA tumors were not detected in the TDLNs. Finally, sentinel lymph node metastasis is a hallmark of human disease and represents a mechanism whereby tumor antigen is delivered directly to the TDLN. While migration of tumor-resident DCs cannot be definitively ruled out it is important to note that since cross-presentation is favored by high doses of antigen it seems improbable that small numbers of DCs (below the level of detection by fluorescent bead transfer) carrying tumor antigen to the TDLN would be sufficient to induce a detectable tumor-specific CD8+ T-cell response. Thus in the absence of DC migration, small numbers of tumor cells may be the source of antigen for cross-presentation in the TDLN. Consistent with this finding, tumor cells have been detected in TDLNs where DCs mediate cross-presentation of tumor antigen.5,43
Our observation that small numbers of tumor cells in TDLNs can escape detection by microscopy or sensitive PCR methods suggests that such methods may miss the presence of antigen-bearing tumor cells as a source of antigen delivery to nodes. This is consistent with a number of studies showing that isolated tumor cells can migrate to other sites in the body without forming solid metastatic deposits.44-47
One way that this could happen would be for those micrometastases to be controlled by local CTL mechanisms. Indeed we show that effector CTL are generated in TDLN and remain localized in that location. This observation, along with our finding that occult tumor cells only become manifest when T cells are depleted from the TDLN population, suggests that these locally generated CTL control the growth of these tumor cells while delivering sufficient antigen to the cross presentation pathway.
These data have several important implications. First, lymph nodes that are considered by pathologists to be tumor negative (‘N0 disease’),48 may actually have barely detectable numbers of tumor cells which are actually delivering antigen and cross priming tumor-specific T cells. Second, the localized TDLN CTL response may be controlling the spread of tumor cells to distal sites, that is, the presence of low-level metastasis to TDLNs may not per se be a negative indicator but would be the source of tumor-restricting CTL. Interestingly, these cross primed CTL in the lymph node would have been missed if systemic CTL activity, measured in peripheral blood or spleen, had been relied upon as the readout for T-cell priming. Third, given the above, the TDLN is likely to be a ‘checkpoint’ for immunoselection of tumor cells that are resistant to CTL lysis, by, for example, loss of class I expression or production of T-cell suppressive molecules. Indeed studies of metastatic deposits compared with primary sites support this notion.49 Of course such selection would only apply to those tumors that spread via the DLNs rather than directly via the bloodstream, as has been described.50 Finally, TDLN removal is occurring daily in thousands of cancer patients and our data showing that the TDLN is active in cross priming and limiting tumor spread support the notion that such removal cannot be a null event and requires further investigation.
In conclusion, these studies suggest that tumor antigens are delivered to TDLNs not by migratory DCs, which appear to be arrested by the tumor, but by extremely low levels of tumor cells themselves which are controlled by locally induced CTL. These results have implications for our understanding of the selection of immuno-resistant tumor variants and for cancer lymph node surgery.
Materials and Methods
Animals
BALB/c (H-2d) mice were obtained from the Animal Resources Centre (Canning Vale, Western Australia) and maintained under specific pathogen free conditions. Balb/c Clone 4 (CL4) TCR-transgenic mice, which express a TCR specific for the H-2d-restricted peptide IYSTVASSL (residues 518–526) of A/PR/8/34 (H1N1) influenza virus hemagglutinin (HA),were generated and screened as previously described.51 Animal experiments were conducted according to The University of Western Australia Animal Ethics Committee guidelines.
Tumor cells and inoculation
AB1-HA (H-2d) is a mouse mesothelioma cell line expressing the hemagglutinin (HA) molecule of influenza virus A/PR/8/34 (H1N1) and were maintained in culture as previously described.3 AB1HA tumors were grown subcutaneously (s.c.) following injection of 5x105 viable cells into the left flank. In some experiments tumors were resected on day 16 following inoculation.
Ex vivo antigen presentation assay
DCs were enriched from TDLNs of AB1HA bearing mice and isolated as previously described.35 Briefly DCs were purified using CD11c microbeads according to the manufacturer’s instructions (Miltenyi Biotec). Enriched lymph node populations were routinely > 85% CD11c positive. HA-specific CD8+ T cells were purified from CL4 TCR transgenic mice using the Miltenyi Biotec CD8+ T-cell isolation kit according to the manufacturer’s instructions (Miltenyi Biotec). Enriched cells were routinely > 90% CD8+ T cells. CFSE-labeling of T cells was performed as described previously.52 Serial dilutions of purified DCs were incubated with CFSE-labeled CD8+ CL4 T cells for 60h in vitro. Proliferation was analyzed by CFSE dilution using flow cytometry (FACS Calibur, BD Biosciences).
In vivo antigen presentation “Lyons-Parish” Assay
A total of 1–2x107 CFSE-labeled HA-specific CL4 TCR-transgenic splenocytes were injected intravenously (i.v.) into recipient mice. TDLNs were harvested 3 d following adoptive transfer and counter-stained with anti-CD8-PECy5.5 (53–6.7, BD Biosciences) prior to flow cytometry (FACS Calibur, BD Biosciences).
In vivo CTL assay
Detection of HA-specific in vivo CTL was performed as previously described.53 Briefly, Balb/c splenocytes were divided into two populations, one of which was pulsed with 1 µg/ml HA peptide (IYSTVASSL). Cells were labeled with CFSE using a final concentration of 5 µM CFSE for peptide-pulsed cells (CFSEhigh) and 0.5 µM for unpulsed cells (CFSElow). CFSEhigh and CFSElow cells were pooled in equal proportions and injected i.v. into recipients. Lymph nodes and spleens were harvested 18h later and analyzed by FACS for recovery of CFSEhigh and CFSElow cells. The percentage killed was determined using the following formula: 100 x (1 – (CFSEhigh events/CFSElow events)).
DC tracking experiment
AB1HA tumor-bearing mice were injected i.t. with 2x107 Fluoresbrite YG microbeads (Polysciences) in 20μL saline. Lymph nodes and tumors were harvested at the indicated time points, counter-stained with anti-CD11c-APC (N418, BioLegend) then analyzed by flow cytometry (FACS Calibur, BD Biosciences).
Tracking tumor cell migration
For in vivo tracking of tumor cells, AB1HA cells were labeled with 1µM of the lipophilic tracer, DiI (D282, Molecular Probes) according to the manufacturer’s instructions, and 5x105 DiI+ cells were injected s.c. Lymph nodes and tumors were harvested at the indicated time points and single cell suspensions were stained with anti-CD11c-APC specific antibody (N418, 117310; BioLegend) then analyzed by flow cytometry.
Tumor outgrowth experiment
TDLNs were harvested and pooled from 5–10 mice. T cells were depleted by incubation with anti-CD3 IgG (17A2, BD Biosciences) followed by sheep anti-rat Dynabeads (Invitrogen) and magnetic removal of positive cells. Intact or T-cell depleted TDLN cells were cultured at 1x106 cells/well in culture media with antibiotic selection (400µg/ml geneticin) and outgrowth of tumor cells evaluated by visual inspection. .
PCR Assay
Balb/c splenocytes were seeded with different numbers of AB1HA tumor cells ranging from 1 in 2x104 to 1 in 1x106 splenocytes. RNA was isolated from these samples using Trizol (Invitrogen) as per manufacturer’s instructions. HA expression, was assayed by real-time PCR using SuperScript III (Invitrogen), SYBR green (QIAGEN) and HA primers (5′-CAATTGGGGAAATGTAACATCGCC-3′ and 5′-AGCTTTGGGTATGAGCCCTCCTTC-3′). Expression of the housekeeping gene GAPDH was also measured (5′-GAAGGTCGGTGTGAACGGATT-3′ and 5′-CGGAAGGGGCGGAGATGATGA-3′).
Statistical Analysis
Statistical significance was calculated using GraphPad PRISM (San Diego, CA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20493
References
- 1.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–64. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 2.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–14. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 3.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, et al. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–45. [PubMed] [Google Scholar]
- 4.Robinson BW, Lake RA, Nelson DJ, Scott BA, Marzo AL. Cross-presentation of tumour antigens: evaluation of threshold, duration, distribution and regulation. Immunol Cell Biol. 1999;77:552–8. doi: 10.1046/j.1440-1711.1999.00876.x. [DOI] [PubMed] [Google Scholar]
- 5.van Mierlo GJD, Boonman ZFHM, Dumortier HMH, den Boer AT, Fransen MF, Nouta J, et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–9. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 6.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 7.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–34. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martín-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol. 2009:31–49. doi: 10.1007/978-3-540-71029-5_2. [DOI] [PubMed] [Google Scholar]
- 9.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pachéco Y, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–9. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 10.Preynat-Seauve O, Schuler P, Contassot E, Beermann F, Huard B, French LE. Tumor-infiltrating dendritic cells are potent antigen-presenting cells able to activate T cells and mediate tumor rejection. J Immunol. 2006;176:61–7. doi: 10.4049/jimmunol.176.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, et al. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol Immunother. 2008;57:1665–73. doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4:585–93. [PubMed] [Google Scholar]
- 13.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 14.Gerner MY, Casey KA, Mescher MF. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J Immunol. 2008;181:155–64. doi: 10.4049/jimmunol.181.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerner MY, Mescher MF. Antigen processing and MHC-II presentation by dermal and tumor-infiltrating dendritic cells. J Immunol. 2009;182:2726–37. doi: 10.4049/jimmunol.0803479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida T, Oyama T, Carbone DP, Gabrilovich DI. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J Immunol. 1998;161:4842–51. [PubMed] [Google Scholar]
- 17.Lucas AD, Halliday GM. Progressor but not regressor skin tumours inhibit Langerhans’ cell migration from epidermis to local lymph nodes. Immunology. 1999;97:130–7. doi: 10.1046/j.1365-2567.1999.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preynat-Seauve O, Contassot E, Schuler P, French LE, Huard B. Melanoma-infiltrating dendritic cells induce protective antitumor responses mediated by T cells. Melanoma Res. 2007;17:169–76. doi: 10.1097/CMR.0b013e3281844531. [DOI] [PubMed] [Google Scholar]
- 19.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 20.Carlson GW, Murray DR, Lyles RH, Staley CA, Hestley A, Cohen C. The amount of metastatic melanoma in a sentinel lymph node: does it have prognostic significance? Ann Surg Oncol. 2003;10:575–81. doi: 10.1245/ASO.2003.03.054. [DOI] [PubMed] [Google Scholar]
- 21.Gobardhan PD, Elias SG, Madsen EV, Bongers V, Ruitenberg HJ, Perre CI, et al. Prognostic value of micrometastases in sentinel lymph nodes of patients with breast carcinoma: a cohort study. Ann Oncol. 2009;20:41–8. doi: 10.1093/annonc/mdn535. [DOI] [PubMed] [Google Scholar]
- 22.Imoto S, Ochiai A, Okumura C, Wada N, Hasebe T. Impact of isolated tumor cells in sentinel lymph nodes detected by immunohistochemical staining. Eur J Surg Oncol. 2006;32:1175–9. doi: 10.1016/j.ejso.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Rena O, Carsana L, Cristina S, Papalia E, Massera F, Errico L, et al. Lymph node isolated tumor cells and micrometastases in pathological stage I non-small cell lung cancer: prognostic significance. Eur J Cardiothorac Surg. 2007;32:863–7. doi: 10.1016/j.ejcts.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Scheunemann P, Stoecklein NH, Hermann K, Rehders A, Eisenberger CF, Knoefel WT, et al. Occult disseminated tumor cells in lymph nodes of patients with gastric carcinoma. A critical appraisal of assessment and relevance. Langenbecks Arch Surg. 2009;394:105–13. doi: 10.1007/s00423-008-0369-4. [DOI] [PubMed] [Google Scholar]
- 25.Graham DB, Stephenson LM, Lam SK, Brim K, Lee HM, Bautista J, et al. An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells. J Exp Med. 2007;204:2889–97. doi: 10.1084/jem.20071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargadon KM, Brinkman CC, Sheasley-O’neill SL, Nichols LA, Bullock TNJ, Engelhard VH. Incomplete differentiation of antigen-specific CD8 T cells in tumor-draining lymph nodes. J Immunol. 2006;177:6081–90. doi: 10.4049/jimmunol.177.9.6081. [DOI] [PubMed] [Google Scholar]
- 27.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci U S A. 1999;96:2233–8. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolkers MC, Stoetter G, Vyth-Dreese FA, Schumacher TN. Redundancy of direct priming and cross-priming in tumor-specific CD8+ T cell responses. J Immunol. 2001;167:3577–84. doi: 10.4049/jimmunol.167.7.3577. [DOI] [PubMed] [Google Scholar]
- 29.Straten P, Dahl C, Schrama D, Pedersen LO, Andersen MH, Seremet T, et al. Identification of identical TCRs in primary melanoma lesions and tumor free corresponding sentinel lymph nodes. Cancer Immunol Immunother. 2006;55:495–502. doi: 10.1007/s00262-005-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu P, Spiotto MT, Lee Y, Schreiber H, Fu YX. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J Exp Med. 2003;197:985–95. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuenca A, Cheng F, Wang H, Brayer J, Horna P, Gu L, et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63:9007–15. [PubMed] [Google Scholar]
- 32.Preynat-Seauve O, Contassot E, Schuler P, Piguet V, French LE, Huard B. Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res. 2007;67:5009–16. doi: 10.1158/0008-5472.CAN-06-4494. [DOI] [PubMed] [Google Scholar]
- 33.Bai X-F, Gao J-X, Liu J, Wen J, Zheng P, Liu Y. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res. 2001;61:6860–7. [PubMed] [Google Scholar]
- 34.Boonman ZFHM, van Mierlo GJD, Fransen MF, Franken KLMC, Offringa R, Melief CJM, et al. Intraocular tumor antigen drains specifically to submandibular lymph nodes, resulting in an abortive cytotoxic T cell reaction. J Immunol. 2004;172:1567–74. doi: 10.4049/jimmunol.172.3.1567. [DOI] [PubMed] [Google Scholar]
- 35.McDonnell AM, Prosser AC, van Bruggen I, Robinson BW, Currie AJ. CD8alpha+ DC are not the sole subset cross-presenting cell-associated tumor antigens from a solid tumor. Eur J Immunol. 2010;40:1617–27. doi: 10.1002/eji.200940153. [DOI] [PubMed] [Google Scholar]
- 36.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 37.Zinkernagel RM. On cross-priming of MHC class I-specific CTL: rule or exception? Eur J Immunol. 2002;32:2385–92. doi: 10.1002/1521-4141(200209)32:9<2385::AID-IMMU2385>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 39.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 40.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–55. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 41.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–61. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 43.Boonman ZF, van Mierlo GJ, Fransen MF, de Keizer RJ, Jager MJ, Melief CJ, et al. Maintenance of immune tolerance depends on normal tissue homeostasis. J Immunol. 2005;175:4247–54. doi: 10.4049/jimmunol.175.7.4247. [DOI] [PubMed] [Google Scholar]
- 44.Kruck S, Gakis G, Stenzl A. Disseminated and circulating tumor cells for monitoring chemotherapy in urological tumors. Anticancer Res. 2011;31:2053–7. [PubMed] [Google Scholar]
- 45.Lianidou ES, Markou A. Circulating tumor cells as emerging tumor biomarkers in breast cancer. Clin Chem Lab Med. 2011;49:1579–90. doi: 10.1515/CCLM.2011.628. [DOI] [PubMed] [Google Scholar]
- 46.Lianidou ES, Markou A. Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin Chem. 2011;57:1242–55. doi: 10.1373/clinchem.2011.165068. [DOI] [PubMed] [Google Scholar]
- 47.Paterlini-Bréchot P. Organ-specific markers in circulating tumor cell screening: an early indicator of metastasis-capable malignancy. Future Oncol. 2011;7:849–71. doi: 10.2217/fon.11.32. [DOI] [PubMed] [Google Scholar]
- 48.Clobes H, Fossâ SD, Waehre H, Jocham D, Berner A. The immunohistochemical assessment of occult regional lymph node metastases in patients with T3pN0M0 prostate cancer before definitive radiotherapy. BJU Int. 2000;85:270–5. doi: 10.1046/j.1464-410x.2000.00406.x. [DOI] [PubMed] [Google Scholar]
- 49.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59:1071–9. [PubMed] [Google Scholar]
- 52.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 53.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–46. [PubMed] [Google Scholar]