Abstract
The success of immunotherapy relies on the participation of all arms of the immune system and the role of CD4+ T lymphocytes in preventing tumor growth is now well established. Understanding how tumors evade immune responses holds the key to the development of cancer immunotherapies. In this review, we discuss how MHC Class II expression varies in cancer cells and how this influences antitumor immune responses. We also discuss the means that are currently available for harnessing the MHC Class II antigen presentation pathway for the development of efficient vaccines to activate the immune system against cancer.
Keywords: HLA, MHC class II, cancer, immunotherapy, tumor, vaccine
Tumors and the Immune System
The interplay between immune and tumor cells is complex. Various genetic immunodeficiency syndromes have been linked to an increased incidence of tumors in mice.1 Moreover, many tumors downregulate the expression of MHC molecules, suggesting a role for the immune system in controlling the progression and evolution of cancer.2 Accordingly, solid tumors, stromal cells and neighboring tissues often are infiltrated by a vast array of immune cells.3 Generally, the magnitude of the T-cell infiltrate correlates with good prognosis.
The very recent FDA approval of sipuleucel-T (an autologous antigen-presenting cell-based vaccine) and the development of therapeutic antibodies that modulate T-cell responses announce the future of cancer immunotherapy as promising and bright.4 However, understanding why the immune system sometimes fails to destroy tumors is the key to the development of efficient anticancer vaccines, be they therapeutic or prophylactic. Impairments in the presentation or recognition of tumor-associated antigens (TAAs) in the context of either MHC class I or class II molecules certainly favors the evasion of tumor cells from immunosurveillance. However, we must bear in mind that antitumor responses may favor the growth of the fittest cancer cells through immunoediting.5
In this age of proteomic, genomic and other “omic” approaches, it has become fairly straightforward to characterize tumor cells in great details. Thus, the extent to which these cells differ from their normal counterparts, both at the genetic and epigenetic levels, has become quite apparent.6 Most tumor cell antigens are usually presented in the absence of a microbial aggression and the lack of danger signals may thus prevent the initiation of a protective immune response. This review will address the importance of helper T cells in cancer immunotherapy and will summarize current knowledge of the MHC class II antigen processing and presentation machinery in tumors.
Role of Adaptive CD4+ T-cell Responses in Tumor Eradication
MHC Class II molecules are crucial for the activation of CD4+ T cells. Patients affected by the Bare lymphocyte syndrome (BLS) Type II (MHC Class II deficiency) usually die of infections at a young age, making it difficult to assess cancer incidence.7 Still, evidence supporting the role of CD4+ T cells in the antitumor response is compelling, in both mice and humans. Tumor eradication following immunization with cancer cells or specific peptides relies on a functional CD4+ T-cell effector compartment, even for MHC Class II-negative tumors.8 A role for helper T cells in the direct mobilization of effector cytotoxic T lymphocytes (CTLs) to virus-infected tissues has recently been demonstrated. Such interplay may also prove to be critical in some cancers.9
It is well known that CD4+ helper T cells can directly mediate cytotoxicity against tumor cells.10 However, CD4+ helper T-cell activation generates regulatory T cells (Tregs) which may limit the success of immunotherapy in vivo.11 The exact role of pro-inflammatory interleukin (IL)-17-producing (Th17) cells, which have first been identified in the murine tumor microenvironment, remains to be established.12 According to studies performed in IL-17-deficient mice, Th17 cells may either promote or prevent tumor growth.
In recent years, the search for new TAAs has intensified, in part because of their importance as biomarkers in cancer diagnosis.13 Based on these discoveries, multiple therapeutic cancer vaccines designed to stimulate helper T cells have been developed. Despite evidence supporting the role of helper T-cell responses in tumor eradication, clinical studies in which melanoma patients received either Class I- or Class II-restricted peptides have yielded discordant results regarding the impact of Class II epitopes.14 The generation of effective helper T-cell responses requires a deeper understanding of the fine tuning of T-cell receptor (TCR)-transduced signals. For instance, modulating the functional avidity during antigen presentation might impact the generation of memory CD4+ T-cell responses.15 Given that avidity is an influencing factor in the escape of some CD4+ T cells from the induction of central tolerance, self-reactive clones can be enrolled in the fight against cancer by careful vaccination.16
Tumor Cells as Antigen-presenting Cells
At some point in their natural history, most tumors are able to present antigens and act as antigen-presenting cells (APCs).17 However, the lack of co-stimulatory molecules on tumor cells promote tolerance, thus exerting detrimental effects. Many solid tumors do not express MHC Class II and the involvement of CD4+ T cells depends mainly on infiltrating APCs that either pick up available antigens or engulf tumor cells. IL-2 and interferon γ (IFNγ)-producing tumor-infiltrating lymphocytes (TILs) help create an inflammatory, delayed type hypersensitivity (DTH)-type of microenvironment, thereby enabling tumor clearance through bystander killing.18 A tumor expressing MHC Class II could amplify such an immune response.
Why Does Antitumor T-cell Responses Often Prove Defective?
Considering the diversity of defense mechanisms that contribute to antitumor immunity, it is surprising that spontaneously arising cancer cells can proliferate to an extent that is lethal to the host. Hence, the mechanisms that facilitate immune evasion are likely to also hinder the efficacy immunotherapy. Such mechanisms include the presence of increased numbers of regulatory T cells (Tregs), reduced adhesion, reduced expression of co-stimulatory molecules, increased expression of FAS ligand (FASL) by tumor cells, the presence of inhibitory factors or regulatory cytokines such as transforming growth factor β (TGFβ) and altered signal transduction pathways in TILs, resulting in T-cell unresponsiveness.19 Although IFNγ undoubtedly favors an antitumor inflammation and promotes MHC Class II expression, it also has immunosuppressive effects. In many cells types, expression of indoleamine 2,3-dioxygenase-1 (IDO1) is strongly induced by IFNγ, and less so by Type I IFNs (IFNα and IFNβ).20 IDO, an intracellular heme-containing enzyme that catalyzes the initial, rate-limiting step in tryptophan degradation along with the kynurenine pathway, plays an important immunoregulatory role by inhibiting T lymphocyte functions and reprogramming Tregs.21 The importance of IDO1 in human cancers is now well documented.20
Subversion of MHC Class II Antigen Presentation in Tumors
Overview of the exogenous antigen presentation pathway
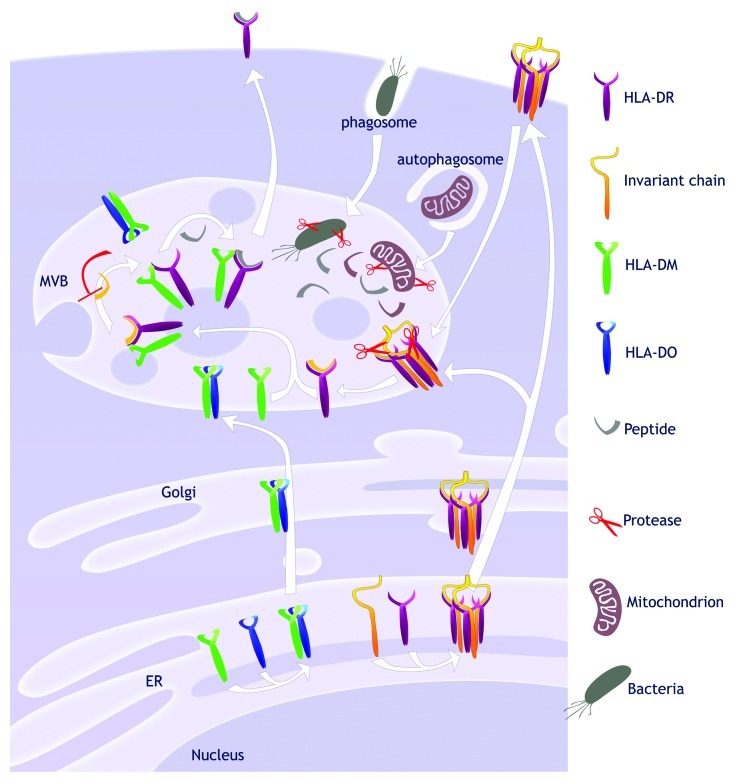
As opposed to MHC Class I, classical MHC Class II molecules (HLA-DR, -DP and -DQ) bind the invariant chain (Ii) and do not associate with peptides in the endoplasmic reticulum (ER) (Fig. 1). The Ii chaperone associates with folding MHC Class II, occupying the peptide-binding groove and preventing aggregation.22 Ii is then degraded in endosomes, ultimately leaving only a short Class II-associated invariant chain peptide (CLIP) inside the MHC Class II groove.
Figure 1. Antigen presentation pathway by MHC Class II. The MHC II-related proteins are synthesized in the endoplasmic reticulum (ER). Three MHC Class II αβ heterodimers bind to a trimer of invariant chains, thus forming a nonameric complex. That complex is directed to the multivesicular bodies (MVBs) directly via the trans-Golgi network or from the cell surface. In the MVBs, proteases degrade the invariant chain until only a small fragment called CLIP remains in the peptide-binding groove. Degradative processes in the acidic compartment generate peptides from the material acquired through phagocytosis or autophagy. The non-classical MHC Class II HLA-DM and -DO dimerize in the ER and localize in MVBs. Free HLA-DM interacts with HLA-DR and mediate peptide exchange following the inward budding of the limiting membrane. The peptide-loaded MHC Class II can then egress to the cell surface where antigen presentation takes place.
The peptide-binding groove of most MHC Class II molecules must be freed by the action of the non-classical MHC Class II HLA-DM. In most resting APCs, the function of HLA-DM is negatively regulated by HLA-DO.23
Endogenous TAAs can gain access to MHC Class II-loading compartments by multiple distinct means. For example, transmembrane proteins from the plasma membrane can be endocytosed and sent to lysosomes for degradation. Cytoplasmic and nuclear antigens can be engulfed by autophagy and hence they can encounter classical MHC Class II and HLA-DM.24 The MHC Class II antigen processing pathway can be significantly altered as part of the tumorigenesis process, thereby precluding efficient presentation of T-cell epitopes.
Patterns of MHC Class II expression in tumor cells
During the past 30 y, much research has focused on describing and characterizing the expression pattern of MHC Class II in mouse and human tumor cell lines or primary samples of various origins. Studies have yielded mixed results, mostly due to confounding factors that include tumor type, origin and source. As such, the prognostic value of MHC Class II expression is certainly not universal.
MHC Class II molecules are often expressed in tumors including colorectal and breast carcinomas. However, the correlation between such expression and clinical outcome has yet to be elucidated.25 Given that the breast epithelium does not typically express MHC Class II molecules, it is believed that the MHC expression phenotype arises in response to hormones or cytokines.26 In contrast, expression of key components of the MHC Class II pathway is often lost in MHC II+ cells.27 Moreover, differential constitutive or inducible expression of MHC Class II isotypes, mainly DR and DQ, occurs in many tumor types.28 In this context, many functional studies have addressed the capacity of MHC II+ tumor cells to present antigens. For instance, despite high levels of surface MHC Class II, peripheral blood B cells from B-cell chronic lymphocytic leukemia (B-CLL) patients have been shown to be poor stimulators in mixed lymphocyte reactions (MLR) and to have a limited capacity to present a model soluble antigen.29 Altogether, these results suggest that the impact of MHC Class II on disease outcome is the result of a delicate balance between intrinsic tumor factors and host factors regulating the immune response.
How do tumors of different types, origins and from different patients acquire different phenotypes regarding MHC Class II expression? The answer to this question lies into the transcriptional and post-transcriptional mechanisms regulating the expression of MHC Class II molecules as well as their various chaperones. As a general rule, genes involved in MHC Class II antigen presentation are co-regulated by the Class II transactivator (CIITA) (Fig. 2).30 CIITA binds to promoter elements involved in both constitutive MHC Class II expression and IFNγ-mediated induction. Some tumors do not upregulate MHC Class II molecules in response to IFNγ. This functional deficit may be due to defects in the CIITA synthesis, either at transcription, mRNA translation or protein stability levels. Very recently, reduced MHC Class II expression, as seen in some lymphomas, has been attributed to fusion transcripts caused by genomic breaks in the CIITA gene.31 BLIMP1, a transcription regulator expressed in plasma cells, downregulates CIITA transcription. BLIMP1 expression does not always show an inverse correlation with MHC Class II expression as CIITA is upregulated in multiple myeloma cells by IFNγ.32
Figure 2. Transcriptional regulation of MHC Class II genes. The binding of interferon γ (IFNγ) to its receptor at the cell surface leads to the transcriptional activation of the Class II transactivator (CIITA). This transcription factor binds to the promoters of the invariant chain and MHC Class II genes. BLIMP-1 can block the transcription at such promoters by directly inhibiting CIITA upregulation. The expression can of the invariant chain can also be modulated by NF-κB in response to various pro-inflammatory signals. On the other hand, the binding of interleukin-10 (IL-10) to its receptor at the cell surface triggers the upregulation of MARCH1.
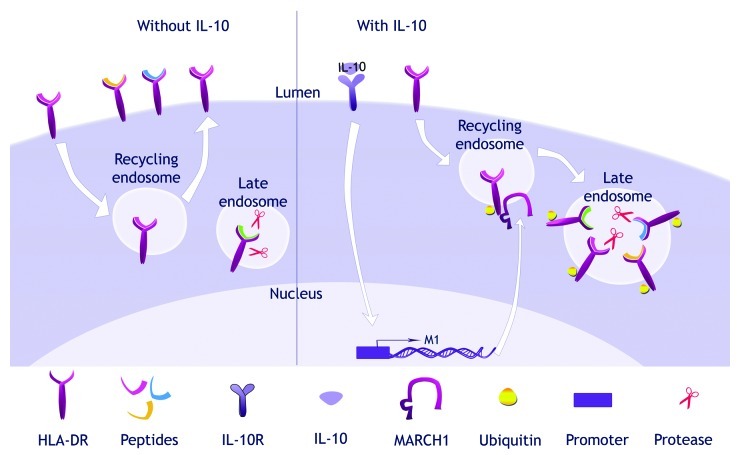
Finally, the cell surface exposure of MHC Class II molecules can be regulated, either indirectly, by modifications in the activity of chaperones, or directly, following the interaction with ubiquitin ligases of the membrane-associated RING-CH (MARCH) family. MARCH1 and MARCH8 can add ubiquitin to the cytoplasmic tail of MHC Class II molecules, leading to MHC Class II intracellular sequestration and degradation (Fig. 3).33 While MARCH8 is expressed rather ubiquitously, MARCH1 is induced by IL-10 in monocytes.34 MARCH1 is also expressed in resting murine and human B lymphocytes.35 Future studies will establish whether the limited exposure of MHC Class II molecules in some tumors is linked to the presence of MARCH proteins.
Figure 3. Post-translational regulation of MHC Class II trafficking. From the cell surface, the mature peptide-MHC Class II complexes are endocytosed and recycle back to the surface. In the presence of interleukin-10 (IL-10), MARCH1 is upregulated. MARCH1 and MHC Class II molecules interact in recycling endosomes and the MARCH1-mediated ubiquitination of MHC Class II prevents recycling. MHC Class II molecules are redirected to lysosomal compartments where they are degraded.
Patterns of Ii expression in tumor cells
In normal and neoplastic cells, the pattern of Ii expression generally correlates with that of MHC Class II molecules, even at the final stage of B cell maturation, when neither molecule is expressed. However, additional analyses revealed numerous instances of discordant expression patterns for these two molecules (see,36 for example). The Ii and MHC Class II genes share common CIITA-dependent regulatory elements. In addition, the human and mouse Ii promoters contain two functional NF-kB/Rel-binding sites, which either activate or inhibit expression depending on the cell type.37
The level of proteins, the proportion of the various isoforms, and the presence of cleavage products are some of the variables influencing the expression of Ii in various tumor types. In humans, Ii exists in four isoforms that originate from alternative splicing and alternative translation initiation sites.22 Translated from the most 5′ AUG triplet, the Iip35 isoform encodes an RxR (Arg-x-Arg) ER retention motif that is masked upon MHC Class II binding and Ii phosphorylation by protein kinase C (PKC).22 Intriguingly, high levels of Ii, and especially of Iip35, were found in hairy cell leukemia (HCL) and some B-CLL patients .38 Such an increase correlated with a high proportion of MHC Class II molecules bound to Iip35, and it was postulated that this tight association might prevent the binding of endogenous tumor antigens.39
The impact of Ii on endogenous antigen presentation by MHC Class II molecules has been mainly addressed in the context of tumor vaccines. Tumor cells genetically engineered to express MHC Class II can be very efficient in activating the immune system, provided that they do not express Ii.40 It is assumed that, in the absence of Ii, the palette of antigens (including TAAs) capable of binding MHC Class II molecules increases over a wider range of compartments. Ii is expressed by many hematological malignancies and the Ii-specific humanized monoclonal antibody milatuzumab is now used as immunotherapeutic agent.41
Patterns of HLA-DM and HLA-DO expression in tumors
The combined action of HLA-DM and HLA-DO affects the level of CLIP at the cell surface.23 When Ii is normally expressed, CLIP levels are inversely and directly proportional to HLA-DM and HLA-DO levels, respectively. Because CLIP prevents the binding of antigenic peptides, these non-classical chaperones have a profound impact on the immune response. Yet, the importance of HLA-DM is still the object of an intense debate, as it was shown in a mouse model that tumor cells transfected with MHC Class II and HLA-DM, with or without Ii, can be highly immunogenic.42 Thus, it is likely that HLA-DM plays a critical role only in the context of Ii expression.
HLA-DM is co-regulated with HLA-DR. Interestingly, low CLIP occupancy of MHC Class II molecules has been reported in a number of malignancies. In tumor cells, expression of HLA-DM has been associated with a Th1 cytokine profile and shown to predict better survival in breast carcinoma patients.43 This is in line with the role of HLA-DM in reducing CLIP at the cell surface, thereby avoiding Th2 polarization.44 Leukemic blasts also lack CLIP at the cell surface, which promotes the activation of specific CD4+ Th1 cells.45 In addition, some pre-B acute lymphocytic leukemia (ALL) (namely ETV6-AML1) display low amounts of CLIP, perhaps inducing a favorable immune response that delays relapse.46 More recently, microarray studies of ovarian cancer cells revealed that high HLA-DMβ expression correlates with improved survival.47 On the other hand, Reed-Sternberg cells in malignant Hodgkin’s disease and myeloid leukemic blasts present high levels of CLIP, which in the latter case predicts poor clinical outcomes.48
Little is known about the potential implication of HLA-DO in antitumor responses. Interestingly, an amino acid change in HLA-DOα was discovered in a patient affected by chronic myeloid leukemia (CML).49 However, this mutation did not appear to affect the function of HLA-DO. In B-CLL, HLA-DO gene expression is often increased. Although higher HLA-DO transcript levels did not translate into higher protein levels, increased gene expression was still deemed to correlate with poor survival.50 Further studies aimed at identifying and characterizing components of the MHC Class II antigen presentation pathway will allow to better grasp the impact of CLIP and peptide loading on clinical parameters of tumor immunology.
Modulation of MHC Class II accessory molecules in tumors
Presentation of peptides in normal cells depends on efficient synthesis, sorting and processing of antigens as well as on the proper trafficking and maturation of MHC Class II molecules. In tumor cells, intrinsic modifications of a cellular compartment and its components such as lipids and enzymes are likely to influence, directly or indirectly, the processing, loading and presentation of antigens to T cells. Several examples of such potentially clinically-relevant perturbations are given below.
Defects in autophagy have been associated with cellular transformation. Because autophagy has been intimately linked to antigen processing by MHC Class II molecules in a variety of systems,51 autophagy-deficient tumors are likely to exhibit defects in the processing of certain antigens.52 Endosomal/lysosomal protease regulation can have a tremendous negative impact on the generation of T-cell epitopes and on Ii degradation.53
As mentioned above, many murine tumor cell lines do not express or upregulate the MHC Class II antigen presentation machinery in response to IFNγ. In melanoma, the absence of IFNγ-inducible lysosomal thiol reductase (GILT) disrupts T-cell recognition of immunodominant epitopes.54 Additionally, in head and neck cancer cells, CIITA does not induce cathepsin S, a cysteine protease involved in the late stage of Ii processing.55 As new alterations are continuously found in tumor cells, the characterization of their effects on adaptive responses in the context of immune evasion will undoubtedly uncover many surprises.
Counteracting the Subversion of Antigen Presentation
Several methods have been considered to maximize antigen presentation. For instance, tumor cells expressing MHC molecules are being used as vaccines. However, the more common approach is to transfer natural or artificial APCs that have been manipulated in vitro to display defined antigens (loaded under controlled conditions). Other in vivo approaches are being developed to limit the manipulations of host cells and to avoid cumbersome personalized immunotherapy. In this last section, we will address the needs to discover additional TAAs, improve cellular vaccines and define alternative methods to effectively stimulate CD4+ T cells.
Discovery of novel TAAs and T-cell epitopes
Tumor-specific antigens (TSAs) may result from gene mutations or from the expression of alternative open reading frames resulting from chromosomal rearrangements.56 In recent years, TSAs and TAAs have been discovered at a regular pace. TAAs are often found in normal tissues. Thus, while breaking tolerance to these antigens through vaccination should result in tumor recognition, it may also lead to autoimmunity. It should be kept in mind that other treatments such as chemotherapy have the potential to modify the proteome of cancer cells and provide new targets for immunotherapy.57
The genetic diversity at the MHC I and II loci is another hurdle in the development of effective immunotherapy. The identification of new epitopes recognized in the context of a series of isotypes and alleles should open the door to a more universal use of immunotherapy. Defining immunopeptidomes specific to different cancer-patient combinations should produce valuable information. Mass spectrometry is used to map MHC Class I and II binding antigens. As the sensitivity and efficacy of this analytical method constantly improve, peptides of very low abundance should be identified more easily, enabling the definition of novel TSAs that may have originated from processes including alternative splicing.58
Cellular vaccines
A variety of cell-based approaches has been considered as a means to increase cancer-specific immune responses. These therapeutic protocols are mostly based on the transfer of modified tumor cells, APCs or engineered T cells.
Tumor vaccines
The rationale underlying tumor vaccines is that tumor cells, although poorly immunogenic themselves, express a full complement of endogenous TAAs. To increase T-cell activation, tumor vaccines can be genetically modified with elements of the MHC Class II processing and presentation machinery.59 Treatment of cells with cytokines that promote the processing of endogenous (even nuclear) antigens through autophagy may increase the variety of T-cell epitopes generated in tumor-cell vaccines.60
Interestingly, many groups reported that Ii expression was detrimental to the presentation of endogenous antigens in mouse and human tumor cells. For example, the depletion of Ii by various means has been shown to increase the presentation of some antigens and to improve the efficacy of immunotherapy.40
As mentioned above, because many tumors do not express either classical or non-classical MHC Class II, they need to be further manipulated in vitro. IFNγ upregulates the MHC Class II antigen presentation machinery as well as more than 200 additional genes.61 In the presence of IFNγ, some tumors gain full antigen presentation properties (see above). For tumors that do not respond to IFNγ, genetic engineering can be considered. However, although complete rejection and antitumor memory have been demonstrated in mice immunized with CIITA-expressing tumor cell lines,62 other studies have cast doubts on the efficiency of such an approach.63 In addition, the introduction of CIITA has been achieved in cellular vaccines, yet some tumors do not fully respond to this transactivator and some genes have been reported to remain silent.64 Under such conditions, gene expression profiles should be carefully monitored to ensure that the desired antigen presentation machinery is upregulated.
Dendritic cell-based vaccines
One of the most promising therapeutic cancer vaccines is based on dendritic cells (DCs).65 Different methods have been used to display specific T-cell epitopes on APCs. For instance, tumor cell lysates can be pulsed onto DCs,66 or recombinant antigens can be coupled to monoclonal antibodies directed against DC surface receptors.67 In this approach, the choice of the receptor is critical, given that most receptors only allow for presentation on either MHC I or MHC Class II.68,69 Moreover, because of their heterogeneity, some DC subsets are more efficient than others at MHC Class I cross-presentation or MHC Class II presentation, which adds a further level of complexity to this approach.69
Synthetic peptides that correspond to carefully selected epitopes constitute the most useful antigens. Their formulation has evolved in recent years. For example, multi-epitope Trojan antigen peptide vaccines and peptides with overlapping CD4-specific and CD8-specific epitopes can induce both CTL and helper immune responses.70 However, even though empty MHC Class II molecules are expressed at the surface of DCs, their loading is rather inefficient. Chemical agents that can break hydrogen bonds linking low affinity peptides to HLA-DR have recently been discovered.71 Moreover, small molecules that can enhance the catalytic activity of HLA-DM have been identified.72 Other genetic approaches aimed at delivering antigens to DCs for the induction of a CD4+ T-cell response have been described.73
B cell-based vaccines
Alternative sources of APCs have also been evaluated in vitro. For instance, B lymphocytes stimulated by the CD40 ligand (CD40L) proliferate in high numbers74 and display a wider array of MHC Class II epitopes due to lowered HLA-DM/HLA-DO ratios.75 B cells serve as efficient APCs for the expansion of TAA-specific CD8+ and CD4+ T cells. Interestingly, not only can B cells present MHC Class II epitopes independently of the specificity of their B-cell receptor (BCR), when pulsed exogenously, but they can also promote MHC Class I cross-presentation.76
Surrogate APCs
To overcome the need for living autologous hematopoietic cells in immunotherapy, alternative ways whereby only basic antigen presentation requirements are expressed on “artificial” supports have been developed. Such alternatives include cellular systems such as fibroblasts and Drosophila cells77 or acellular artificial APCs that consist of microbeads, liposomes or exosomes.78,79
Adoptive T-cell therapy — Ex vivo-expanded and genetically engineered T cells
To counteract the production of immunosuppressive cytokines by tumors, autologous T cells normally are activated and expanded ex vivo before adoptive transfer. Clinical remissions have been observed in melanoma patients treated with CD4+ T cells expanded ex vivo in the presence of tumor antigens.80 The benefit of using CD4+ T cells was recently demonstrated in a MHC Class II-negative ovarian cancer model.81
Efficiency can be maximized by expressing high affinity TAA-specific recombinant TCRs in recipient human T cells. Such T-cell clones can expand, secrete cytokines and lyse target cells.82 Still, the loss of HLA molecules at the surface of tumor cells represents an important barrier for both conventional and engineered MHC-restricted T cells. One way of bypassing the TCR-MHC axis that has been developed relies on chimeric antigen receptors (CARs). The rationale of this approach is that the TAA-specific binding domain of a single-chain (scFv) antibody endows T cells with a defined specificity and the intracellular portion of the chimeric receptor triggers signal transduction upon ligand binding. Using recombinant DNA technology, different types of CARs have been generated with various combinations of specific parts of the TCR/CD3 complex, immunoglobulins (usually derived from a mouse B-cell hybridoma) and intracellular domains of co-stimulators such as CD28, 41BB or OX40.83
One hurdle in the development of antibody-based CARs is the need to target TAAs that are displayed at the cell surface. Fortunately, the search for novel specificities in different types of tumors is ongoing, as monoclonal antibodies are used in anticancer therapy and as prognostic tools.84 Recombinant monoclonal antibody-superantigen fusion proteins have also been used to activate large fractions of the T-cell repertoire at the tumor site.85
Conclusions
To understand the role of CD4+ T cells in the antitumor response, as well as that played by CD4+ Tregs, studies in humans will need to decipher the pathways leading to the generation of various helper T-cell subsets, and to the presentation of immunogenic (as opposed to tolerogenic) MHC Class II epitopes. Also, we must develop new methods to increase antigen presentation via the in vivo targeting of immunogens. For example, electrodes have been designed for the in vivo DNA delivery by direct electroporation.86 Such an approach may seem extreme, but we will need more ingenious ideas to spark the field of cancer vaccination and, as usual, only imagination will be a limit to innovation.
Acknowledgments
MCBD was supported by a studentship from the Cole Foundation. This work was supported by a grant from the Canadian Institutes of Health Research and the Cancer Research Society Inc. to JT and grants from the Canadian Institutes of Health Research and the Cancer Research Society Inc. to RL.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21205
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60:1161–71. doi: 10.1007/s00262-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33:335–40. doi: 10.1007/s00281-011-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–9. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim MS, Elenitoba-Johnson KS. The molecular pathology of primary immunodeficiencies. J Mol Diagn. 2004;6:59–83. doi: 10.1016/S1525-1578(10)60493-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corthay A, Skovseth DK, Lundin KU, Røsjø E, Omholt H, Hofgaard PO, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–83. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quezada SA, Peggs KS. Tumor-reactive CD4+ T cells: plasticity beyond helper and regulatory activities. Immunotherapy. 2011;3:915–7. doi: 10.2217/imt.11.83. [DOI] [PubMed] [Google Scholar]
- 11.Nizar S, Copier J, Meyer B, Bodman-Smith M, Galustian C, Kumar D, et al. T-regulatory cell modulation: the future of cancer immunotherapy? Br J Cancer. 2009;100:1697–703. doi: 10.1038/sj.bjc.6605040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–9. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Peng B, Lu Y, Xu W, Qian W, Zhang JY. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun Rev. 2011;10:331–5. doi: 10.1016/j.autrev.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khong HT, Yang JC, Topalian SL, Sherry RM, Mavroukakis SA, White DE, et al. Immunization of HLA-A*0201 and/or HLA-DPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J Immunother. 2004;27:472–7. doi: 10.1097/00002371-200411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caserta S, Kleczkowska J, Mondino A, Zamoyska R. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J Immunol. 2010;185:6545–54. doi: 10.4049/jimmunol.1001867. [DOI] [PubMed] [Google Scholar]
- 16.Huber V, Benkhoucha M, Huard B. Evidence for a repertoire of functional untolerized CD4+ T cells specific for melanoma-associated antigens. Scand J Immunol. 2011;74:80–6. doi: 10.1111/j.1365-3083.2011.02548.x. [DOI] [PubMed] [Google Scholar]
- 17.Mortara L, Frangione V, Castellani P, De Lerma Barbaro A, Accolla RS. Irradiated CIITA-positive mammary adenocarcinoma cells act as a potent anti-tumor-preventive vaccine by inducing tumor-specific CD4+ T cell priming and CD8+ T cell effector functions. Int Immunol. 2009;21:655–65. doi: 10.1093/intimm/dxp034. [DOI] [PubMed] [Google Scholar]
- 18.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207:2469–77. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer WH, thor Straten P, Terheyden P, Becker JC. Function and dysfunction of CD4(+) T cells in the immune response to melanoma. Cancer Immunol Immunother. 1999;48:363–70. doi: 10.1007/s002620050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Stumptner-Cuvelette P, Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim Biophys Acta. 2002;1542:1–13. doi: 10.1016/S0167-4889(01)00166-5. [DOI] [PubMed] [Google Scholar]
- 23.Alfonso C, Liljedahl M, Winqvist O, Surh CD, Peterson PA, Fung-Leung WP, et al. The role of H2-O and HLA-DO in major histocompatibility complex class II-restricted antigen processing and presentation. Immunol Rev. 1999;172:255–66. doi: 10.1111/j.1600-065X.1999.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 24.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–6. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 25.Durrant LG, Ballantyne KC, Armitage NC, Robins RA, Marksman R, Hardcastle JD, et al. Quantitation of MHC antigen expression on colorectal tumours and its association with tumour progression. Br J Cancer. 1987;56:425–32. doi: 10.1038/bjc.1987.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabibzadeh SS, Sivarajah A, Carpenter D, Ohlsson-Wilhelm BM, Satyaswaroop PG. Modulation of HLA-DR expression in epithelial cells by interleukin 1 and estradiol-17 beta. J Clin Endocrinol Metab. 1990;71:740–7. doi: 10.1210/jcem-71-3-740. [DOI] [PubMed] [Google Scholar]
- 27.Momburg F, Herrmann B, Moldenhauer G, Möller P. B-cell lymphomas of high-grade malignancy frequently lack HLA-DR, -DP and -DQ antigens and associated invariant chain. Int J Cancer. 1987;40:598–603. doi: 10.1002/ijc.2910400504. [DOI] [PubMed] [Google Scholar]
- 28.Oldford SA, Robb JD, Watson PH, Drover S. HLA-DRB alleles are differentially expressed by tumor cells in breast carcinoma. Int J Cancer. 2004;112:399–406. doi: 10.1002/ijc.20441. [DOI] [PubMed] [Google Scholar]
- 29.Dazzi F, D’Andrea E, Biasi G, De Silvestro G, Gaidano G, Schena M, et al. Failure of B cells of chronic lymphocytic leukemia in presenting soluble and alloantigens. Clin Immunol Immunopathol. 1995;75:26–32. doi: 10.1006/clin.1995.1048. [DOI] [PubMed] [Google Scholar]
- 30.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109(Suppl):S21–33. doi: 10.1016/S0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 31.Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377–81. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao M, Flynt FL, Hong M, Chen H, Gilbert CA, Briley NT, et al. MHC class II transactivator (CIITA) expression is upregulated in multiple myeloma cells by IFN-gamma. Mol Immunol. 2007;44:2923–32. doi: 10.1016/j.molimm.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohmura-Hoshino M, Goto E, Matsuki Y, Aoki M, Mito M, Uematsu M, et al. A novel family of membrane-bound E3 ubiquitin ligases. J Biochem. 2006;140:147–54. doi: 10.1093/jb/mvj160. [DOI] [PubMed] [Google Scholar]
- 34.Thibodeau J, Bourgeois-Daigneault MC, Huppé G, Tremblay J, Aumont A, Houde M, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38:1225–30. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galbas T, Steimle V, Lapointe R, Ishido S, Thibodeau J. MARCH1 down-regulation in IL-10-activated B cells increases MHC class II expression. Cytokine. 2012;59:27–30. doi: 10.1016/j.cyto.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Degener T, Momburg F, Möller P. Differential expression of HLA-DR, HLA-DP, HLA-DQ and associated invariant chain (Ii) in normal colorectal mucosa, adenoma and carcinoma. Virchows Arch A Pathol Anat Histopathol. 1988;412:315–22. doi: 10.1007/BF00750257. [DOI] [PubMed] [Google Scholar]
- 37.Brown AM, Linhoff MW, Stein B, Wright KL, Baldwin AS, Jr., Basta PV, et al. Function of NF-kappa B/Rel binding sites in the major histocompatibility complex class II invariant chain promoter is dependent on cell-specific binding of different NF-kappa B/Rel subunits. Mol Cell Biol. 1994;14:2926–35. doi: 10.1128/mcb.14.5.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiro RC, Sairenji T, Humphreys RE. Identification of hairy cell leukemia subset defining p35 as the human homologue of Ii. Leuk Res. 1984;8:55–62. doi: 10.1016/0145-2126(84)90031-6. [DOI] [PubMed] [Google Scholar]
- 39.Narni F, Kudo J, Mars W, Calabretta B, Florine DL, Barlogie B, et al. HLA-DR-associated invariant chain is highly expressed in chronic lymphocytic leukemia. Blood. 1986;68:372–7. [PubMed] [Google Scholar]
- 40.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol Immunother. 2008;57:389–98. doi: 10.1007/s00262-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frolich D, Blabetafeld D, Reiter K, Giesecke C, Daridon C, Mei HE, et al. The anti-CD74 humanized monoclonal antibody, milatuzumab, which targets the invariant chain of MHC II complexes, alters B-cell proliferation, migration, and adhesion molecule expression. Arthritis Res Ther. 2012;14:R54. doi: 10.1186/ar3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci U S A. 1997;94:6886–91. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldford SA, Robb JD, Codner D, Gadag V, Watson PH, Drover S. Tumor cell expression of HLA-DM associates with a Th1 profile and predicts improved survival in breast carcinoma patients. Int Immunol. 2006;18:1591–602. doi: 10.1093/intimm/dxl092. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi P, Hengeveld R, Zechel MA, Lee-Chan E, Singh B. The functional role of class II-associated invariant chain peptide (CLIP) in its ability to variably modulate immune responses. Int Immunol. 2000;12:757–65. doi: 10.1093/intimm/12.6.757. [DOI] [PubMed] [Google Scholar]
- 45.van Luijn MM, van den Ancker W, Chamuleau ME, Zevenbergen A, Westers TM, Ossenkoppele GJ, et al. Absence of class II-associated invariant chain peptide on leukemic blasts of patients promotes activation of autologous leukemia-reactive CD4+ T cells. Cancer Res. 2011;71:2507–17. doi: 10.1158/0008-5472.CAN-10-3689. [DOI] [PubMed] [Google Scholar]
- 46.Jastaniah WA, Alessandri AJ, Reid GS, Schultz KR. HLA-DM expression is elevated in ETV6-AML1 translocation-positive pediatric acute lymphoblastic leukemia. Leuk Res. 2006;30:487–9. doi: 10.1016/j.leukres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Callahan MJ, Nagymanyoki Z, Bonome T, Johnson ME, Litkouhi B, Sullivan EH, et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res. 2008;14:7667–73. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chamuleau ME, Souwer Y, Van Ham SM, Zevenbergen A, Westers TM, Berkhof J, et al. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–50. doi: 10.1158/0008-5472.CAN-04-1350. [DOI] [PubMed] [Google Scholar]
- 49.van Lith M, van Ham M, Neefjes J. Novel polymorphisms in HLA-DOA and HLA-DOB in B-cell malignancies. Immunogenetics. 2002;54:591–5. doi: 10.1007/s00251-002-0500-6. [DOI] [PubMed] [Google Scholar]
- 50.Souwer Y, Chamuleau ME, van de Loosdrecht AA, Tolosa E, Jorritsma T, Muris JJ, et al. Detection of aberrant transcription of major histocompatibility complex class II antigen presentation genes in chronic lymphocytic leukaemia identifies HLA-DOA mRNA as a prognostic factor for survival. Br J Haematol. 2009;145:334–43. doi: 10.1111/j.1365-2141.2009.07625.x. [DOI] [PubMed] [Google Scholar]
- 51.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335–41. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watts C, Moss CX, Mazzeo D, West MA, Matthews SP, Li DN, et al. Creation versus destruction of T cell epitopes in the class II MHC pathway. Ann N Y Acad Sci. 2003;987:9–14. doi: 10.1111/j.1749-6632.2003.tb06028.x. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein OG, Hajiaghamohseni LM, Amria S, Sundaram K, Reddy SV, Haque A. Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma. Cancer Immunol Immunother. 2008;57:1461–70. doi: 10.1007/s00262-008-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meissner M, Whiteside TL, Kaufmann R, Seliger B. CIITA versus IFN-gamma induced MHC class II expression in head and neck cancer cells. Arch Dermatol Res. 2009;301:189–93. doi: 10.1007/s00403-008-0922-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang RF, Wang X, Rosenberg SA. Identification of a novel major histocompatibility complex class II-restricted tumor antigen resulting from a chromosomal rearrangement recognized by CD4(+) T cells. J Exp Med. 1999;189:1659–68. doi: 10.1084/jem.189.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang JT, Liu Y. Use of comparative proteomics to identify potential resistance mechanisms in cancer treatment. Cancer Treat Rev. 2007;33:741–56. doi: 10.1016/j.ctrv.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berkers CR, de Jong A, Ovaa H, Rodenko B. Transpeptidation and reverse proteolysis and their consequences for immunity. Int J Biochem Cell Biol. 2009;41:66–71. doi: 10.1016/j.biocel.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 59.Anderson KS. Tumor vaccines for breast cancer. Cancer Invest. 2009;27:361–8. doi: 10.1080/07357900802574421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.English L, Chemali M, Desjardins M. Nuclear membrane-derived autophagy, a novel process that participates in the presentation of endogenous viral antigens during HSV-1 infection. Autophagy. 2009;5:1026–9. doi: 10.4161/auto.5.7.9163. [DOI] [PubMed] [Google Scholar]
- 61.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 62.Frangione V, Mortara L, Castellani P, De Lerma Barbaro A, Accolla RS. CIITA-driven MHC-II positive tumor cells: preventive vaccines and superior generators of antitumor CD4+ T lymphocytes for immunotherapy. Int J Cancer. 2010;127:1614–24. doi: 10.1002/ijc.25183. [DOI] [PubMed] [Google Scholar]
- 63.Martin BK, Frelinger JG, Ting JP. Combination gene therapy with CD86 and the MHC class II transactivator in the control of lung tumor growth. J Immunol. 1999;162:6663–70. [PubMed] [Google Scholar]
- 64.Baton F, Deruyffelaere C, Chapin M, Prod’homme T, Charron D, Al-Daccak R, et al. Class II transactivator (CIITA) isoform expression and activity in melanoma. Melanoma Res. 2004;14:453–61. doi: 10.1097/00008390-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1513–37. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 67.Pudney VA, Metheringham RL, Gunn B, Spendlove I, Ramage JM, Durrant LG. DNA vaccination with T-cell epitopes encoded within Ab molecules induces high-avidity anti-tumor CD8+ T cells. Eur J Immunol. 2010;40:899–910. doi: 10.1002/eji.200939857. [DOI] [PubMed] [Google Scholar]
- 68.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 69.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 70.Lu J, Higashimoto Y, Appella E, Celis E. Multiepitope Trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J Immunol. 2004;172:4575–82. doi: 10.4049/jimmunol.172.7.4575. [DOI] [PubMed] [Google Scholar]
- 71.Falk K, Lau JM, Santambrogio L, Esteban VM, Puentes F, Rotzschke O, et al. Ligand exchange of major histocompatibility complex class II proteins is triggered by H-bond donor groups of small molecules. J Biol Chem. 2002;277:2709–15. doi: 10.1074/jbc.M109098200. [DOI] [PubMed] [Google Scholar]
- 72.Nicholson MJ, Moradi B, Seth NP, Xing X, Cuny GD, Stein RL, et al. Small molecules that enhance the catalytic efficiency of HLA-DM. J Immunol. 2006;176:4208–20. doi: 10.4049/jimmunol.176.7.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonehill A, Heirman C, Thielemans K. Genetic approaches for the induction of a CD4+ T cell response in cancer immunotherapy. J Gene Med. 2005;7:686–95. doi: 10.1002/jgm.713. [DOI] [PubMed] [Google Scholar]
- 74.Banchereau J, de Paoli P, Vallé A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–2. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 75.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 76.Leclerc D, Beauseigle D, Denis J, Morin H, Paré C, Lamarre A, et al. Proteasome-independent major histocompatibility complex class I cross-presentation mediated by papaya mosaic virus-like particles leads to expansion of specific human T cells. J Virol. 2007;81:1319–26. doi: 10.1128/JVI.01720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butler MO, Ansén S, Tanaka M, Imataki O, Berezovskaya A, Mooney MM, et al. A panel of human cell-based artificial APC enables the expansion of long-lived antigen-specific CD4+ T cells restricted by prevalent HLA-DR alleles. Int Immunol. 2010;22:863–73. doi: 10.1093/intimm/dxq440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steenblock ER, Wrzesinski SH, Flavell RA, Fahmy TM. Antigen presentation on artificial acellular substrates: modular systems for flexible, adaptable immunotherapy. Expert Opin Biol Ther. 2009;9:451–64. doi: 10.1517/14712590902849216. [DOI] [PubMed] [Google Scholar]
- 79.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 80.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nesbeth YC, Martinez DG, Toraya S, Scarlett UK, Cubillos-Ruiz JR, Rutkowski MR, et al. CD4+ T cells elicit host immune responses to MHC class II-negative ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol. 2010;184:5654–62. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29:550–7. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almåsbak H, Lundby M, Rasmussen AM. Non-MHC-dependent redirected T cells against tumor cells. Methods Mol Biol. 2010;629:453–93. doi: 10.1007/978-1-60761-657-3_28. [DOI] [PubMed] [Google Scholar]
- 84.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–4. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dohlsten M, Kalland T, Gunnarsson P, Antonsson P, Molander A, Olsson J, et al. Man-made superantigens: Tumor-selective agents for T-cell-based therapy. Adv Drug Deliv Rev. 1998;31:131–42. doi: 10.1016/S0169-409X(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 86.Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20:1269–78. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]